Introduction
Adult stem cells, although not totipotent, are able
to differentiate into specific precursor and terminal cells. Bone
marrow-derived mesenchymal stem cells (BMSCs), as seed cells for
bone tissue engineering, possess the generality of stem cells with
self-replicating, amplification and multilineage differentiation
potential. In vitro and in vivo studies have
indicated that culture-expanded BMSCs are capable of
differentiation along osteogenic, chondrogenic and adipogenic
lineages, as well as into cardiomyocytes, skeletal muscle and
neural precursors (1–9). In the present study, BMSCs were
induced to differentiate into osteogenic, adipogenic and neural
lineages in vitro. The cells were then implanted into
subcutaneous pockets on the dorsa of nude mice to form new bone and
cartilage tissues.
Materials and methods
Isolation and culture of human BMSCs
The BMSCs were isolated using our previously
described methods (10). Briefly,
bone marrow (10 ml) was obtained from the iliac crest of voluntary
donors from whom informed consent had been obtained. The aspirate
was diluted at a ratio of 1:2 in Dulbecco’s modified Eagle’s
medium-low glucose (DMEM-LG; Gibco, Carlsbad, CA, USA). The
mononuclear cell layer was removed from the interface, washed twice
and suspended in DMEM at 107 cells/ml subsequent to
density gradient centrifugation (density of 1.077 g/ml, Ficoll) at
400 × g for 20 min. Each 25-cm2 flask (Corning Inc., One
Riverfront Plaza, Corning, NY, USA) contained DMEM with 10% fetal
bovine serum (FBS; Gibco) supplemented with 1%
penicillin/streptomycin (Gibco). The non-adherent cells were
discarded and the adherent cells were washed with
phosphate-buffered saline (PBS; Gibco) on the second day. The cells
were then cultured in DMEM with antibiotics and 10% FBS in a
humidified incubator (37°C, 5% CO2) with renewal of the
culture medium every 3 days. The medium containing 10% FBS was
replaced every 3 or 4 days. At ∼50% confluence, the cells were
suspended using a 0.25% trypsin/0.02% EDTA solution (Sigma, St.
Louis, MO, USA) and replated at ∼5,000 cells/cm2. The
cells were split every 5–7 days following the first passage. The
cells were subsequently subjected to analysis following three or
four passages.
Multilineage differentiation assays in
vitro
Osteogenic, adipogenic, neurogenic and chondrogenic
differentiation were induced according to the reported methods
(6), with certain
modifications.
Osteogenic differentiation
Three or four passages of BMSCs were adjusted to a
concentration of 1×105/ml and cultured on a 6-well
culture plate (1 ml/well). The cells were cultured in a humidified
incubator (37°C, 5% CO2) with renewal of the culture
medium every 3 days. The cells were incubated in a differentiation
medium for 2–4 weeks once the cells had reached 100% confluence,
during which time the medium was changed every 2–3 days. The
differentiation medium was as follows: DMEM-LG supplemented with
10% FBS, 1 μM dexamethasone (Sigma), 50 μg/ml
ascorbic acid (Sigma), 10 mM sodium β-glycerophosphate (Sigma) and
1% penicillin/streptomycin (Sigma). The cells were fixed with
ice-cold 70% ethanol and stained with Alizarin Red S (Amresco,
Solon, OH, USA), as well as the von Kossa stain, to detect
mineralization (calcium deposits). The alkaline phosphatase (ALP)
activity was also tested.
Adipogenic differentiation
The cells were first grown to 100% confluence and
then incubated for 3 days in an induction medium consisting of
DMEM-LG supplemented with 10% FBS, 100 μM indomethacin
(Sigma), 0.1 μM dexamethasone, 0.5 mM
3-isobutyl-1-methylxanthine (IBMX, Sigma), 10 μg/ml human
insulin (Sigma) and 1% penicillin/streptomycin. The cells were
incubated in the induction and maintenance media for >2 weeks
and then fixed with 4% paraformaldehyde for 30 min at room
temperature and stained with Oil Red O, as well as Sudan Black B
(Amresco), to detect fat deposition.
