Introduction
Cancer invasion and metastasis are difficult
problems to overcome in cancer intervention. Genomics and
transcriptional technology have been used to study the resected
liver specimens of patients with hepatocellular carcinoma, as well
as the molecular genetic features and gene expression profile in
nude mouse and cell models of metastatic human hepatocellular
carcinoma. It was identified that changes to the genes associated
with liver metastasis occurred in the primary tumor stage and
confirmed that osteopontin (OPN) had a significant predictive value
and that it was the key transfer factor in hepatocellular carcinoma
(1,2). This provided a new basis for the
early diagnosis of hepatocellular carcinoma and for post-operative
non-surgical intervention. These studies primarily answered the
question of what invasion and metastasis of hepatocellular
carcinoma are, but there have been few clinical studies concerning
drug intervention in the invasion and metastasis of hepatocellular
carcinoma. The present study aimed to evaluate whether thalidomide
was able to inhibit the invasion and metastasis of hepatocellular
carcinoma.
Thalidomide was first widely popularized and applied
in West Germany in 1953 as a non-barbiturate sedative-hypnotic,
mainly for the prevention of morning sickness. However, due to the
teratogenic events (the side-effects were confined to pregnant
women) associated with the drug, its use was forbidden in 1961. In
1991, D’Amato et al(3)
identified that the teratogenic effect of thalidomide was related
to the inhibition of new blood vessel formation. Subsequently,
thalidomide was once more a focus of attention due to its effects
in certain malignant tumors, particularly multiple myelomas.
Thalidomide has also been identified to have extensive immune
regulatory and anti-angiogenic effects. In 1998, thalidomide was
approved by the FDA for use in clinical trials. There have been
worldwide studies on thalidomide intervention in malignant tumors,
however this cheap and well-known drug has commonly been limited in
its use due to its unknown mechanism of action and the lack of
support from evidence-based medical studies (4–7).
Thalidomide may act via a series of cascading
effects with OPN involving new cell signaling pathways or media to
control the expression and molecular behavior of intercellular
substances in the hepatocellular carcinoma tumor microenvironment,
and thereby directly or indirectly repress the invasion and
metastasis of hepatocellular carcinoma. The elucidation of its
mechanism may facilitate significant improvements in
structure-activity studies of thalidomide and promote its use in
tumor translational medicine.
Materials and methods
General data
A total of 36 BALB/C male nude mice (aged 6–7 weeks
old), were purchased from the Laboratory Animal Center of the
Medical College of Guangzhou Medical University. The MHCC97 cells
were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
The present study was carried out in strict accordance with the
recommendations in the National Institutes of Health Guide for the
Care and Use of Laboratory Animals. The protocol for animal use was
reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) of the First People’s Hospital Affiliated to
Guangzhou Medical University (Guangzhou, China).
Establishment of the nude mouse model of
hepatocellular carcinoma
Following successful anesthesia, the nude mice were
fixed in the prone position and conventional skin disinfection was
performed. A 1-cm straight incision was made next to the spine 0.5
cm from the left costal margin, descending layer by layer into the
abdomen. The incision was bluntly drawn to expose the spleen and
then 0.1 ml MHCC97 single cell suspension was drawn up into a 1-ml
insulin syringe and injected 0.3–0.4 cm into the spleen. Subsequent
to being injected with 0.02 ml (∼8×106 cells per mouse),
the syringe was quickly withdrawn. The conditions of the mice were
observed, including whether or not there was bleeding or intestinal
oppression in the spleen and whether there was any contortion in
the intestinal tract or mesentery. The abdomen was then closed
layer by layer using a needle and 5-0 sutures.
Partial hepatectomy and post-operative
management of the nude mice
A partial hepatectomy of the liver was performed 14
days after splenic subcapsular inoculation with the MHCC97 cells.
The nude mice were fed separately following the hepatectomy. The
number of mice in each cage was also reduced as far as possible,
generally to two mice in each cage. At one week post-hepatectomy,
the mice were already being fed with sterile solid food, thereafter
they were fed by injections of sterile water or physiological
saline.
