Introduction
Restenosis (RS) following percutaneous coronary
intervention (PCI) is a type of repair response to local vascular
injury and is a local vascular reconstruction comprehensively
mediated by various cytokines (1,2).
Vascular smooth muscle cell (VSMC) proliferation is a complex
biological process finely controlled by various cytokines (3). Early growth response factor-1
(Egr-1), a member of the immediate-early gene family, is an
important nuclear transcription factor that regulates the
expression of various cell proliferation-related genes and promotes
cell proliferation and migration (4). Deoxyribozymes (DNA enzymes, DRz) are
deoxyribose molecules with enzyme activity. Due to their
phosphoesterase activity, DRz are able to catalyze the splicing of
specific RNA (5) and may be used
as an effective treatment tool for various stages of a disease. The
present study elucidates the mechanism by which an Egr-1-specific
DNAzyme (10–23 DNA enzyme, ED5) inhibits smooth muscle cell
proliferation by observing, at the cellular and molecular levels,
the effect of ED5 on the expression of Egr-1 and PCNA following the
transfection of VSMCs. This study also proposes a novel gene
therapy technique for the prevention of RS following PCI.
Materials and methods
Materials
Healthy Wistar rats, aged 5–6 years and weighing
120–150 g, were used in the present study. ED5 and ED5SCR were
synthesized by Takara Biotechnology (Dalian) Co., Ltd. (China). The
ED5 sequence was 5′-CCGCTGCCAGGCTAGC TACAACGACCCGGACGT-3′ and the
ED5SCR sequence was 5′-GCCAGCCGCGGCTAGCTACAACGATGGCTCCAC-3′. The
two sequences were purified by polyacrylamide gel electrophoresis,
lyophilized and recovered. The 5′ and 3′ ends were sodium-modified
and the 5′ ends of certain ED5 molecules were labeled with
fluorescein isothiocynate (FITC).
Cell culture and identification
The rat thoracic aorta was quickly removed under
aseptic conditions following intraperitoneal injection of 10%
chloral hydrate and immediately rinsed with aseptic
phosphate-buffered saline (PBS) containing penicillin (100 g/ml)
and streptomycin (100 g/ml). After stripping the extravascular
connective tissue and epineurium, the vessels were longitudinally
cut off and the internal membrane was positioned tilted upward in
the glass culture dish. Trypsin (0.25%) was smeared onto the
endometrium for 1 min and then the digestion was terminated with a
medium containing 20% fetal calf serum (FCS) to achieve a
transparent, thinner and tougher mesosome. The mesosome was cut
into 1 mm2 sized tissue blocks. The endometrial sections
were positioned tilted upward in the plastic culture dish and
separated from each other by 5 mm. These tissue blocks were left to
stand at 37°C in a 5% CO2 saturated humidity incubator
for 1–2 h and submerged in 2 ml medium after firm adhesion. After
5–7 days, 10% FCS medium was used to extract the cells from the
tissue blocks. Third to fifth generation cells were selected for
the experiments. This study was conducted in accordance with the
declaration of Helsinki. This study was conducted with approval
from the Ethics Committee of The First Affiliated Hospital of
Xinxiang Medical University (Weihui, China).
Cell identification
Immunocytochemical identification of α-smooth muscle
anti-actin was performed. The morphology of the cells was observed
under an inverted phase-contrast microscope. Cells were
streptavidin-peroxidase labeled with horseradish peroxidase, with
positive staining shown by a tan/yellow cytoplast.
Experimental groups
Three groups were used in the study: the control
group, the ED5 group and the ED5SCR group. Different sub-groups
according to various monitoring indicators and transfection time
were also employed.
Transfection
Serum- and antibiotic-free Dulbecco’s modified
Eagle’s medium (DMEM) was placed in a sterile Eppendorf tube, mixed
with an additional quantity of FuGENE6 and then incubated at room
temperature for 5 min. FuGENE6 and oligonucleotides (ED5, ED5SCR)
were mixed at 3:1 (volume: mass), with incubation at room
temperature for 15 min. Upon reaching 70% fusion, the VSMCs were
cultured for 30 h in serum- and antibiotic-free DMEM and then
transfected with 0.1 μmol/l transfection complex. After 18
h, 10% FCS and antibiotic-free DMEM were exchanged for the second
transfection. Continuous culture for 1–3 days was performed for
monitoring indicators. Fluorescence microscopy revealed that the
transfected cells displayed the yellow-green fluorescence of
FITC.