Neurogenic differentiation
The cells were grown to 100% confluence and then
incubated for 24 h in a pre-induction medium consisting of DMEM-LG
supplemented with 20% FBS and 1 mM/l β-mercaptoethanol (BME),
followed by incubation for 5 h in an induction medium consisting of
DMEM-LG supplemented with 5 mM/l BME. The neuroblasts were examined
by toluidine blue staining and glial fibrillary acidic protein
(GFAP) immunohistochemical staining.
Chondrogenic differentiation
Three or four passages of BMSCs were adjusted to a
concentration of 1×106/ml and cultured in a
75-cm2 flask using a humidified incubator (37°C, 5%
CO2) with renewal of the culture medium every 3 days.
The cells were incubated in a differentiation medium for 10 days
once the cells had reached 100% confluence, during which time the
medium was changed every 2–3 days. The differentiation medium
comprised DMEM-LG supplemented with 10% FBS, 10 ng/ml transforming
growth factor-β1 (TGF-β1; Sigma), 6.25 μg/ml insulin, 6.25
μg/ml transferrin (Sigma), 0.1 μM dexamethasone, 50
μg/ml ascorbic acid and 1% penicillin/streptomycin.
Osteogenesis and cartilage tissue
formation in vivo
Three or four passages of BMSCs were incubated in an
osteogenic and a chondrogenic differentiation medium for 10 days,
respectively, during which time the medium was changed every 2–3
days. A 300-μl cell suspension (4×107 cells/ml,
osteoblast or chondroblast) was used to inoculate multiple sites of
a coral scaffold which was placed in an incubator for 2 days prior
to implantation. The composites of the osteoblast or chondroblast
coral scaffolds were then implanted into subcutaneous pockets on
the dorsa of nude mice to form new bone and cartilage tissues. All
in vivo mouse implantation experiments were performed in
accordance with our institutional guidelines for animal care and
use. A total of 18 nude mice (Guangzhou University of Chinese
Medicine, Guangzhou, China) were randomly divided into three groups
(n=6 animals/group), namely the osteoblast- and
chondroblast-scaffold groups and the cell-free scaffold group. The
mice were sacrificed by anesthesia overdose at 6 or 9 weeks
post-surgery. The scaffolds were then removed for analysis. The
implanted scaffolds were assessed using radiographic, histological
and immunohistochemical methods.
Results
Osteogenic differentiation
The BMSCs were isolated by density gradient
centrifugation and purified by adherent separation to obtain an
ample amount of the BMSCs with a uniform appearance.
The cells were cultured in a differentiation medium
for 2–4 weeks to induce osteogenic differentiation. The appearance
of the cells changed in the first 3–5 days from long spindles to
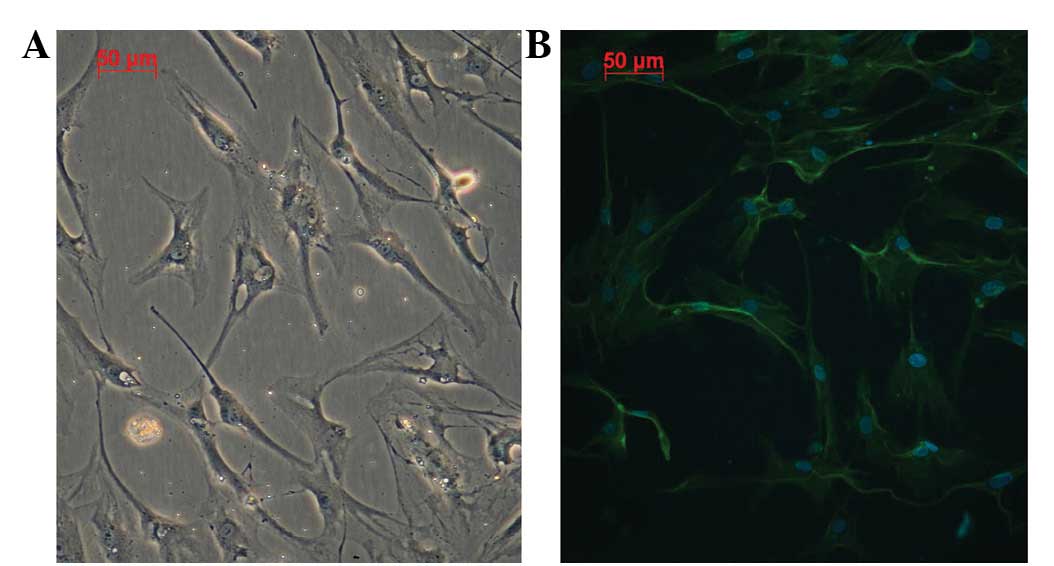
polygonal or irregularly shaped conformations (Fig. 1A). A cluster of cells demonstrating
a growth tendency was present and a visible change to the
cytoskeleton was observed (Fig.
