Introduction
The Wnt gene family encodes evolutionarily conserved
proteins that regulate processes in adults and in embryonic
development (1–4). The Wnt signaling pathway impacts the
development of the liver, pancreas, intestines, lungs and other
endodermal organs, and the development of the mesodermal organs in
early embryonic development by appropriate spatial and temporal
mechanisms (5–9). Numerous WNT signaling pathways are
involved in the signal transduction process and the canonical
Wnt/β-catenin signaling pathway is the best characterized pathway
in this process.
The Wnt/β-catenin signaling pathway is topic of
great interest in biomedicine. Numerous studies have confirmed that
the key activator of the Wnt/β-catenin signaling pathway is the
nuclear translocation of β-catenin. As a key signaling protein, its
accumulation in the cytoplasm and translocation into the nucleus
mark the activation of this signaling pathway (10,11).
However, few studies have addressed its role in the generation and
development of gastrointestinal tissues. In the current study, we
investigated the primitive guts of Sprague Dawley (SD) rats during
the middle and late embryonic periods and the gastrointestinal
tissues of rats during the perinatal period. Using
immunohistochemical methods and a clear understanding of the normal
developmental pattern from morphological and histological
characterizations, we examined the temporal expression pattern of
β-catenin in the Wnt/β-catenin signaling pathway in the
gastrointestinal tissues of embryonic and adult rats. Furthermore,
we discuss the role of the pathway in gastrointestinal
development.
Materials and methods
Animals
SD rats (20 females and 10 males) were purchased
from the animal center of the Cancer Institute of the Chinese
Academy of Medical Sciences (Beijing, China), and bred at the
facility of laboratory animals in Tianjin University (Tianjin,
China). All experimental procedures were carried out according to
the regulations and internal biosafety and bioethics guidelines of
Tianjin Medical University and the Tianjin Municipal Science and
Technology Commission (Tianjin, China). The rats were housed
together each night and vaginal secretions were obtained the next
morning. Day 0 of gestation was marked as the day when sperm was
discovered. Since the average fertility cycle of rats is 28 days,
the integrated embryos were removed by cesarean section from
anesthetized female rats at days 13, 18 and 21 of gestation and
then fixed in 4% paraformaldehyde. Additionally, the
gastrointestinal tissues of rats at days 1, 3, 7 and 28 of age were
removed and fixed in 4% paraformaldehyde.
Immunohistochemistry
After the embryos and the gastrointestinal tissues
of rats were fixed, paraffin-embedded tissue sections were used for
the examination of β-catenin expression. The sections were dewaxed,
treated with 3% H2O2 for 10 min and incubated
with the appropriate antibody (1:200 dilutions; Signalway Antibody,
College Park, MD, USA) overnight at 4°C. Biotinylated secondary
antibody (1:200 dilutions; Signalway Antibody) was added at room
temperature for 1 h, which was followed by incubation with
ABC-peroxidase for an additional hour. After washing with Tris
buffer, the sections were incubated with 3,3′-diaminobenzidine
(DAB, 30 mg dissolved in 100 ml Tris buffer containing 0.03%
H2O2) for 5 min, rinsed in water and
counterstained with hematoxylin.
The average positive rates of β-catenin were
determined by examining slices in each of 10 randomly selected
fields that were chosen by the same observer. The β-catenin
staining in the cytoplasm and the nucleus of each cell was analyzed
according to Duncan et al(12).
Statistical analysis
The statistical analysis was performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). To compare the
expression-positive rates of β-catenin at various time points, a
χ2 test was applied to compare the numerical data among
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
β-catenin expression in the cytoplasm and
nuclei of gastric cells at various time points
Fig. 1 shows the
expression of β-catenin in gastric cells. First, β-catenin
expression was observed in the cytoplasm at each of the selected
time points and the expression-positive rates were 6% (day 13 of
gestation), 8% (day 18 of gestation), 62% (day 21 of gestation),
89% (day 1 of age), 94% (day 3 of age), 75% (day 7 of age) and 2%
(day 28 of age). After statistical comparisons were conducted
between any two samples, the differences in the expression-positive
rate among samples from days 13 and 18 of gestation and day 28 of
age were not statistically significant (P>0.05) due to the low
positive rates of these samples. However, comparing each of these
data with data obtained from day 21 of gestation and days 1, 3 and
7 of age, respectively, the differences were statistically
significant (χ2=408.73, P<0.001). The positive rates
were higher in the latter samples. Secondly, in the nuclei of the
samples taken at the 7 chosen time points, the expression of
β-catenin was only observed in the rats at day 21 of gestation and
days 1, 3 and 7 of age, which corresponded to positive rates of
12%, 81%, 86% and 90%, respectively. Statistical comparisons
between any two samples showed that the differences between the
sample at day 21 of gestation and the latter three samples were
statistically significant (χ2=186.65, P<0.001),
whereas the differences between the samples taken at days 1, 3 and
7 of age were not statistically significant (P>0.05). The
positive rate of the sample taken at day 21 of gestation was low,
whereas the rates of the latter three samples were high (Fig. 2).
