Introduction
The incidence of pulmonary tuberculosis (PTB) has
increased globally, particularly in developing countries. Injuries
from PTB have worsened, causing more complex therapeutic
difficulties. The mortality rate for PTB has continued to rise.
Clinically, severe bronchostenosis or bronchial obstruction of the
trachea, main bronchus and lobar bronchi often occur prior to,
during and following the treatment of endobronchial tuberculosis.
The accompanying lesions may result in atelectasis of the left
lung, right lung or lung lobes, which limits the efficacy of the
anti-PTB therapy. Thus, certain PTB patients suffer from clinical
symptoms, including severe chest distress, shortness of breath and
dyspnea. Medication is unsuccessful for these patients and
pulmonary function fails to recover following surgery. Pulmonary
lobectomy of the lung or the lung lobes, performed when the medical
condition is stable, often has severe negative consequences in
patients (1). At the Respiratory
Department of Tangdu Hospital, The Forth Military Medical
University (Xi’an, China), reliable and effective anti-PTB therapy
has been combined with sequential therapy through electric
coagulation, cryotherapy (2) and
balloon dilation (3–9) using an electronic video bronchoscope
to treat patients with bronchostenosis or bronchial obstruction
without stenting (10–14). This technique yielded encouraging
results prior to, during and following the treatment of
endobronchial tuberculosis. The technique primarily eliminated the
bronchostenosis or bronchial obstruction, healed atelectasis and
preserved the pulmonary function with satisfactory therapeutic
effects. Our complex method varies slightly from previously
reported methods (15,16).
Patients and methods
Clinical data
A total of 56 subjects with bronchostenosis or
bronchial obstruction caused by endobronchial tuberculosis were
selected for this study. These subjects were treated sequentially
with electric coagulation, cryotherapy and balloon dilation using
an electronic video bronchoscope. The procedures were performed
during outpatient consultation or inpatient hospitalization in the
Department of Cardiovascular Medicine, First Affiliated Hospital,
Medical College of Xi’an Jiaotong University, China. The subjects
were treated from January 2007 to December 2009. The recovery of
the patients was monitored for a year. The 56 patients consisted of
21 males and 35 females, aged 5–56 years (mean, 26.8±12.5 years).
The diagnosis of endobronchial tuberculosis was confirmed by biopsy
pathology with an electronic video bronchoscope (model 1T-240 or
-260, Olympus, Tokyo, Japan) or by the detection of tubercle
bacillus in the patient’s sputum aided by the tuberculin test
(PPD test). The stenosis sites in the bronchi or lobar bronchi are
specified in Table I. All patients
provided informed consent. The treatment method was verified by the
Ethics Committee of Tangdu Hospital and the procedure was approved.
Five of the 56 patients completed the entire course of anti-PTB
therapy and then the use of anti-PTB drugs was stopped. These
patients only exhibited bronchostenosis, which was later corrected
through balloon dilation. The other 51 patients continued with the
anti-PTB treatment. Several patients presented poor treatment
efficacy when evaluated via electronic video bronchoscopy. These
patients were then subjected to a modified and reinforced therapy
and the therapeutic effects were positive. All 56 cases received
the tuberculin test and the results are presented in Table II. The surgery staff wore N97 masks
for self-protection and no evidence of cross-infection was
observed.
 | Table IDistribution of the sites of bronchial
perforation or bronchostenosis. |
Table I
Distribution of the sites of bronchial
perforation or bronchostenosis.
| Sites | N (%) | Bronchostenosis | Bronchial
perforation |
|---|
| Trachea | 12 (16.9) | 12 | 0 |
| Left main
bronchus | 11 (15.5) | 8 | 3 |
| Right main
bronchus | 13 (18.3) | 10 | 3 |
| Right upper lobar
bronchus | 11 (15.5) | 9 | 2 |
| Right middle lobar
bronchus | 6 (8.5) | 4 | 2 |
| Right lower lobar
bronchus | 7 (9.9) | 5 | 2 |
| Left upper lobar
bronchus | 6 (8.5) | 4 | 2 |
| Left lower lobar
bronchus | 5 (7.0) | 4 | 1 |
| Total | 71 | 56 | 15 |
 | Table IIDistribution of the tuberculin test
(PPD) results. |
Table II
Distribution of the tuberculin test
(PPD) results.
| Diameter of
induration (mm) | 0–5 | 6–10 | 11–20 | >20 | Total |
|---|
| Number of cases | 21 | 11 | 15 | 9 | 56 |
Treatment with anti-PTB medicine
All patients received a combination of anti-PTB
drugs. The appropriate anti-PTB drug combination (0.3 g isoniazid,
0.45 g rifampicin, 0.75 g ethambutol and 0.5 g pyrazinamide taken
three times daily; a dose increase or reduction was made for
several patients) switched to 2HRZE/9HR after two months (0.3 g
isoniazid and 0.45 g rifampicin). This was administered under
constant observation during the entire treatment period. Patients
with endobronchial erosion received isoniazid and dexamethasone
through ultrasonic gas-atomization technology. Patients who were
resistant to the anti-PTB drug combination were provided with
second-line anti-tuberculosis drugs [ethionamide, physiological
saline (PS), para-amino-salicylate and levofloxacin].