1B). The deposition of calcium, an indicator of osteogenic
differentiation, was determined by von Kossa and Alizarin Red S
staining following 2–3 weeks of incubation in a differentiation
medium. The von Kossa staining showed an uneven, black-stained
calcified nodule with an unclear boundary (Fig. 2A and B). Alizarin Red S staining
occurred in the sedimentary sections, indicating the deposition of
calcium (Fig. 2C). An assay for
ALP activity, an independent indicator of osteoblast
differentiation, was conducted following the induction of the
differentiation process. The results showed that the mean ALP
activity increased moderately between days 1 and 7 and further
increased between days 7 and 14, reaching a peak on day 14 and
declining thereafter. The results of optical density were as
follows (mean ± SD): day 1, 0.082±0.004; day 7, 0.171±0.008; day
14, 0.467±0.014; and day 28, 0.301±0.037. These results strongly
suggest that BMSCs are able to differentiate into osteogenic
cells.
Adipogenic differentiation
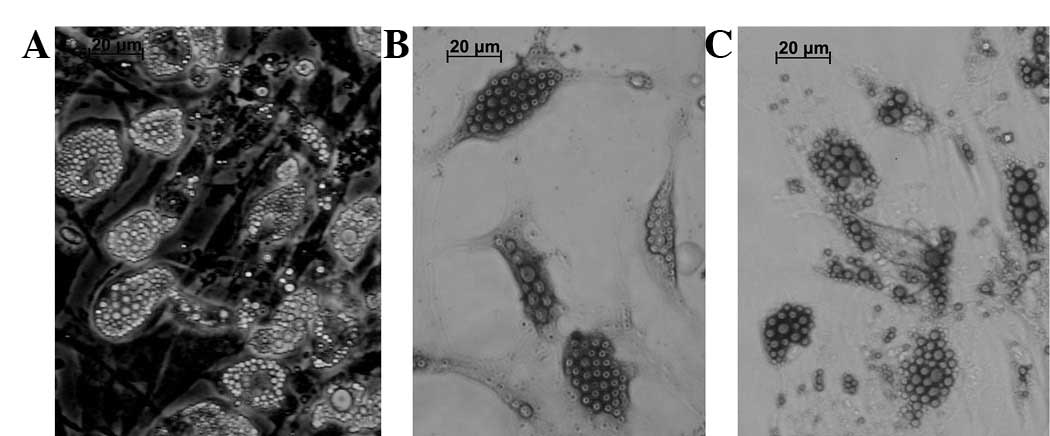
The cells were incubated in an induction medium for
2 weeks to induce adipogenic differentiation. The cells were
stained with Oil Red O and Sudan Black B to detect lipid
production. The cells changed in appearance from long spindles to
polygonal shapes and became enlarged following 3–5 days incubation
in a differentiation medium (Fig.
3A). Certain cells became round and spherical on day 7 and
round, translucent lipid drops were observed in the cytoplasm.
Numerous cells containing abundant lipids (adipocytes, Fig. 3A) were observed following 14 days
of adipogenic differentiation. Positive staining with Sudan Black B
and Oil Red O was observed (Fig. 3B
and C). Lipid drops were distributed inside and outside of the
cytoplasm as revealed by the Sudan Black B (Fig. 3B) and Oil Red (Fig. 3C) staining. In total, >50% of
the adipocytes were induced in the cell populations.