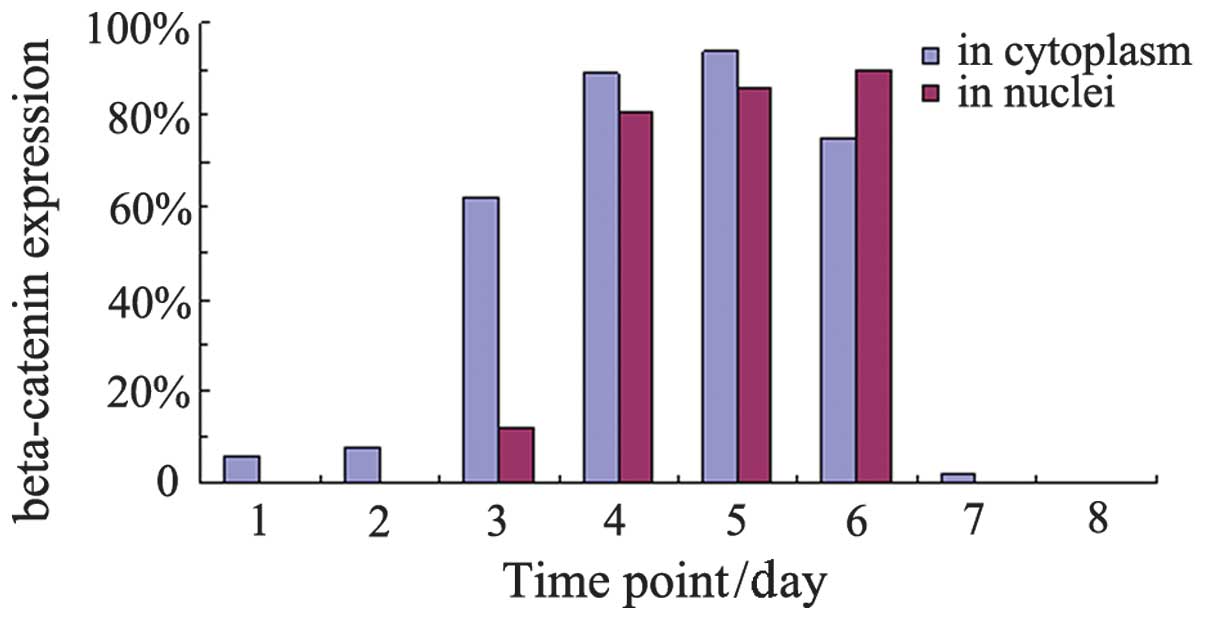 | Figure 2β-catenin expression in the cytoplasm
and nuclei of gastric cells at various time points. Time points 1,
2, 3, 4, 5, 6 and 7 represent days 13, 18 and 21 of gestation and
days 1, 3, 7 and 28 of age, respectively. Series 1, β-catenin
expression in the cytoplasm of gastric cells at various time
points, Series 2, β-catenin expression in the nuclei of gastric
cells at various time points. |
β-catenin expression in the cytoplasm and
nuclei of intestinal cells at various time points
Fig. 3 shows
β-catenin expression in the cytoplasm and nuclei of intestinal
cells. β-catenin expression was observed in the cytoplasm of cells
taken at the 7 time points and the positive rates were 36% (day 13
of gestation), 88% (day 18 of gestation), 96% (day 21 of
gestation), 97% (day 1 of age), 97% (day 3 of age), 37% (day 7 of
age) and 24% (day 28 of age). After a statistical analysis of these
data was conducted, the differences in the expression-positive rate
among samples at day 13 of gestation and days 7 and 28 of age were
not statistically significant since the positive rates were too low
(P>0.05). However, when each of these samples was compared with
samples taken at day 18 and 21 of gestation and days 1 and 13 of
age, the differences were statistically significant
(χ2=311.16, P<0.001). The positive rate was higher in
the latter samples. β-catenin expression was also observed in the
nuclei of intestinal samples taken from rats at days 18 and 21 of
gestation and days 1, 3 and 7 of age. Using statistical analysis,
we identified that the differences between the sample taken at day
7 of age and each of the other four samples were statistically
significant (χ2=204.37, P<0.001), whereas the
differences among days 18 and 21 of gestation and days 1 and 3 of
age were not (P>0.05). The positive rates were low for the
samples taken at day 7 of age, whereas the rates were high for the
samples taken at the other four time points (Fig. 4).