Microtrauma and video
bronchoscopy
The topical treatments included sequential electric
coagulation with an argon plasma coagulator (ARCO-3000; Söring
GmbH, Quickborn, Germany), cryotherapy using a cryotherapy machine
(Dräger, Lübeck, Germany) and dilation using high-pressure balloons
of different diameters (Johnson & Johnson, New Brunswick, NJ,
USA).
Electric coagulation
The main granulation tissue that was not frozen and
eliminated was selected and the tissue was coagulated using the
argon plasma coagulator until it carbonized. The process was
repeated several times.
Cryotherapy
The main necrotic tissues that it was possible to
freeze and eliminate by cryotherapy were selected, so according to
the ice ball mechanism, the tissues would be swollen. The ice ball
is formed in the cryotherapy detection head at the beginning of
cryotherapy. The freezing detecting head was placed in one point
for one to two minutes each time. A section with bronchostenosis
was treated repeatedly several times. The treatment was normally
administered once a week.
Balloon dilation
Numerous methods of balloon dilation exist (4–9). The
majority of methods involve continuous dilation for 2 min and the
highest pressure of dilation is <2 atm. Our method is entirely
different from these methods. Our novel high-pressure balloon
dilation method includes the following steps: insertion of the
video bronchoscope, insertion of the guide wire along the
gravitational duct of the bronchoscope, removal of the
bronchoscope, reinsertion of the bronchoscope, insertion of the
high-handed balloon along the guide wire, injection of
physiological saline through the balloon duct with a booster pump
to increase the pressure in the balloon (the pressure of balloon
increased from 0 to 14 atm) and removal of the video bronchoscope.
The patient was seated for 40 min for continuous dilation. Then,
the bronchoscope was inserted again and the balloon and guide wire
were removed following observation of the bronchus. Finally, the
bronchoscope was removed. Balloon dilation was not performed if the
diameter of the bronchial stenosis was greater than two-thirds of
the diameter of the normal bronchus.
Sequential therapy for bronchostenosis
with a video bronchoscope
Sequential therapy was performed. A topical
anesthetic spray with 2% tetracaine and a 2% lidocaine drip were
administered through a bronchoscope instead of general anesthesia.
For patients with bronchostenosis caused by endobronchial ‘cheesy’
necrosis, the necrotic tissues were frozen and excised and the
bronchial wall was frozen. The procedure was repeated until the
intima healed and became smooth. For patients with endobronchial
‘cheesy’ necrosis accompanied by bleeding, the necrotic tissues
were refrigerated and excised once the bleeding had stopped using
the argon plasma coagulator and the bronchial wall was treated
through cryotherapy until the intima became smooth. In cases where
bronchostenosis remained following these procedures, a
high-pressure balloon was utilized (pressure, 4–12 kPa) to dilate
the inner diameter of the bronchus to recover its pulmonary
function and to relieve symptoms, including dyspnea. A 64-row
computed tomography (CT) examination for bronchial imaging was
performed on patients with bronchial obstruction to assess whether
the distant bronchus was obstructed. In cases where the bronchus
was open, the site of stenosis was treated with cryotherapy to
reduce the size of the closure. Then, the closure was reopened
using the argon plasma coagulator. When the opening became larger,
cryotherapy was performed until the intima became smooth, followed
by the balloon dilation method (5,17,18)
to maximize the recovery of the inner bronchus. If the distant
bronchus is obstructed, the above mentioned procedure is not
applicable. Normal lung inflation, as well as lung function, was
gradually restored when medications were administered.
Timing of bronchoscopic examination
and treatment
The first examination was conducted on patients upon
admittance to hospital and the first video bronchoscope treatment
followed as necessary. The treatment was based on the damaged
tissue of the local bronchi. The succeeding treatments were
administered once a week until the damage was alleviated. Then, the
treatment was provided once every two weeks or once a month. When
the damaged focus disappeared completely, the patients received
their last examination and were asked to stop taking all the
anti-tuberculosis medicine.