Neurogenic differentiation
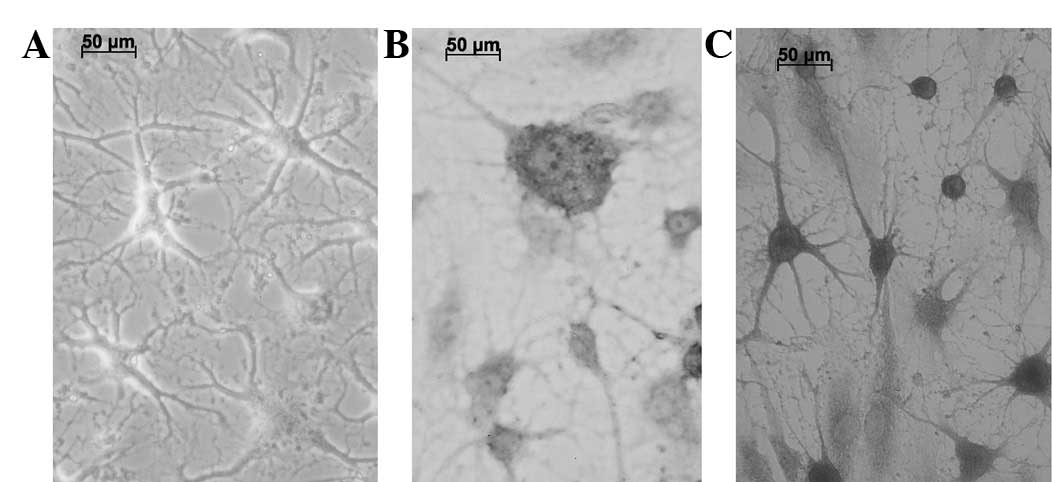
The BMSCs were incubated in a pre-induction medium
for 24 h and then in an induction medium for 5 h to induce
neurogenic differentiation. The cells withdrew to form neuron-like
cells with axon- and dendrite-like processes, instead of the
spindle shapes (Fig. 4A). These
cells presented a strong refractive trait, the Nissl bodies were
displayed as a deep blue by toluidine blue staining (Fig. 4B) and the nucleus was nearly
colorless. Positive immunohistochemical staining for GFAP was
observed (Fig. 4C).
Osteogenesis and cartilage tissue
formation in vivo
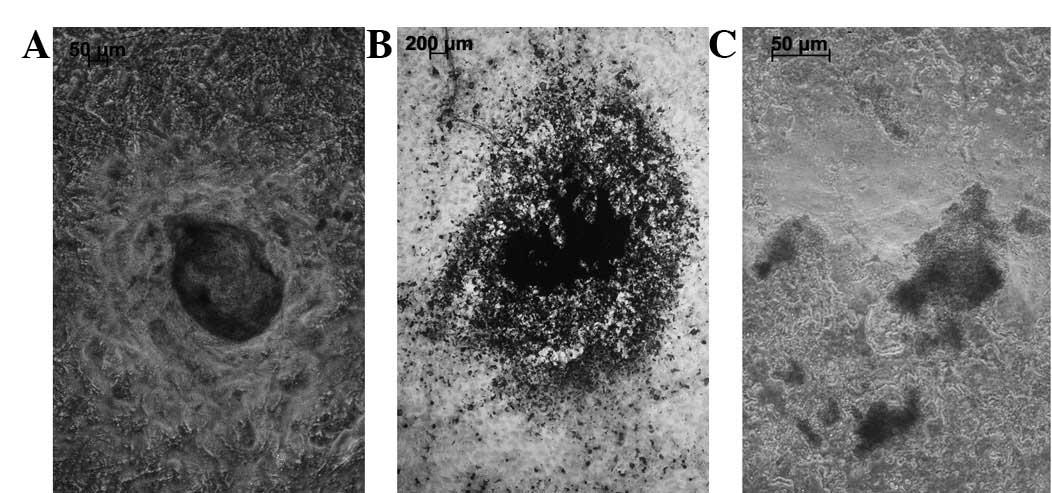
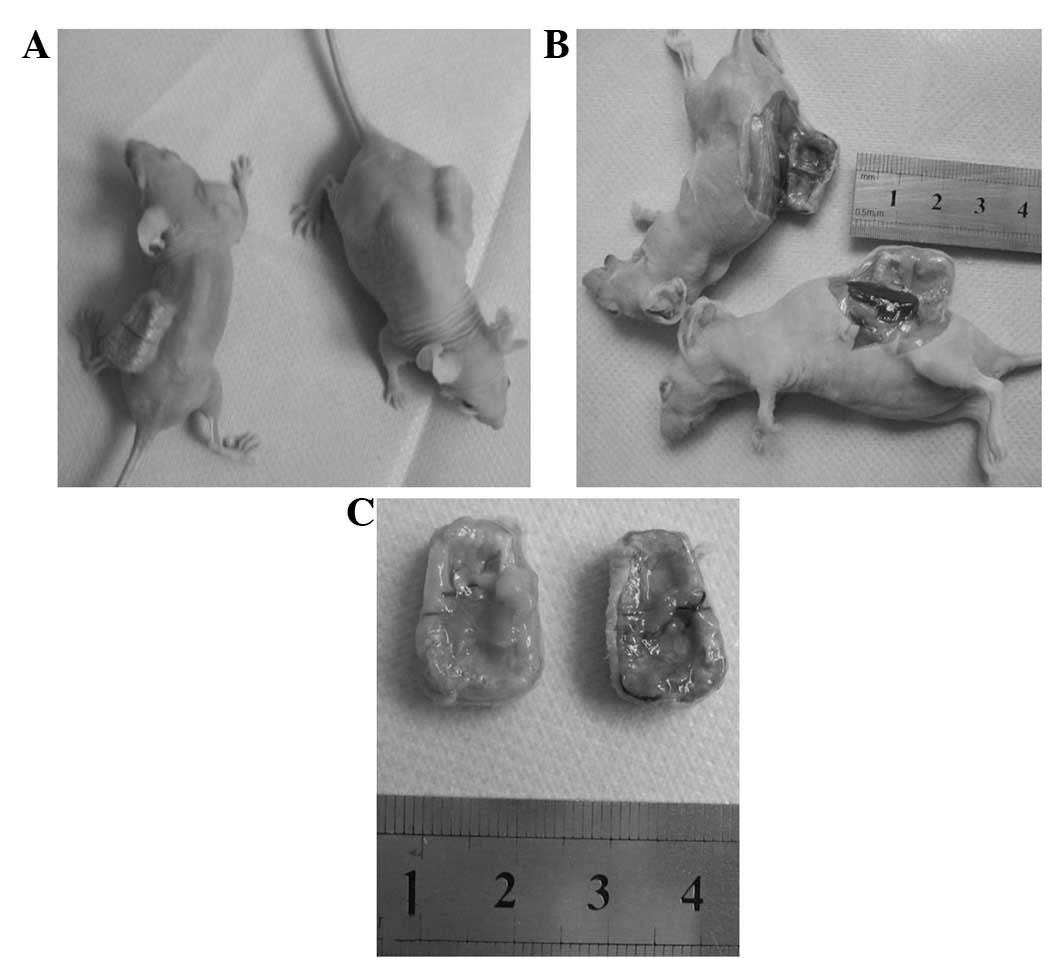
The visual inspection and X-ray results showed that
the in vivo scaffold specimens in all three groups
maintained the initial shape of the coral scaffold. The scaffold
specimen was dark red, hard and bonelike in the osteoblast-scaffold
group (Fig. 5). By contrast, the
scaffold specimen had a translucent surface, resembling cartilage,
in the chondroblast-scaffold group (Fig. 5). However, in the cell-free
scaffold group, the scaffold specimen displayed only fibrous coral
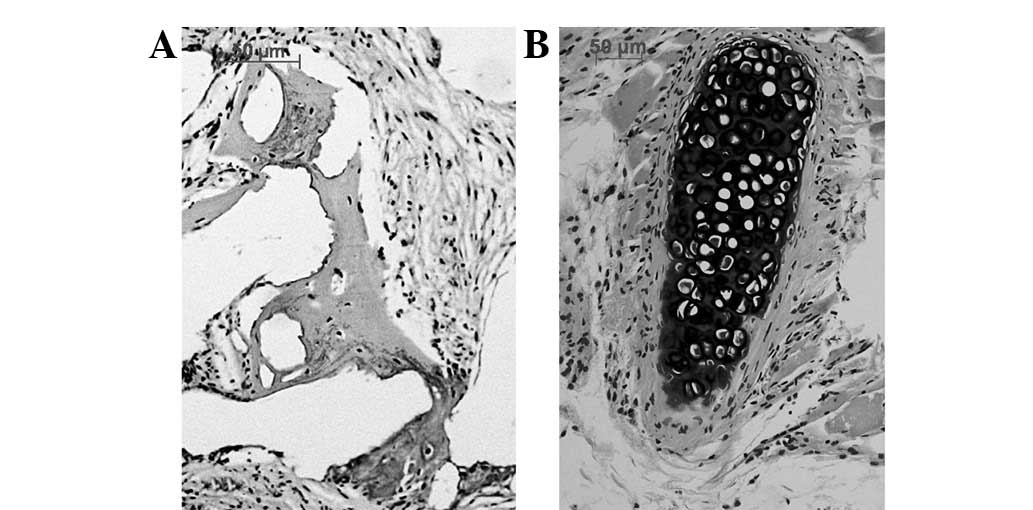
tissue growth. H&E staining indicated new bone formation but no
new cartilage was formed in the osteoblast-scaffold group (Fig. 6A). Islands of cartilage tissue
(Fig. 6B) were present in the
chondroblast-scaffold group. The distribution and arrangement of
the new bone and island cartilage tissues was disordered. The