Animal groups and specimens
Following the establishment of the hepatocellular
carcinoma model, the nude mice were randomly divided into three
groups, each consisting of 12 mice. Subsequent to the partial
hepatectomy, the early intervention group were administered 5 mg/kg
thalidomide as an immediate post-operative intervention, with
continuous administration for one week. Specifically, the
post-operatively anesthetized mice were fixed in an injection frame
and 0.1 ml (150 mg/ml) thalidomide was injected into the tail vein
with a 1-ml syringe. The late intervention group were treated with
0.1 ml (150 mg/ml) thalidomide via injection into the tail vein as
a post-operative intervention one week after the surgery. They were
also provided with continuous administration for one week.
Following the partial hepatectomy, the negative control group were
immediately injected with a placebo of 0.1 ml 0.9% physiological
saline into the tail vein as the replacement intervention.
Continuous administration was also provided for one week. The mice
were sacrificed immediately prior to dissection. The liver tumor
tissues were dissected, separated and fixed in formalin 21 days
after the partial hepatectomy. The OPN contents of the liver tumors
and paracarcinomatous tissues were detected using
immunohistochemistry.
Immunohistochemistry
Conventional perfusion fixation with 4%
paraformaldehyde was performed on the mice. Samples were cut into
slices. The primary antibody (rabbit anti-OPN antibody) was diluted
with serum diluent (bovine serum albumin 1.00 g, 0.01 M PBS 100 ml
and hydraulic nano 0.08 g) and added to the slices, which were kept
at 4°C for 24–48 h. Subsequent to blotting the antibody, the slices
were washed three times with 0.01 M KPBS for 5 min. In each group
there were negative control groups, which included non-diluted
primary antibodies (rabbit anti-OPN antibodies), secondary
antibodies diluted with 0.01 M KPBS (anti-rabbit antibodies) and
antibodies containing ABC or other complexes. The slices were
incubated at room temperature for 2 h, washed three times with 0.01
M KPBS for 5 min and then rapidly washed three times with distilled
water. DAB chromogen liquid was added to aid immunohistochemical
analysis. Following dehydration with gradient alcohol, the slices
were made transparent, sealed and images were captured.
Calculation of results
The results of the staining process were judged
using Bresalier semi-quantitative formulae. A total of 10 fields of
view were randomly selected in each slice using high magnification
(×200) and the double-blind method. The fields of view were divided
into four grades according to the cell staining intensity and then
scored as follows. Negative cells (-) showed no coloration (0
point); weakly positive cells (+) were light yellow (1 point);
moderately positive cells (++) were claybank in color (2 points);
and strongly positive cells (+++) were brown (3 points). The number
of fields of view of each strength were counted and the average
stain strength of each slice was calculated according to the
following formula: IS (intensity score) = Σ [(0 × F0) + (1 × F1) +
(2 × F2) + (3 × F 3)], F = % × 10 fields of view.
Statistical analysis
Statistical analyses were performed using SPSS
version 14.0 (SPSS, Inc., Chicago, IL, USA). The data are presented
as mean ± SD and a t-test and one-way analysis of variance (ANOVA)
was used for the comparison of the data between the groups.
P<0.0091 was considered to indicate a statistically significant
result.