Standard effect of
bronchostenosis
The diameter of the bronchus before and after
bronchoscopic balloon dilation and before the patient was cured of
PTB was measured. Then, the patients were asked to stop taking all
treatments (∼18 months after treatment). The diameters of the
bronchoscopes were considered as the control values. The diameter
of the Olympus BF260 video bronchoscope was 4.9 mm and of the
Olympus 1T260 bronchoscope was 5.9 mm. The diameters of the bronchi
were measured, recorded and compared with the diameter of the
bronchoscopes.
Standard effect of bronchostenosis on
bronchial diameter
A bronchial diameter that was one-third to
two-thirds the size of a normal bronchus following treatment
indicated that the treatment was effective. The diameter of the
cured bronchus was determined and compared with the diameter of the
normal bronchus. If the diameter of the cured bronchus was less
than one-third of the diameter of the normal bronchus, then the
treatment was not effective.
Results
Clinical effect
Fifty-three of the 56 cases (94.6%) of
bronchostenosis recovered to varying degrees. The mucous membranes
healed and became smooth. Symptoms, including chest distress,
shortness of breath and dyspnea, improved. Thirteen of the 15 cases
(86.9%) of bronchial obstruction reopened. The majority of the
pulmonary atelectatic tissues were dilated. The overall rate of
recovery for all cases was 90.4%. In two of the cases, it was not
possible to reopen the long segment of bronchial obstruction and
dilate the pulmonary tissues distal to the obstruction; thus, these
two patients discontinued the treatment. The patients underwent
cryotherapy 20 times in a period of six months and had five
sessions of balloon dilation within the same time period. The
high-pressure balloons were selected in a sequence according to the
inner diameter of the bronchus with bronchostenosis or bronchial
obstruction (3–15 mm) during therapy. The opening of the bronchus
was dilated as much as possible to allow the inner diameter of the