cell-free scaffold group displayed only host cell growth within the
pores of the scaffold but no bone or cartilage tissues were
observed. The immunohistochemical stain demonstrated that the newly
formed bone displayed type I collagen expression in contrast to the
type II collagen expressed by the cartilage.
Discussion
Osteogenic, chondrogenic and adipogenic
differentiation have been the most common methods used to identify
whether analyzed cell populations are capable of multilineage
differentiation. Pittenger et al(6) utilized clonally derived human
mesenchymal stem cells (hMSCs) and osteogenic, adipogenic and
chondrogenic differentiation assays to demonstrate that clonally
derived hMSCs undergo differentiation to these three lineages. Of
the six tested clonally derived populations, three (50%)
differentiated into all three lineages, whereas two populations
differentiated into the adipogenic and osteogenic lineages and one
population became only osteogenic. These results demonstrated that
BMSCs are capable of multilineage differentiation. Sudo et
al(8) identified that the
majority of the distinct populations of primary fibroblast-like
cells (MPCs or MSCs) derived from various human tissues, including
the lung, skin, umbilical cord and amniotic membrane tissues,
contained cells that are able to differentiate into at least one
mesenchymal lineage, including osteoblasts, chondrocytes and
adipocytes.
A previous study has indicated that the expression
of genes and proteins related to osteoblasts, including ALP, bone