Immunocytochemistry and western blot
analysis
The cell slides were created at 4°C and fixed with
75% alcohol for 30 min prior to storing at −20°C. Following the
instructions of the streptavidin-peroxidase kit, rabbit polyclonal
anti-rat Egr-1 antibody (1:100 dilution) and PCNA antibody (1:200
dilution) were used. For 3,3′-diaminobenzidine (DAB) staining, a
tan-yellow cytoplast or nucleus indicated positive staining. The
cell slides were counterstained with hematoxylin, differentiated
with hydro-chloric acid alcohol, dehydrated with graded ethanol,
vitrified with dimethylbenzene and sealed with gum. The integral
optical density value was analyzed and calculated by a MetaMorph
image analysis system (three pieces were removed from each group
and five different fields of view were freely selected for each
piece). A total of 50 μg total extracted protein was
separated by polyacrylamide gel electrophoresis, transferred and
blocked. Egr-1 polyclonal antibody (Santa Cruz Biotechnology Inc.,
Santa Cruz, CA, USA; 1:500) and PCNA monoclonal antibody (Boster
Biological Technology Ltd., Fremont, CA, USA; 1:500) were
individually added for overnight incubation. Incubation was then
repeated with secondary antibodies labeled with horseradish
peroxidase and dyed for observation.
Statistical analysis
SPSS 13.0 statistical software (SPSS Inc., Chicago,
IL, USA) was used to perform statistical analyses, with mean ±
standard deviation as the measurement data. Single factor analysis
of variance was used to compare the means among the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
VSMC identification
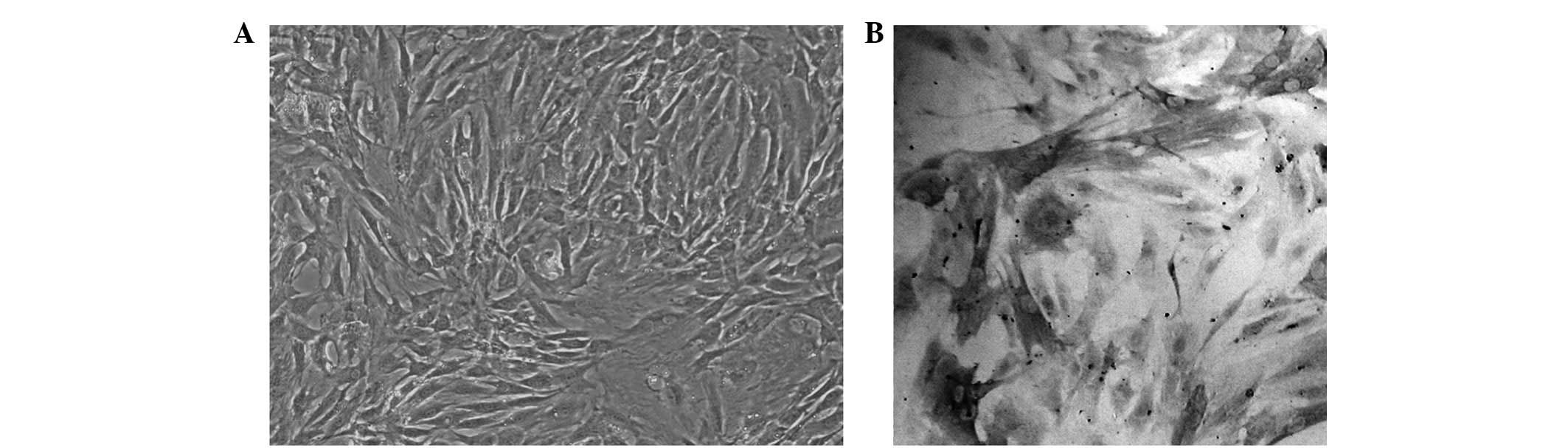
Morphological observation revealed that after
primary culture for 5–6 days, long spindle-shaped cells with strong
cytoplasmic refractivity, oval nucleus and rich cytoplasm migrated
out from the tissues. Part of these cells overlapped, forming
typical VSMC ‘peak-to-valley’ growth (Fig. 1A) (6). Alpha smooth muscle actin (α-SM-actin)
immunocytochemical staining revealed an abundance of tan-yellow
myonemes in the cytoplasm, which were aligned parallel to the
vertical axis of the cells. The nuclei were light blue following
counterstaining with hematoxylin, proving that the cells obtained
were VSMCs with high purity (Fig.
1B).