bronchus to return to its normal size.
Typical cases
Case 1
A 22-year-old female was diagnosed on March 4, 2009
with double PTB and bronchial tuberculosis marked by a month of
coughing, expectoration, chest distress and shortness of breath.
Anti-PTB treatment (isoniazid, rifampicin, ethambutol and
pyrazinamide) was administered; however, it produced poor results.
Through an electronic video bronchoscope, ‘cheesy’ necrosis was
noted in the carina, right main bronchus, end of the left main
bronchus, opening of the upper, middle and lower lobe of the right
lung and the opening of the left lower lobe, which obstructed the
trachea. Combined with a treatment of isoniazid (0.1 g) and sodium
chloride (20 ml) and through the ultrasonic gas-atomization
technology, cryotherapy was conducted 19 times through an
electronic video bronchoscope. The patient’s condition improved.
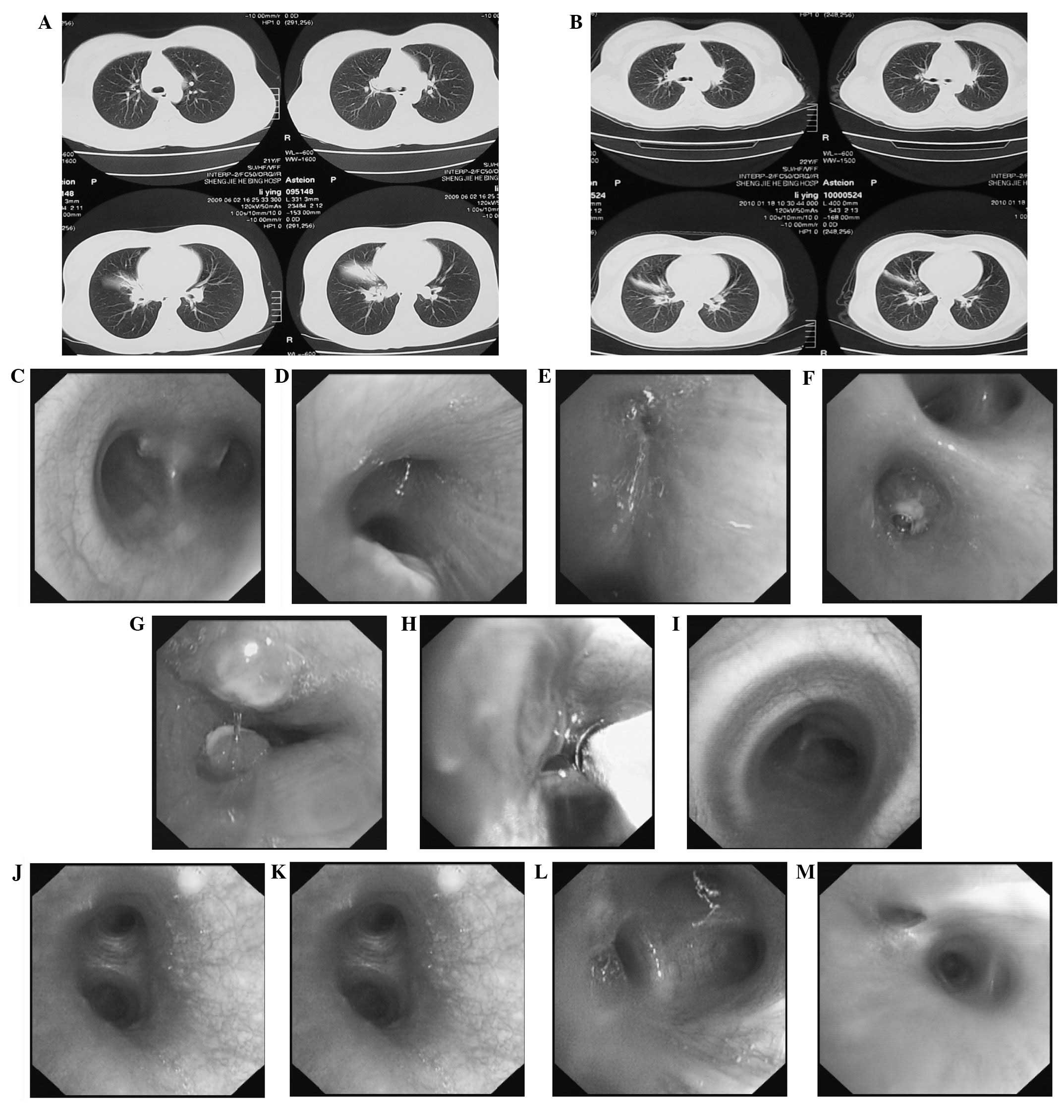
Fig. 1A and B show CT images
before and after cryotherapy, respectively. Fig. 1C–G, Fig. 1H and Fig. 1I–M show bronchoscopic images
before, during and after cryotherapy, respectively.
Case 2
A 43-year-old male was diagnosed on March 10, 2009
with PTB in the right upper lobe and bronchial tuberculosis marked
by two months of coughing and expectoration. The patient underwent
treatment in another hospital for three months; however, the
treatment was ineffective. Through an electronic video
bronchoscope, granulation tissue covering the right upper lobar
bronchus was noted, which obstructed the majority of the right
upper lobe. Through the combination of isoniazid (0.1 g), sodium
chloride (20 ml), ultrasonic gas-atomization technology (twice a
day for two months) and anti-PTB therapy (isoniazid, rifampicin,
ethambutol and pyrazinamide), cryotherapy with an electronic video
bronchoscope was conducted 17 times. The patient’s condition
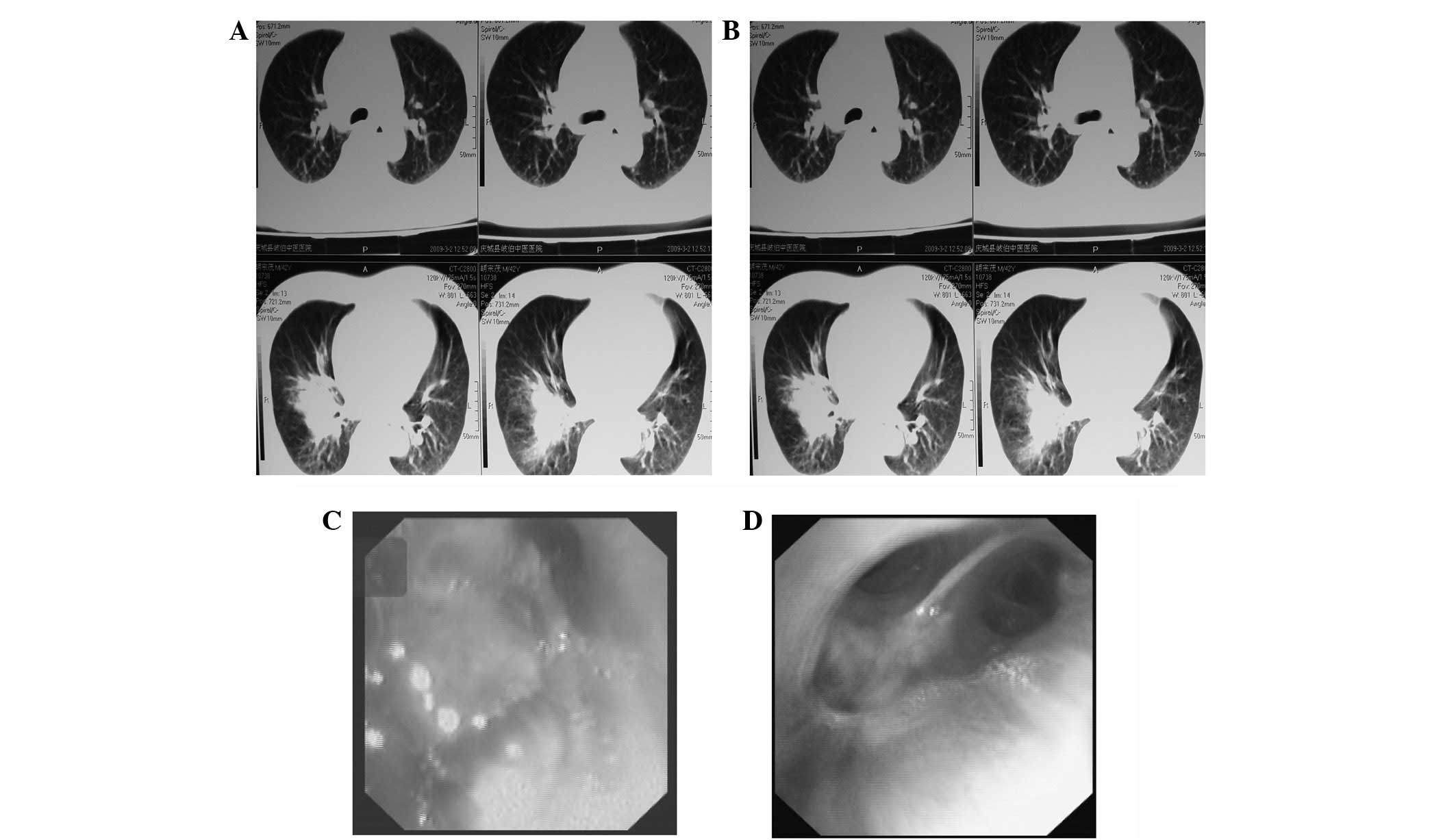
improved. Fig. 2A and B present CT
images before and after cryotherapy, respectively. Fig. 2C and D present bronchoscopic images
before and after treatment, respectively.