Results
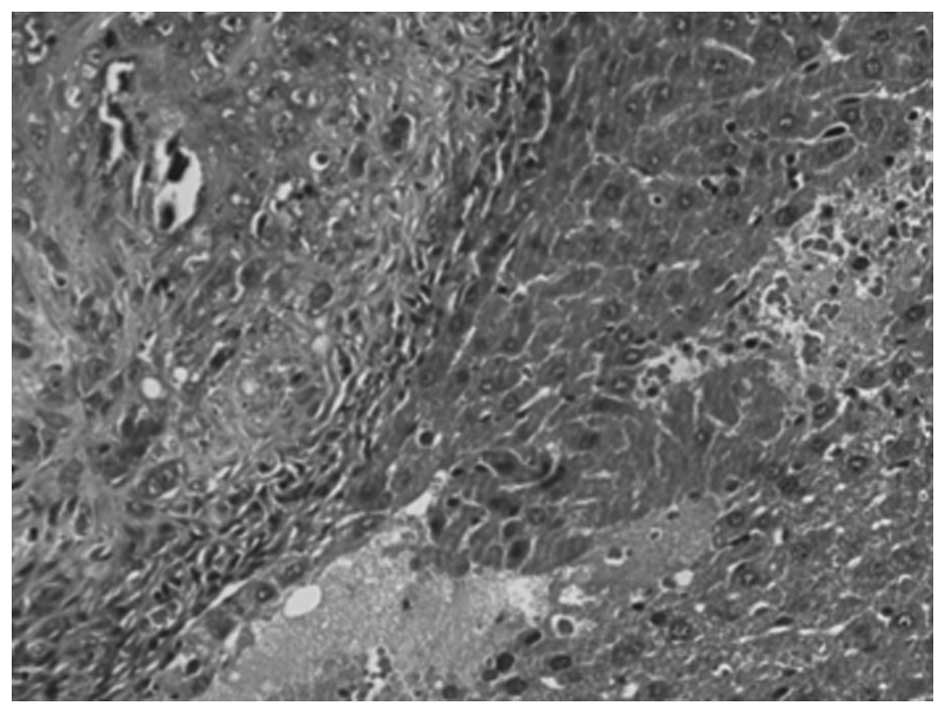
HE staining
Yellow-white nodules were present in the livers of
all 36 experimental animals that underwent partial hepatectomy
subsequent to being sacrificed. The specimens of liver tumor tissue
in all 36 cases were identified to be hepatic carcinoma following
HE staining (Fig. 1).
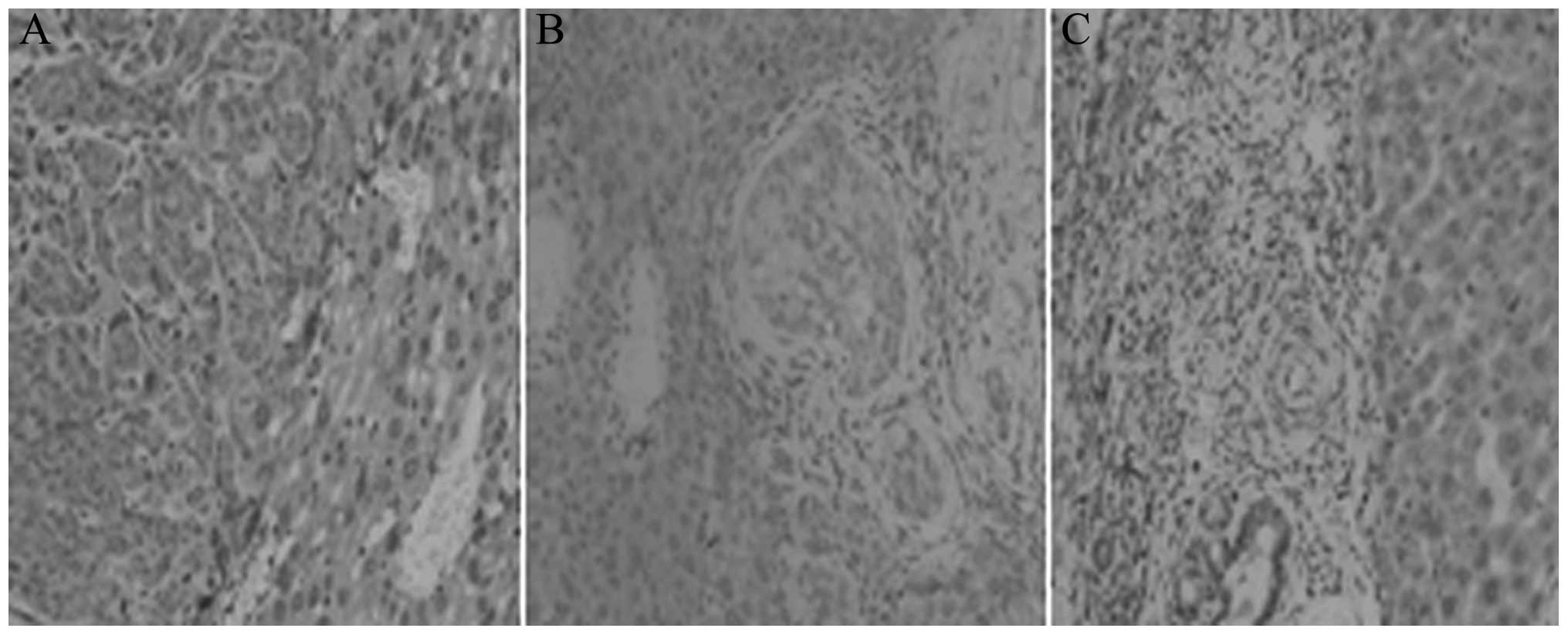
Immunohistochemistry results
As shown in Fig. 2,
the OPN-positive cells in the tumor tissues of the early
intervention group were fewer in number than those in the
pericarcinomatous tissues. However, the OPN content in the
pericarcinomatous liver cells in the late intervention group was
lower than that in the tumor tissues.