Transfection efficiency and cell survival
rate
After being transfected twice, the cells presented
the yellow-green fluorescence of FITC when observed by fluorescence
microscopy. The transfection efficiencies of ED5 (70±1.25%) and
ED5SCR (72±1.63%) were high, according to the statistical analyses.
The VSMCs were continually cultured for 72 h following
transfection, without the presence of numerous apoptotic or
necrotic cells, and achieved a cell survival rate of >99%.
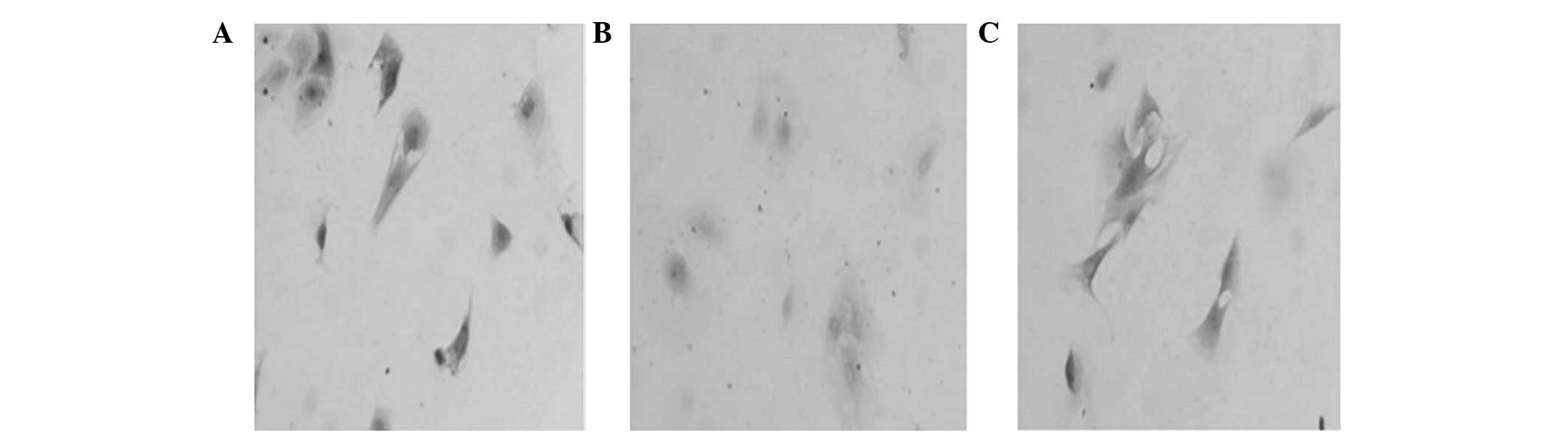
Egr-1 protein expression
Cell chemical staining and western blot analysis
revealed that Egr-1 protein in the three groups was expressed most
strongly 1 h after serum stimulation (Fig. 2); however, a declining trend was
observed over time (Table I).
Egr-1 protein expression in the ED5 group was inhibited and this
inhibition was statistically significant compared with the two
control groups at four different time points.
 | Table IOptical density change of Egr-1 in
each group at various time points. |
Table I
Optical density change of Egr-1 in
each group at various time points.
| Group | 1 h | 4 h | 24 h | 48 h | 72 h |
|---|
| Control | 44.15±4.21 | 31.71±1.90 | 22.18±1.35 | 13.48±0.73 | 11.09±0.76 |
| ED5 | 29.23±2.13a | 20.27±2.09a | 14.37±1.37a | 8.46±0.86a | 10.27±0.21 |
| ED5SCR | 44.54±3.37 | 33.04±2.50 | 22.06±2.20 | 13.58±1.09 | 10.45±1.30 |
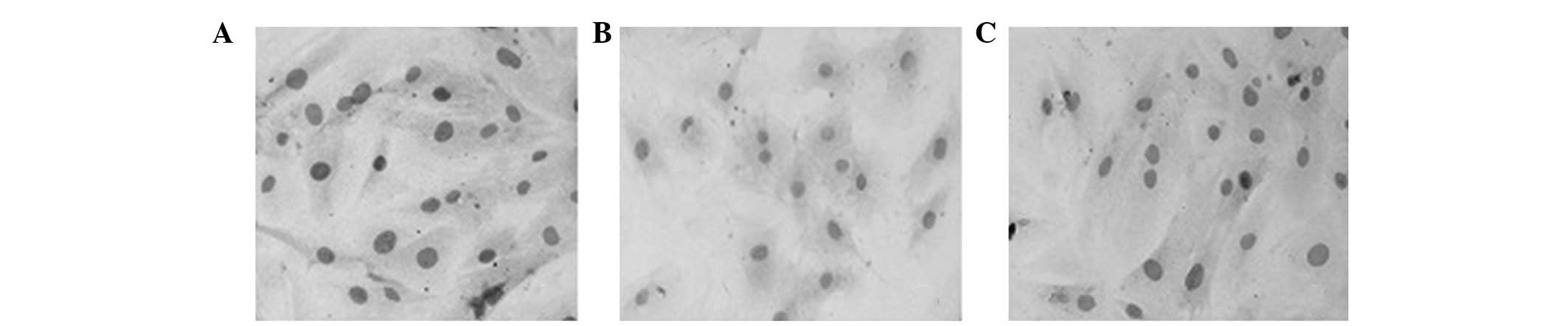
PCNA expression
Cell chemical staining and western blot analysis
revealed that the expression of PCNA protein in all three groups
began at 4 h, peaked at 24 h, and thereafter demonstrated a
slightly declining trend over time (Fig. 3 and Table II). The level of PCNA protein
expression in the ED5 group was inhibited to a certain extent at
the four different time points.