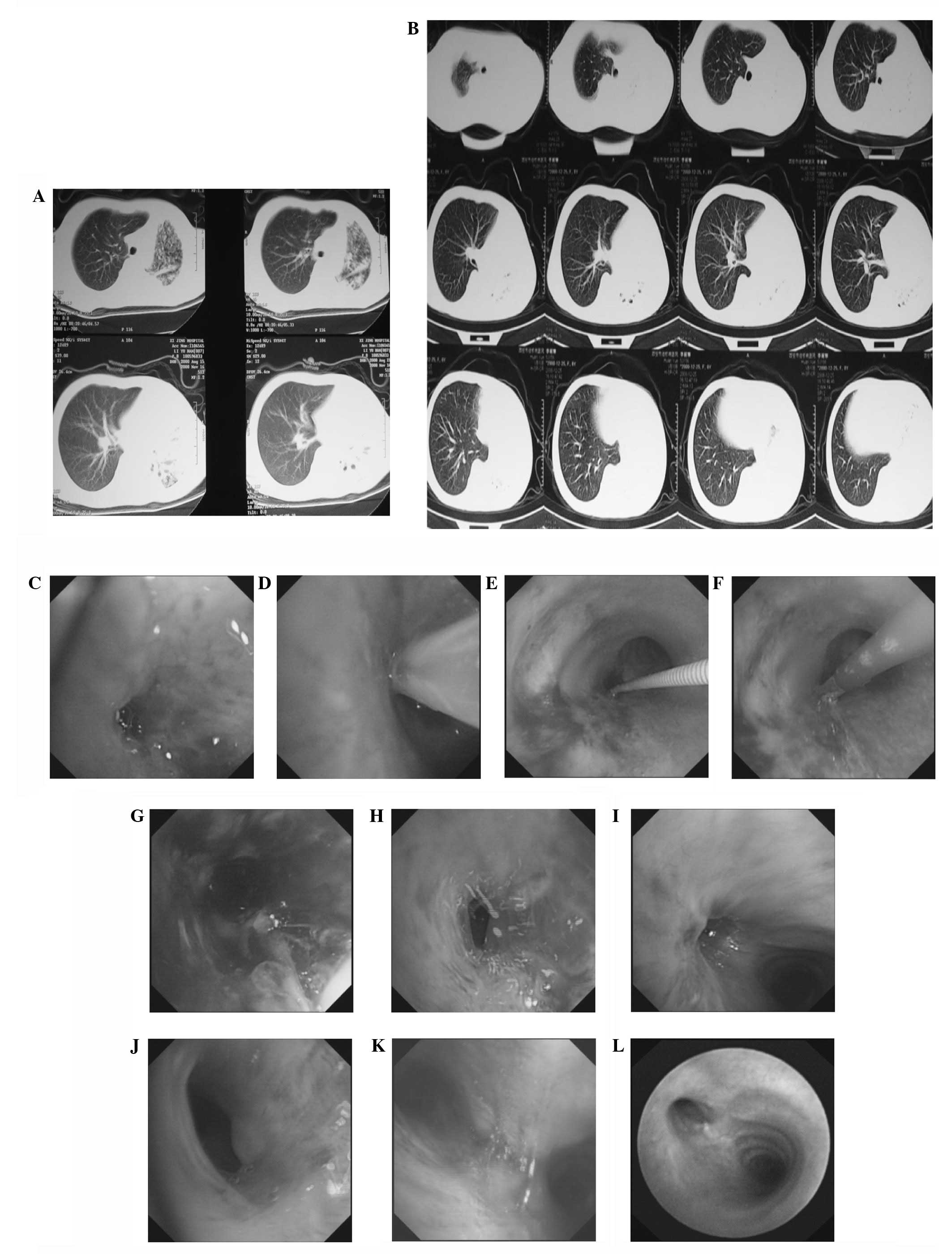
Case 3
A shadow in the left lung of an 8-year-old female
was detected by chest CT. The patient reported two months of
coughing and shortness of breath and was diagnosed with left lung
tuberculosis and endobronchial tuberculosis based on the discovery
of tubercle bacillus in the sputum. The patient experienced left
pulmonary closure following treatment with anti-tuberculosis
medicine for two weeks. The diameter of the left main bronchus was
2 mm as determined by electric bronchoscopy. The patient was
subjected to balloon dilation three times following cryotherapy and
argon coagulation. The atrophy lung lobar in the patient’s left
lung stretched. Images of this case are shown in Fig. 3.
Discussion
Endobronchial tuberculosis is one of the main causes
of incurable PTB. Bronchostenosis or bronchial obstruction leads to
pulmonary closure and resistance to treatment. The bronchi of the
majority of patients are destroyed, obstructed and even closed as a
result of PTB, which causes the pulmonary function to gradually
weaken. Several patients also exhibit severe clinical symptoms or
even disabilities and a number succumb to PTB. Pulmonary lobectomy
is performed on patients suffering from repeated infections. If PTB
is not contained appropriately prior to pulmonary lobectomy,
bronchopleural fistula may occur during the procedure, followed by
repeated thoracic infections that cause mortality. In the past,
argon plasma coagulators and microwave coagulators or electric
coagulators have been utilized for bronchostenosis. However, these
electric approaches often result in the repeated growth of
granulation tissue and destruction or collapse of the bronchial
walls, which negatively affects the ventilation of the lung, thus
compromising the effectiveness of the therapies.
Cryotherapy combined with high-pressure balloon
dilation has been used to treat malignant bronchostenosis
domestically and abroad (19–21).
The outcomes differ each time; however, they are generally