Immunohistochemistry results (qualitative
determination)
The OPN level in the tumor tissues of the early
intervention group (1.079±0.345) was significantly lower than that
of the negative control group (2.775±0.094; Table I: F=269.57, P<0.05). The OPN
level in the tumor tissues of the late intervention group
(1.898±0.342) was also significantly lower than that of the
negative control group (2.775±0.094; Table II: F=73.318, P<0.05) and the OPN
level in the tumor tissues of the late intervention group
(1.898±0.342) was significantly lower than that of the early
intervention group (1.079±0.345; Table
III: F=34.12, P<0.05). In the negative control group without
thalidomide treatment, OPN (2.775±0.094) was highly expressed in
the hepatocellular carcinoma tissues and was at a significantly
higher level than that in the pericarcinomatous tissues (Table IV: F=328.74, P<0.05).
 | Table IAnalysis of variance of the value of
immunohisto-chemistry in the early intervention and negative
control groups. |
Table I
Analysis of variance of the value of
immunohisto-chemistry in the early intervention and negative
control groups.
| Group | Sum of squares | Degree of
freedom | Square of mean | F-value | P-value |
|---|
| Inter | 17.26 | 1 | 17.255 | | |
| Intra | 1.408 | 22 | 0.064 | 269.57 | 0.0091 |
| Total | 18.66 | 23 | | | |
 | Table IIAnalysis of variance of the value of
immunohistochemistry in the late intervention and negative control
groups. |
Table II
Analysis of variance of the value of
immunohistochemistry in the late intervention and negative control
groups.
| Group | Sum of squares | Square of mean | F-value | P-value |
|---|
| Inter | 4.611 | 4.611 | | |
| Intra | 1.384 | 0.063 | 73.318 | 0.0184 |
| Total | 5.995 | | | |
 | Table IIIResults of analysis of variance of the
value of immunohistochemistry in the early intervention and late
intervention groups. |
Table III
Results of analysis of variance of the
value of immunohistochemistry in the early intervention and late
intervention groups.
| Group | Sum of squares | Degree of
freedom | Square of mean | F-value | P-value |
|---|
| Inter | 4.026 | 1 | 4.026 | | |
| Intra | 2.596 | 22 | 0.118 | 34.12 | 0.0372 |
| Total | 6.622 | 23 | | | |
 | Table IVAnalysis of variance of the value of
immunohisto-chemistry in the tumor tissues and pericarcinomatous
tissue of the negative control group. |
Table IV
Analysis of variance of the value of
immunohisto-chemistry in the tumor tissues and pericarcinomatous
tissue of the negative control group.