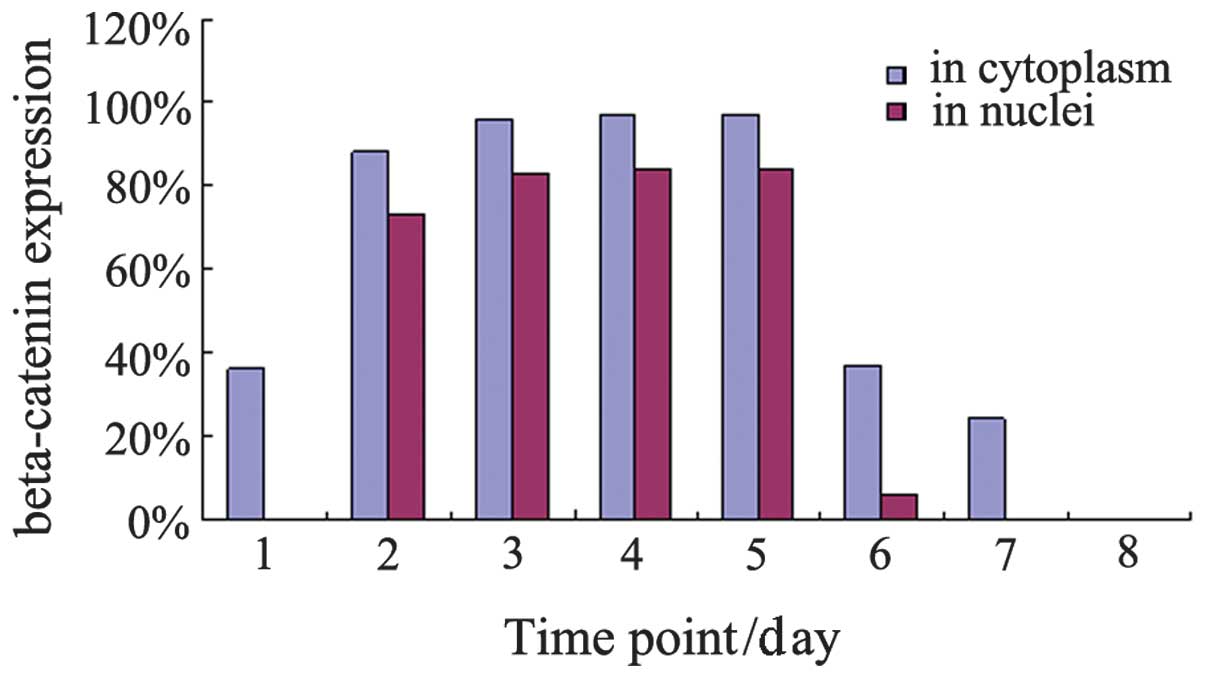 | Figure 4β-catenin expression in the cytoplasm
and nuclei of intestinal cells at various time points. Series 1,
β-catenin expression in the cytoplasm of intestinal cells at
various time points. Series 2, β-catenin expression in the nuclei
of intestinal cells at various time points. Time points 1, 2, 3, 4,
5, 6 and 7 represent days 13, 18 and 21 of gestation and days 1, 3,
7 and 28 of age, respectively. |
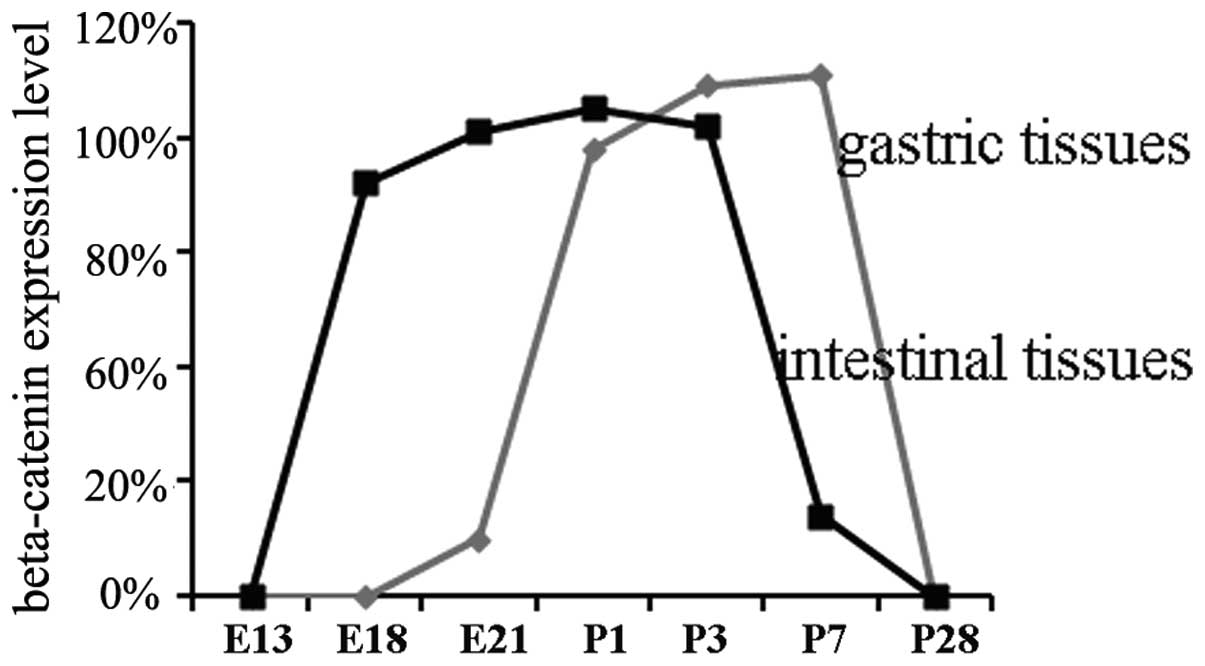
Expression pattern of β-catenin in
various tissues and developmental phases
In gastric cells, β-catenin had moved from the
cytoplasm into the nuclei by day 21 of gestation. The expression
reached a peak level at days 1, 3 and 7 of age, and then the
expression began to decrease at day 7 of age. Within intestinal
cells, β-catenin moved from the cytoplasm into the nuclei by day 18
of gestation, reached a peak level at day 21 of gestation and days
1 and 3 of age, and then began to decrease at day 3 of age.
β-catenin moved from the cytoplasm into the nuclei and reached a
peak level in intestinal tissues earlier than in gastric tissues.
This time point corresponded to when the signal intensity began to
decrease (Fig. 5).
Discussion
Nusse et al(13) reported the first Wnt gene when the
authors induced breast cancer into mice using the mouse mammary
tumor virus (MMTV). Since this discovery in 1982, 19 types of human
Wnt genes have been identified (14). The Wnt signaling pathway has also
been shown to be divided into three main branches: i) the canonical
Wnt/β-catenin signaling pathway. This pathway regulates the