positive. The argon plasma coagulator (22) is utilized for the treatment for
stenosis in the main bronchi; however, it is rarely regarded as an
addition to the combined cryotherapy and balloon dilation therapy.
When the argon plasma coagulator and microwave coagulator or
electric coagulator are used for the electric coagulation of
bronchostenosis caused by endobronchial tuberculosis (15), the granulation tissues grow
repeatedly, leading to undesirable effects. We combined electric
coagulation with refrigeration in this study, supplemented with
balloon dilation to provide a solution to the growth of granulation
tissues. Successful results were achieved. Cryotherapy decreased
the local temperature to −80°C, terminated the blood supply to the
granulation tissues and formed a microthrombus that obstructed the
blood vessels and halted the growth of the granulation tissues.
Cryotherapy also froze the cells, quickened the infiltration of
inflammatory cells into the granulation tissues and the necrosis of
the granulation tissues, and promoted the recovery of the intima of
the bronchus. The combination of electric coagulation, cryotherapy
and balloon dilation provides a solution to the growth of unwanted
granulation tissues caused by PBT and bronchostenosis. Fifty-three
of the 56 cases (94.6%) of bronchostenosis in our study recovered
to varying degrees. The mucous membranes of the patients healed and
became smooth. Symptoms, including chest distress, shortness of
breath and dyspnea, also improved. Thirteen of the 15 cases (86.9%)
of bronchial obstruction reopened. The majority of the pulmonary
atelectatic tissues were dilated. The overall rate of recovery was
90.4%. In two of the cases, it was not possible to reopen the long
segment of bronchial obstruction; therefore, the treatment was
discontinued.
Several studies have shown that cryotherapy destroys
cell structure, mitigates edema, suppresses the growth of tissues
by reducing blood supply and activates the inflammation of tissues
to hasten the death of local tissues or cells (3,4).