| Group | Sum of squares | Degree of
freedom | Square of mean | F-value | P-value |
|---|
| Inter | 9.767 | 1 | 9.767 | | |
| Intra | 0.654 | 22 | 0.03 | 328.74 | 0.0064 |
| Total | 10.42 | 23 | | | |
Discussion
OPN is a secretory phosphorylated glycoprotein, with
a relative molecular mass of ∼44 kDa, containing ∼300 amino acid
residues, of which aspartic acid, serine and glutamic acid residues
account for a high proportion. It has been demonstrated that OPN is
largely synthesized and secreted in malignant tumor cells,
particularly in hepatocellular carcinoma (1,2). The
intramolecular structure, RGD (Arg-Gly-Asp), is an unique sequence
that improves cell adhesion in proteins. Through the RGD cell
adhesion sequence, OPN may interact with important tumor metastasis
factors, including integrin, CD44, vascular endothelial growth
factor/epidermal growth factor receptor (VEGF/EGFR), matrix
metalloproteinases (MMPs), fibronectin (FN), survivin, transforming
growth factor (TGF), tumor necrosis factor (TNF) and urokinase-type
plasminogen activator (uPA) to promote cell chemotaxis, adhesion
and migration (8–17). Budhu et al(18) and Pan et al(19) put forward the theory that OPN was a
significant factor in hepatocellular carcinoma metastasis and that
it may be a molecular marker of intrahepatic metastasis. These
authors also suggested that OPN may act via the PI3K/NF-Kβ cell
signaling pathway. In our preliminary studies (3,20,21),
the excessive expression of OPN was was identifed to be closely
correlated with the early metastasis and relapse of hepatocellular
carcinoma, which is a significant factor in a poor prognosis
following hepatectomy. OPN had varying expression levels in
hepatoma cells with different metastatic potentials and was not
expressed in normal hepatic cells. These differences were
statistically significant. OPN was confirmed to have utility as a
sensitive index for predicting micro-metastases in early
hepatocellular carcinoma. OPN may be considered as a bridge
connecting primary and metastatic tumors in hepatocellular
carcinoma.
It has been reported (22) that thalidomide prevents basic
fibroblast growth factor (bFGF) and VEGF from inducing
angiogenesis, which may involve multiple pathways. However, the
specific mechanism by which thalidomide inhibits angiogenesis
remains unclear. We have previously attempted to use thalidomide
intervention in 7 patients with unexplained, permanent hematochezia
in the clinic and observed unexpected effects. We speculated that
thalidomide may be related to uPA (23). Thalidomide may block the blood flow
to malignant tumors and the resultant lack of nutrition may then
reduce the growth of tumor cells and cause them to atrophy, thereby
extending the lives of affected patients. Matrix
metalloproteinase-9 (MMP-9) is a significant member of the MMP
family, with the largest molecular weight (92 kDa). MMP-9
decomposes the extracellular matrix (ECM) and is involved in a
number of physiological and pathological processes in the human
body. Collagen types IV, V, VII and X, gelatins and elastin fibers
are its main substrates. MMPs degenerate the basement membrane
barrier and act with various cytokines to promote the formation of
new blood vessels in tumors and the proliferation of tumor cells
(24). MMPs may also participate
in evasion of the host’s immune surveillance to promote the growth
of tumors, as well as participating in invasion and metastasis. We
speculate that thalidomide may affect the activity and expression
of VEGF to reduce the stimulation of endothelial cells which
produce MMP-9. Alternatively, thalidomide may regulate the balance
of MMP-9 and its inhibitors, the tissue inhibitors of MMPs (TIMPs),
to attenuate the cascading matrix degradation process and affect
the activities of MMP-9 and other proteases which aid the
degeneration of the basement membrane and ECM. Reduced vascular
permeability may increase the resistance of MHCC97 cells to
penetration, which would also affect the migration of endothelial
cells and constrain the angiogenesis, infiltration and metastasis
of MHCC97. However, the specific mechanism of action remains
unknown. OPN is highly expressed in most malignant tumors, has been
shown to be an important tumor metastasis factor and is considered
the molecular trigger of invasion and metastasis of hepatocellular
carcinoma due to its teratogenic effects. Thalidomide was abandoned
for numerous years. In recent years, due to its clinical treatment
for certain tumors and obvious therapeutic effect, thalidomide has
gained support, but the mechanism of tumor inhibition is unclear
and widespread clinical application is restricted.This study
selected OPN as a main target and showed that thalidomide is able
to damage human hepatocellular carcinoma cells and downregulate the
expression of osteopontin. The present study has certain scientific
significance and potential social significance. Uncertainty
concerning whether thalidomide is correlated with OPN and the
nature of the correlation remains. In the present study,
thalidomide was applied to modulate the expression of OPN in
hepatocellular carcinoma tissues and the results revealed
predictable effects which indicated that OPN may be one of the
targets of thalidomide. We observed the encouraging appearance that
thalidomide have the imaging efforts, but the deeper mechanism
remains unknown. We suggest that thalidomide may block the
vascularization of the cancer and destroy the circle or cancer
cell. Although no literature has previously reported studies of
thalidomide and OPN, it is likely that there is a inner correlation
between them.