 | Table IIOptical density change of PCNA in each
group at various time points. |
Table II
Optical density change of PCNA in each
group at various time points.
| Group | 4 h | 24 h | 48 h | 72 h |
|---|
| Control | 11.61±1.09 | 19.13±2.84 | 15.64±1.12 | 13.94±1.82 |
| ED5 | 6.20±1.33a | 6.22±0.24a | 7.87±0.79a | 5.79±0.64a |
| ED5SCR | 12.12±1.43 | 18.74±2.20 | 15.73±0.63 | 14.28±1.35 |
Discussion
Over the last 20 years, coronary intervention
treatment has made tremendous progress. Stent implantation has been
used in a wide range of applications and is now applied in >70%
of all coronary intervention treatments (7). The RS rate has been significantly
reduced. In a clinical trial, rapamycin-coated stents demonstrated
good effects in preventing RS (8).
However, the RS and progressive thrombogenesis may occur after drug
stenting (9). Currently, the
mechanism of RS stenting causes endothelial injury, platelet
activation, VSMC proliferation and migration, increased
extracellular matrix and neointimal hyperplasia (10). It has been reported that VSMC
proliferation and migration are the main causes of RS occurrence
(11). Given previous developments
in molecular biology, gene therapies inhibiting smooth muscle cell
proliferation have become important for RS treatment.
Egr-1, a zinc finger transcription factor, is
expressed at low levels or not expressed at all in normal vessel
walls. Egr-1 induces VSMC and endothelial cell expression under
arterial injury and other stimulation, promoting VSMC proliferation
and endometrial thickening (12).
Previous studies have shown that Egr-1 antisense oligonucleotides
or the ‘lure strategy’ successfully suppresses Egr-1 expression
following arterial injury and VSMC proliferation (13,14).
Moreover, in a rat carotid artery balloon injury model, ED5
restrained Egr-1 expression and suppressed VSMC proliferation and
internal membrane thickening (14,15),
which stopped the occurrence and development of RS to a certain
extent. However, the specific mechanism involved in this biological
function remains unclear.
The expression of PCNA, a nuclear peptide
synthesized or expressed only in proliferating cells, begins to
increase in the late G1 phase and then reaches a peak in the S
phase, and is an important index of cell proliferation. Its
expression level is directly proportional to the degree of cell
proliferation; thus, PCNA detection is a reliable index by which to
evaluate the cell proliferation state (16,17).
Inhibiting the core process of cell proliferation/cell cycle by
intervening in the expression of cell cycle-associated proteins is
an effective method of preventing RS (18). We consider that ED5 plays a role in
these processes by regulating PCNA and Egr-1 expression.
The present study demonstrates that Egr-1 promotes
VSMC proliferation. ED5 inhibits Egr-1 and PCNA protein expression
to a certain extent following transfection of VSMCs cultured in
vitro. ED5 also inhibits the proliferation of VSMCs cultured
in vitro, an observation that may lead to a new method of
gene therapy that prevents postoperative RS following PCI.
Currently, RS gene therapy has shown good treatment effects by
regulating various target genes in animal experiments. However, its
successful application in the human body has not yet been reported.
Whether or not treatment of a single target gene is effective in
the complex human body remains to be studied.