expression of target genes of the Wnt/β-catenin signaling pathway
through the β-catenin/T-cell factor (TCF) (15,16).
ii) The planar cell polarity (PCP) pathway. This pathway regulates
cytoskeletal rearrangement within cells, establishes asymmetric
cell polarity and coordinates changes in cell morphology and cell
movement (17). iii) The
Wnt/Ca2+ pathway. This pathway regulates cellular
adhesion and viability (18).
Research on Wnt signal transduction showed that β-catenin, a
protein mediating cellular adhesion and cellular junctions, is
crucial for the Wnt signaling pathway (19,20).
Under normal conditions, the majority of β-catenin in the cytoplasm
binds E-cadherin and cytoskeletal proteins to maintain the
stability of cells. However, some β-catenin binds adenomatous
polyposis coli (APC), glycogen synthase kinase 3β (GSK3β) and axin
and then the ubiquitin-proteasome system through GSK3β
phosphorylation and the β-transducin-repeat-containing protein
(β-TrCP). β-TrCP degrades β-catenin and thereby maintains low
levels of free β-catenin in the cytoplasm that prevent β-catenin
from entering the nucleus to activate target genes (21). When the Wnt signaling pathway is
activated, Wnt binds the receptors Frizzled (Fz) and LRP5/6 to
activate the dishevelled protein (Dvl/Dsh) within cells. Dvl then
inhibits the phosphorylation of β-catenin by GSK3β. The
unphosphorylated β-catenin remains stable in the cytoplasm,
accumulates gradually, moves into the nucleus and then binds
transcription factors of the TCF/LEF family to control the
transcription and expression of downstream target genes and fully
promote the development of tissues.