Certain scholars (25) believe that in order to clarify the
molecular pharmacological mechanism of thalidomide, emphasis should
be placed on the upstream molecules of NF-κB, including searching
for a specific thalidomide-binding factor to inhibit the
phosphorylation of NF-κB. NF-κB is a protein factor, which
specifically combines with the enhancer κB sequence of the κ-light
chain gene in immunoglobulin located in extracts of B-cell nuclei.
NF-κB has been identified to play a significant role in the
occurrence and development of a number of diseases. The
anti-apoptotic and immune activation functions of NF-κB and its
ability to promote cell proliferation are potentially factors that
lead to normal cells becoming malignant. Thalidomide may attenuate
the phosphorylation process induced by TNF-α and other factors.
This would inhibit the proteins from separating from NF-κB and
prevent NF-κB from passing into the nucleus, thereby resulting in
immune adjustment and an anti-angiogenic effect. The authors
identified that thalidomide had a potential inhibitory effect on
the NF-κB signaling pathway. A mouse model of hepatic cirrhosis was
established and research was conducted into the effect of
thalidomide on the expression of NF-κB, IKB, inter-cellular
adhesion molecule-I (ICAM-I) and vascular CAM-1 (VCAM-1).
Thalidomide was shown to markedly decrease the expression of NF-κB,
ICAM-I and VCAM-1. Whether OPN was involved in these processes and
how it contributed to them require further study.
In conclusion, as an older drug with a mature
production line, thalidomide is cheap and convenient to use. The
specific mechanism of thalidomide requires clarification if it is
intended to be comprehensively used as a clinical antitumor drug or
to replace or be used in combination with expensive new drugs. This
would assist in correcting the historical prejudices against
thalidomide. Previous studies into thalidomide have shown that it
plays a significant role in the treatment of various difficult and
severe diseases. With the continuous development of clinical and
pharmacological studies, the effects of thalidomide and its
mechanism of action may become clearer and better defined. The
present study supports the positive effect of thalidomide as an
anticancer tratment that is cheap. The present results show that
tholidomide prohibits the liver cancer as it targets
osteopontin.
References
|
1.
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
2.
|
Qin LX and Tang ZY: Recent progress in
predictive biomarkers for metastatic recurrence of human
hepatocellular carcinoma: a review of the literature. J Cancer Res
Clin Oncol. 130:497–513. 2004.PubMed/NCBI
|
|
3.
|
D’Amato RJ, Loughnan MS, Flynn E and
Folkman J: Thalidomide is an inhibitor of angiogenesis. Proc Natl
Acad Sci USA. 91:4082–4085. 1994.
|
|
4.
|
Shortt J, Hsu AK and Johnstone RW:
Thalidomide-analogue biology: immunological, molecular and
epigenetic targets in cancer therapy. Oncogene. Jan 14–2013.[Epub
ahead of print].
|
|
5.
|
Chen YY, Yen HH, Chou KC and Wu SS:
Thalidomide-based multidisciplinary treatment for patients with
advanced hepatocellular carcinoma: a retrospective analysis. World
J Gastroenterol. 18:466–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ang SF, Tan SH, Toh HC, Poon DY, Ong SY,
Foo KF and Choo SP: Activity of thalidomide and capecitabine in
patients with advanced hepatocellular carcinoma. Am J Clin Oncol.