morphogenic proteins, osteocalcin, bone connexins and osteopontin
receptors, are detectable in BMSCs incubated in a differentiated
medium for 2–4 weeks. Newly formed bone was identified 6 weeks
subsequent to the BMSCs being seeded to a biomaterial and implanted
into nude mice. The ability of the various cell populations to
differentiate into particular lineages appears to depend on the
source tissue and induction conditions (8). Chemical inducers, including
dexamethasone, β-glycerophosphate (β-GP) and ascorbic acid, are
essential to cause MSCs to differentiate into osteoblasts (11–14).
Dexamethasone may promote MSC differentiation, as well as
osteocalcin and osteopontin expression, by raising the cAMP level
of MSCs in response to parathyroid hormone and prostaglandin E2.
This chemical inducer is also able to stimulate osteoblast-like
cells to increase insulin-like growth factor secretion, collagen
synthesis and ALP activity (15).
β-GP, as an ALP substrate, provides phosphate ions, activates ALP
activity, promotes the transformation of inorganic phosphorus to
organophosphate and accelerates the formation of a mineralized
extracellular matrix, as well as mineral deposits.
Several studies have compared the osteogenic and
chondrogenic differentiation capacity of BMSCs to those of
adipose-derived stem cells (ADSCs), as well as MSCs derived from
peripheral blood and umbilical cord matrices (7,16–25),
with varying results. The majority of the previous research
findings showed that BMSCs were more advantageous than ADSCs
(7,17,18,21–24).