This study further demonstrates that Egr-1 is an
important nuclear transcription factor that promotes VSMC
proliferation. ED5 was shown to inhibit the proliferation of VSMCs
cultured in vitro by reducing Egr-1 and PCNA protein
expression at the cellular and molecular levels. The findings of
this work provide a new method of gene therapy for the prevention
and treatment of RS.
References
|
1.
|
Clever YP, Cremers B, Krauss B, et al:
Paclitaxel and sirolimus differentially affect growth and motility
of endothelial progenitor cells and coronary artery smooth muscle
cells. EuroIntervention. 7(Suppl K): K32–K42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nakatani M, Takeyama Y, Shibata M, et al:
Mechanisms of restenosis after coronary intervention: difference
between plain old balloon angioplasty and stenting. Cardiovasc
Pathol. 12:40–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yamamoto H, Watanabe T, Miyazaki A, et al:
High prevalence of Chlamydia pneumoniae antibodies and
increased high-sensitive C-reactive protein in patients with
vascular dementia. J Am Geriatr Soc. 53:583–589. 2005.
|
|
4.
|
Rhachigian LM: Early growth response-1 in
cardiovascular pathobiology. Circ Res. 98:186–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Carmi N and Breaker RR: Characterization
of a DNA-cleaving deoxyribozyme. Bioorg Med Chem. 9:2589–2600.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Martin MM, Victor X, Zhao X, McDougall JK
and Elton TS: Identification and characterization of functional
angiotensin II type 1 receptors on immortalized human fetal aortic
vascular smooth muscle cells. Mol Cell Endocrinol. 183:81–91. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cutlip DE, Leon MB, Ho KK, et al: Acute
and nine-month clinical outcomes after ‘suboptimal’ coronary
stenting: results from the Stent Anti-thrombotic Regimen Study
(STARS) registry. J Am Coll Cardiol. 34:698–706. 1999.
|
|
8.
|
Morice MC, Serruys PW, Sousa JE, et al: A
randomized comparison of a sirolimus-eluting stent with a standard
stent for coronary revascularization. N Eng J Med. 346:1773–1780.
2002. View Article : Google Scholar
|
|
9.
|
Holmes DR Jr, Leon MB, Moses JW, et al:
Analysis of 1-year clinical outcomes in the SIRIUS trial: a
randomized trial of a sirolimus-eluting stent versus a standard
stent in patients at high risk for coronary restenosis.
Circulation. 109:634–640. 2004.PubMed/NCBI
|
|
10.
|
Casterella PJ and Teirstein PS: Prevention
of coronary restenosis. Cardiol Rev. 7:219–231. 1999. View Article : Google Scholar
|
|
11.
|
Gordon PC, Gibson CM, Cohen DJ, Carrozza
JP, Kuntz RE and Baim DS: Mechanisms of restenosis and redilatation
within coronary stents: quantitative angiographic assessment. J Am
Coll Cardiol. 21:1166–1174. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ohtani K, Egashira K, Usui M, et al:
Inhibition of neointimal hyperplasia after balloon injury by
cis-element ‘decoy’ of early growth response gene-1 in
hypercholesterolemic rabbits. Gene Ther. 11:126–132. 2004.
|
|
13.
|
Santiago FS, Atkins DG and Khachigian LM:
Vascular smooth muscle proliferation and regrowth after mechanical
injury in vitro are Egr-1/NGFI-A-dependent. Am J Pathol.
155:897–905. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Teng YX, Liu GN and Zhou J: Effect of
DNAzyme targeting early growth response factor-1 on endothelial
function of injured artery in rats. Journal of China Medical
University. 34:511–512. 2005.
|
|
15.
|
Han W and Liu GN: EGR-1 decoy ODNs inhibit
vascular smooth muscle cell proliferation and neointimal
hyperplasia of balloon-injured arteries in rat. Life Sci.
86:234–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
McArthur JG, Qian H, Citron D, et al:
p27–p16 Chimera: a superior antiproliferative for the prevention of
neointimal hyperplasia. Mol Therapy. 3:8–13. 2001.
|
|
17.
|
Miniati DN, Hoyt EG, Feeley BT, Poston RS
and Robbins RC: Ex vivo antisense oligonucleotides to proliferating
cell nuclear antigen and Cdc2 kinase inhibit graft coronary artery
disease. Circulation. 102:237–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Braun-Dullaeus RC, Mann MJ and Dzau VJ:
Cell cycle progression: new therapeutic target for vascular
proliferative disease. Circulation. 98:82–89. 1998. View Article : Google Scholar : PubMed/NCBI
|