35:222–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Garrison LJ Jr, Wang ST, Huang H, et al:
The cost-effectiveness of initial treatment of multiple myeloma in
the u.s. With bortezomib plus melphalan and prednisone versus
thalidomide plus melphalan and prednisone or lenalidomide plus
melphalan and prednisone with continuous lenalidomide maintenance
treatment. Oncologist. 18:27–36. 2013.
|
|
8.
|
Ue T, Yokozaki H, Kitadai Y, et al:
Co-expression of osteopontin and CD44v9 in gastric cancer. Int J
Cancer. 79:127–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wallach RC: Osteopontin as a biomarker for
ovarian cancer. JAMA. 287:3209–3210. 2002.
|
|
10.
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wai PY and Kuo PC: The role of Osteopontin
in tumor metastasis. J Surg Res. 121:228–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Teramoto H, Castellone MD, Malek RL, et
al: Autocrine activation of an osteopontin-CD44-Rac pathway
enhances invasion and transformation by H-RasV12. Oncogene.
24:489–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gao C, Guo H, Downey L, Marroquin C, Wei J
and Kuo PC: Osteopontin-dependent CD44v6 expression and cell
adhesion in HepG2 cells. Carcinogenesis. 24:1871–1878. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Guo H, Marroquin CE, Wai PY and Kuo PC:
Nitric oxide-dependent osteopontin expression induces metastatic
behavior in HepG2 cells. Dig Dis Sci. 50:1288–1298. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang GX, Zhao ZQ, Wang HD and Hao B:
Enhancement of osteopontin expression in HepG2 cells by epidermal
growth factor via phosphatidylinositol 3-kinase signaling pathway.
World J Gastroenterol. 10:205–208. 2004.PubMed/NCBI
|
|
16.
|
Reginato MJ, Mills KR, Paulus JK, et al:
Integrins and EGFR coordinately regulate the pro-apoptotic protein
Bim to prevent anoikis. Nat Cell Biol. 5:733–740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Nishimichi N, Hayashita-Kinoh H, Chen C,
et al: Osteopontin undergoes polymerization in vivo and gains
chemotactic activity for neutrophils mediated by integrin
alpha9beta1. J Biol Chem. 286:11170–11178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Budhu AS, Zipser B, Forgues M, et al: The
molecular signature of metastases of human hepatocellular
carcinoma. Oncology. 69(Suppl 1): 23–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pan HW, Ou YH, Peng SY, et al:
Overexpression of osteopontin is associated with intrahepatic
matastasis, early recurrence, and poorer prognosis of surgically
resected hepatocellular carcinoma. Cancer. 98:119–127. 2003.
View Article : Google Scholar
|
|
20.
|
Lin F, Li Y, Cao J, et al: Overexpression
of osteopontin in hepatocellular carcinoma and its relationships
with metastasis, invasion of tumor cells. Mol Biol Rep.
38:5205–5210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yamazaki H, Suemizu H, Igaya S, et al: In
vivo formation of a glutathione conjugate derived from thalidomide
in humanized uPA-NOG mice. Chem Res Toxicol. 24:287–289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Majumdar S, Lamothe B and Aggarwal BB:
Thalidomide suppresses NF-kappa B activation induced by TNF and
H2O2, but not that activated by ceramide,
lipopolysaccharides, or phorbol ester. J Immunol. 168:2644–2651.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Eichholz A, Merchant S and Gaya AM:
Anti-angiogenesis therapies: their potential in cancer management.
Onco Targets Ther. 3:69–82. 2010.PubMed/NCBI
|
|
24.
|
Stewart EE, Sun H, Chen X, Schafer PH,
Chen Y, Garcia BM and Lee TY: Effect of an angiogenesis inhibitor
on hepatic tumor perfusion and the implications for adjuvant
cytotoxic therapy. Radiology. 264:68–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kappler M, Taubert H, Holzhausen HJ, et
al: Immunohistochemical detection of HIF-1alpha and CAIX in
advanced head-and-neck cancer. Prognostic role and correlation with
tumor markers and tumor oxygenation parmeters. Strahlenther Onkol.
184:393–399. 2008. View Article : Google Scholar : PubMed/NCBI
|