Afizah et al(16) compared
the chondrogenic potential of human BMSCs with that of ADSCs from
the same donors. Qualitative and quantitative methods were used to
assess for variations in the expression of cartilage markers at the
gene and protein levels. The findings suggested that BMSCs were
more suitable than ADSCs for chondrogenesis. Huang et
al(21) compared the
chondrogenic potential of progenitor cells isolated from bone
marrow aspirates and adipose tissue. The findings showed that the
tissue formed by the aggregate culture of the expanded ADSC
population was less cartilaginous and that BMPCs may be a better
choice for progenitor cell-based strategies for cartilage
repair.
In the present study, three or four passages of
BMSCs were induced for differentiation into osteogenic, adipogenic
and neurogenic lineages in vitro, resulting in the formation
of new bone and cartilage tissues in vivo. The deposition of
calcium and an increased ALP activity were detected when the cells
were incubated in an induction medium consisting of dexamethasone,
ascorbic acid and β-GP. These conditions strongly suggest that
BMSCs are able to differentiate into osteogenic cells. Round and
translucent lipid vacuoles were detected in the cytoplasm and
adipogenic differentiation was demonstrated when the cells were
incubated in an induction medium containing indomethacin,
dexamethasone, IBMX and human insulin. These molecules induced the
MSCs to differentiate into adipocytes. IBMX promotes the adipocytic
and neuroblastic differentiation of stem cells, whereas
indo-methacin inhibits the neurogenic differentiation of MSCs. The
MSCs were induced into neurogenic differentiation when basic
fibroblast growth factor (bFGF), BME and IBMX were added to the
basic medium. These findings demonstrated that BMSCs are able to
differentiate into neuroblasts. The BMSCs were incubated in an
osteogenic and a chondrogenic differentiation medium, seeded on a
coral scaffold and implanted in mice in vivo. New bone and
cartilage tissue formation was demonstrated in vivo.
In summary, in the present study, the BMSCs were
incubated in osteogenic, adipogenic and neurogenic media to
differentiate these cells in vitro into osteoblasts,
adipocytes and neuroblasts, respectively, as well as to form new
bone and cartilage tissues in vivo. The results showed that
the fibroblast-like clone separated from the bone marrow of the
ilium possesses the characteristics of stem cells. The study also
demonstrated that the cells isolated from the bone marrow were
homogeneous and that they were able to differentiate with high
fidelity into osteogenic, adipogenic, neurogenic or chondrogenic
lineages. These human BMSCs are also able to form bone and
cartilage tissues when experimentally implanted in vivo and
may thus be used as seed cells in bone tissue engineering.
Acknowledgements
This study was supported by the
National Science Foundation of China (10972242), the Key Clinical
Program of Ministry of Health China [(2010)439], the Sun Yat-Sen
University Clinical Research 5010 Program (2007050) and the Social
Development Program of the Science and Technology Department of
Guangdong Province, China (2007B031505006).
References
|
1.
|
Arthur A, Zannettino A and Gronthos S: The
therapeutic applications of multipotential mesenchymal/stromal stem
cells in skeletal tissue repair. J Cell Physiol. 218:237–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Delorme B and Charbord P: Culture and
characterization of human bone marrow mesenchymal stem cells.
Methods Mol Med. 140:67–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Krampera M, Marconi S, Pasini A, et al:
Induction of neural-like differentiation in human mesenchymal stem
cells derived from bone marrow, fat, spleen and thymus. Bone.
40:382–390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matsuda C, Takagi M, Hattori T, Wakitani S
and Yoshida T: Differentiation of human bone marrow mesenchymal
stem cells to chondrocytes for construction of three-dimensional
cartilage tissue. Cytotechnology. 47:11–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520.
2001.PubMed/NCBI
|
|
6.