Cryotherapy is widely utilized for prostate hyperplasia, prostatic
carcinoma, cervical erosion, renal carcinoma, hepatic masses, and
other diseases (23–27). Cryotherapy using a video
bronchoscope also exhibits favorable effects on lung cancer
(9). However, it does not
demonstrate prompt efficacy since cryotherapy is a very
slow-functioning procedure. Patients may undergo cryotherapy >20
times in a period of several months. Balloon dilation is
administered for bronchostenosis when the condition is finally
stabilized. Patients must complete the entire procedure.
Although bleeding is rarely observed during the
cryotherapy of endobronchial tuberculosis or neoplasms (28), the frozen cutting of the fresh
granulation tissue or neoplasm easily causes bleeding. The argon
plasma coagulator is a good solution to the issue of bleeding.
Frozen cutting is difficult to perform on granulomatous lesions;
however, the lesions may be coagulated first by an argon plasma
coagulator prior to refrigerated or frozen cutting. Given that the
argon plasma coagulator also causes massive hemorrhaging when it
damages the blood vessels, tissues with an abundant supply of blood
or large blood vessels should be protected from the damage.
The diameter of the bronchus with stenosis and that
of the usable balloon should be considered during balloon dilation
to avoid massive hemorrhaging, since a large balloon may rip the
bronchus. The pressure from the balloon and the injection of
thrombin terminates the bleeding and so the bleeding does not cause
a severe adverse outcome. None of the patients in the current study
experienced massive hemorrhaging.
A large rip in the bronchus may lead to re-stenosis
(29) when the bronchial stenosis
heals. When balloon dilation is conducted in the left or right main
bronchus at the carina of the trachea, it is important that the
balloon does not block a large section of the lumen of the main
bronchi. This is necessary to prevent the development of acute
respiratory failure that threatens the patient’s life.
The techniques demonstrated in the current study are
likely to benefit a number of patients (30). Our balloon dilation method is
different from previously described methods. We dilated the
bronchus continuously for 40 min at high pressure (12–14 atm),
which positively affected bronchostenosis. Other balloon dilation
methods dilate the bronchus for only 2 min at low pressure (<2
atm) and normally allow the immediate re-stenosis of the bronchus.
Further studies on this aspect of the treatment should be conducted
to add to the understanding and creation of improved
techniques.
Bronchostenosis or bronchial obstruction resulting
from endobronchial tuberculosis may be treated by electric
coagulation, cryotherapy and balloon dilation with an electronic
video bronchoscope. This sequential therapy has demonstrated the
ability to heal atelectasis and avoid pulmonary lobectomy. Thus, it
is considered an effective method that may be used in clinical
practice.
References
|
1.
|
Bekci TT, Maden E and Emre L: Bronchial
anthracofibrosis case with endobronchial tuberculosis. Int J Med
Sci. 8:84–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mu D, Nan D, Li W, et al: Efficacy and
safety of bronchoscopic cryotherapy for granular endobronchial
tuberculosis. Respiration. 82:268–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kuo SM, Yang ML, Li YC, Tsao TF, Liu SC
and Chen FL: Balloon dilatation in management of postoperative
airway obstruction due to tracheal bronchus associated with right
main bronchial stenosis: emphasizing the role of three-dimensional
computed tomography on preoperative evaluation. Pediatr Pulmonol.
45:730–733. 2010. View Article : Google Scholar
|
|
4.
|
Meng C, Yu HF, Ni CY, et al: Balloon
dilatation bronchoplasty in management of bronchial stenosis in
children with mycoplasma pneumonia. Zhonghua Er Ke Za Zhi.
48:301–304. 2010.(In Chinese).
|
|
5.
|
Shitrit D, Kuchuk M, Zismanov V, Rahman
NA, Amital A and Kramer MR: Bronchoscopic balloon dilatation of
tracheobronchial stenosis: long-term follow-up. Eur J Cardiothorac
Surg. 38:198–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abi-Jaoudeh N, Francois RJ, Oliva VL, et
al: Endobronchial dilation for the management of bronchial stenosis
in patients after lung transplantation: effect of stent placement
on survival. J Vasc Interv Radiol. 20:912–920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tan JH, Fidelman N, Durack JC, et al:
Management of recurrent airway strictures in lung transplant
recipients using AERO covered stents. J Vasc Interv Radiol.
21:1900–1904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nouraei SA, Mills H, Butler CR, Ghufoor K,
Sandhu GS and George PJ: Outcome of treating airway compromise due
to bronchial stenosis with intralesional corticosteroids and
cutting-balloon bronchoplasty. Otolaryngol Head Neck Surg.