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shahdadfar A, Frønsdal K, Haug T, Reinholt
FP and Brinchmann JE: In vitro expansion of human mesenchymal stem
cells: choice of serum is a determinant of cell proliferation,
differentiation, gene expression, and transcriptome stability. Stem
Cells. 23:1357–1366. 2005. View Article : Google Scholar
|
|
8.
|
Sudo K, Kanno M, Miharada K, et al:
Mesenchymal progenitors able to differentiate into osteogenic,
chondrogenic, and/or adipogenic cells in vitro are present in most
primary fibroblast-like cell populations. Stem Cells. 25:1610–1617.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yang H, Xia Y, Lu SQ, Soong TW and Feng
ZW: Basic fibroblast growth factor-induced neuronal differentiation
of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and
transcription factor AP-1. J Biol Chem. 283:5287–5295. 2008.
View Article : Google Scholar
|
|
10.
|
Zheng YH, He T, Kuang SJ, Zhang ZG and Su
K: The isolation and characterization of human bone marrow
mesenchymal stem cells. Int Med Health Guidance News. 16:129–134.
2010.
|
|
11.
|
Coelho MJ and Fernandes MH: Human bone
cell cultures in biocompatibility testing. Part II: efect of
ascorbic acid, beta-glycerophosphate and dexamethasone on
osteoblastic diferentiation. Biomaterials. 21:1095–1102. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cheng SL, Yang JW, Rifas L, Zhang SF and
Avioli LV: Differentiation of human bone marrow osteogenic stromal
cells in vitro: induction of the osteoblast phenotype by
dexamethasone. Endocrinology. 134:277–286. 1994.PubMed/NCBI
|
|
13.
|
Collignon H, Davicco MJ and Barlet JP:
Isolation of cells from ovine fetal long bone and characterization
of their osteoblastic activities during in vitro mineralization.
Arch Physiol Biochem. 105:158–166. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Milne M, Quail JM and Baran DT:
Dexamethasone stimulates osteogenic diferentiation in vertebral and
femoral bone marrow cell cultures: comparison of IGF-1 gene
expression. J Cell Biochem. 71:382–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sakaguchi Y, Sekiya I, Yagishita K and
Muneta T: Comparison of human stem cells from various mesenchymal
tissues: superiority of synovium as a cell source. Arthritis Rheum.
52:2521–2529. 2005.PubMed/NCBI
|
|
16.
|
Afizah H, Yang Z, Hui JH, Ouyang HW and
Lee EH: A comparison between the chondrogenic potential of human
bone marrow stem cells (BMSCs) and adipose-derived stem cells
(ADSCs) taken from the same donors. Tissue Eng. 13:659–666. 2007.
View Article : Google Scholar
|
|
17.
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
De Ugarte DA, Morizono K, Elbarbary A, et
al: Comparison of multi-lineage cells from human adipose tissue and
bone marrow. Cells Tissues Organs. 174:101–109. 2003.PubMed/NCBI
|
|
19.
|
Erickson GR, Gimble JM, Franklin DM, Rice
HE, Awad H and Guilak F: Chondrogenic potential of adipose
tissue-derived stromal cells in vitro and in vivo. Biochem Biophys
Res Commun. 290:763–769. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hayashi O, Katsube Y, Hirose M, Ohgushi H
and Ito H: Comparison of osteogenic ability of rat mesenchymal stem
cells from bone marrow, periosteum, and adipose tissue. Calcif
Tissue Int. 82:238–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Huang JI, Kazmi N, Durbhakula MM, Hering
TM, Yoo JU and Johnstone B: Chondrogenic potential of progenitor
cells derived from human bone marrow and adipose tissue: a
patient-matched comparison. J Orthop Res. 23:1383–1389. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hui JH, Li L, Teo YH, Ouyang HW and Lee
EH: Comparative study of the ability of mesenchymal stem cells
derived from bone marrow, periosteum, and adipose tissue in
treatment of partial growth arrest in rabbits. Tissue Eng.
11:904–912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Liu TM, Martina M, Hutmacher DW, Hui JH,
Lee EH and Lim B: Identification of common pathways mediating
differentiation of bone marrow- and adipose tissue-derived human
mesenchymal stem cells into three mesenchymal lineages. Stem Cells.
25:750–760. 2007.PubMed/NCBI
|
|
24.
|
Winter A, Breit S, Parsch D, et al:
Cartilage-like gene expression in differentiated human stem cell
spheroids: a comparison of bone marrow-derived and adipose
tissue-derived stromal cells. Arthritis Rheum. 48:418–429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002.PubMed/NCBI
|