145:623–627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Carlin BW, Harrell JH II and Moser KM: The
treatment of endobronchial stenosis using balloon catheter
dilatation. Chest. 93:1148–1151. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Saueressig MG, Sanches PR, Macedo Neto AV,
Moreschi AH, Oliveira HG and Xavier RG: Novel silicone stent to
treat tracheobronchial lesions: results of 35 patients. Asian
Cardiovasc Thorac Ann. 18:521–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Higuchi T, Shiraishi T, Hiratsuka M,
Yanagisawa J and Iwasaki A: Successful treatment of bronchial
anastomotic stenosis with modified Dumon Y-stent insertion in lung
transplantation: report of a case. Surg Today. 41:1302–1305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Noppen M, Stratakos G, Amjadi K, et al:
Stenting allows weaning and extubation in ventilator- or
tracheostomy dependency secondary to benign airway disease. Respir
Med. 10:139–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mroz RM, Kordecki K, Kozlowski MD, et al:
Severe respiratory distress caused by central airway obstruction
treated with self-expandable metallic stents. J Physiol Pharmacol.
59:491–497. 2008.PubMed/NCBI
|
|
14.
|
Chung FT, Lin SM, Chou CL, et al: Factors
leading to obstructive granulation tissue formation after ultraflex
stenting in benign tracheal narrowing. Thorac cardiovasc Surg.
58:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fernández-Bussy S, Majid A, Caviedes I,
Akindipe O, Baz M and Jantz M: Treatment of airway complications
following lung transplantation. Arch Bronconeumol. 47:128–133.
2011.
|
|
16.
|
Folch E and Mehta AC: Airway interventions
in the tracheobronchial tree. Semin Respir Crit Care Med.
29:441–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kwon YS, Kim H, Kang KW, et al: The role
of ballooning in patients with post-tuberculosis bronchial
stenosis. Tuberc Respir Dis. 66:431–436. 2009. View Article : Google Scholar
|
|
18.
|
Iwata T, Yamakawa H, Fujiwara T, Matsui Y
and Fujino M: Successfully treated postbronchoplasty bronchial
stenosis using short-interval repeated endobronchial balloon
dilation. Gen Thorac Cardiovasc Surg. 59:627–631. 2011. View Article : Google Scholar
|
|
19.
|
Jin FG, Mu DG, Chu DL, Fu EQ, Xie YH and
Liu TG: Interventional bronchoscopy for the treatment of tracheal
and bronchial tuberculosis. J US-China Med Sci. 6:33–34. 2009.
|
|
20.
|
Vergnon JM, Huber RM and Moghissi K: Place
of cryotherapy, brachytherapy and photodynamic therapy in
therapeutic bronchoscopy of lung cancers. Eur Respir J. 28:200–218.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fernando HC, Dekeratry D, Downie G, et al:
Feasibility of spray cryotherapy and balloon dilation for
non-malignant strictures of the airway. Eur J Cardiothorac Surg.
40:1177–1180. 2011.PubMed/NCBI
|
|
22.
|
Bolliger CT, Sutedja TG, Strausz J and
Freitag L: Therapeutic bronchoscopy with immediate effect: laser,
electrocautery, argon plasma coagulation and stents. Eur Respir J.
27:1258–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Collins NC: Is ice right? Does cryotherapy
improve outcome for acute soft tissue injury? Emerg Med J.
25:65–68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Morrison PR, Silverman SG, Tuncali K and
Tatli S: MRI-guided cryotherapy. J Magn Reson Imaging. 27:410–420.
2008. View Article : Google Scholar
|
|
25.
|
Mehta RM and Cutaia M: The role of
interventional pulmonary procedures in the management of
post-obstructive pneumonia. Curr Infect Dis Rep. 8:207–214. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shinohara K: Cryotherapy. Int J Clin
Oncol. 12:416–426. 2007. View Article : Google Scholar
|
|
27.
|
Stein RJ and Kaouk JH: Renal cryotherapy:
a detailed review including a 5-year follow-up. BJU Int.
99:1265–1270. 2007.PubMed/NCBI
|
|
28.
|
Kaouk JH, Aron M, Rewcastle JC and Gill
IS: Cryotherapy: clinical end points and their experimental
foundations. Urology. 68:38–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Jantz MA and Silvestri GA: Fire and ice:
laser bronchoscopy, electrocautery and cryotherapy. Thoracic
Endoscopy: Advances in Interventional Pulmonology. Simoff MJ,
Sterman DH and Ernst A: Blackwell Publishing; Malden, MA: 2008
|
|
30.
|
Bolliger CT, Mathur PN, Beamis JF, et al:
ERS/ATS statement on interventional pulmonology. European
Respiratory Society/American Thoracic Society. Eur Respir J.
19:356–373. 2002.PubMed/NCBI
|