A previous study suggests that Wnt/β-catenin plays
an important role in the early development of the embryo (22). For example, at the two-cell stage
of Xenopus development, β-catenin is distributed mainly in
the cytoplasm of dorsal cells, whereas during the 16-cell stage to
32-cell stage; it is mainly distributed in the nuclei of dorsal
cells. However, if the β-catenin in the rat embryo is knocked out,
the development of the embryonic primary body axis fails. Few
studies have systematically investigated whether β-catenin plays an
important role during the middle and late embryonic periods and the
early postnatal period.
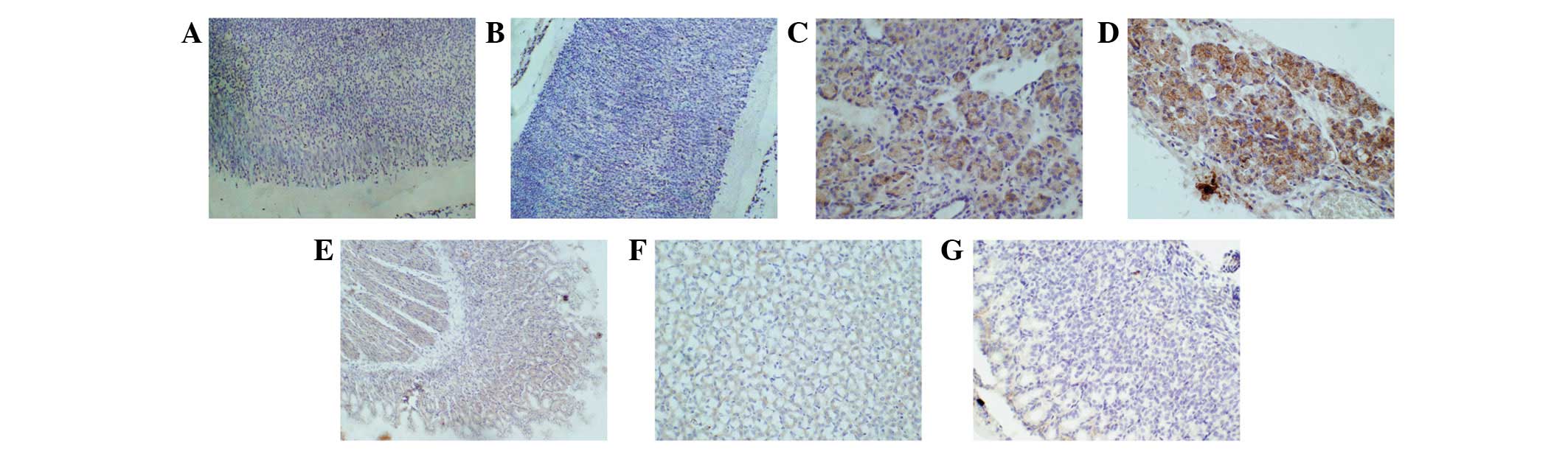
In the longitudinal sections of gastric tissues from
the primitive gut shown in Fig. 1A and
B, we observed a few light brown stains in the cytoplasm of the
mucousal, submucousal, muscular, and serous layers; these stains
showed that β-catenin exists in the cytoplasm of gastric cells at
these two time points, but it was small in quantity and weak in
signal expression. In Fig. 1C–E,
we observed stronger brown stains in gastric cells, and these
stains were observed in the cytoplasm and in the nuclei. The stains
gradually became more evident, and the signal expression increased.
This result showed that β-catenin had moved from the cytoplasm into
the nucleus by day 21 of gestation, which indicated that the
β-catenin signaling pathway was activated at this time point. This
movement had reached a peak in the rats at days 1 and 3 of age. In
Fig. 1F, the positive rates of
brown stains in the gastric cytoplasm and nuclei of the rats at day
7 of age remained at the peak level, but the color was lighter. In
Fig. 1G, the brown stains in the
cytoplasm and nuclei of the rat gastric cells at day 28 of age had
almost disappeared. This result showed that β-catenin expression
had weakened in the gastric cells of the rat at day 7 of age, and a
small amount of β-catenin existed in the cytoplasm of rat gastric
cells at day 28 of age when gastric structures are formed
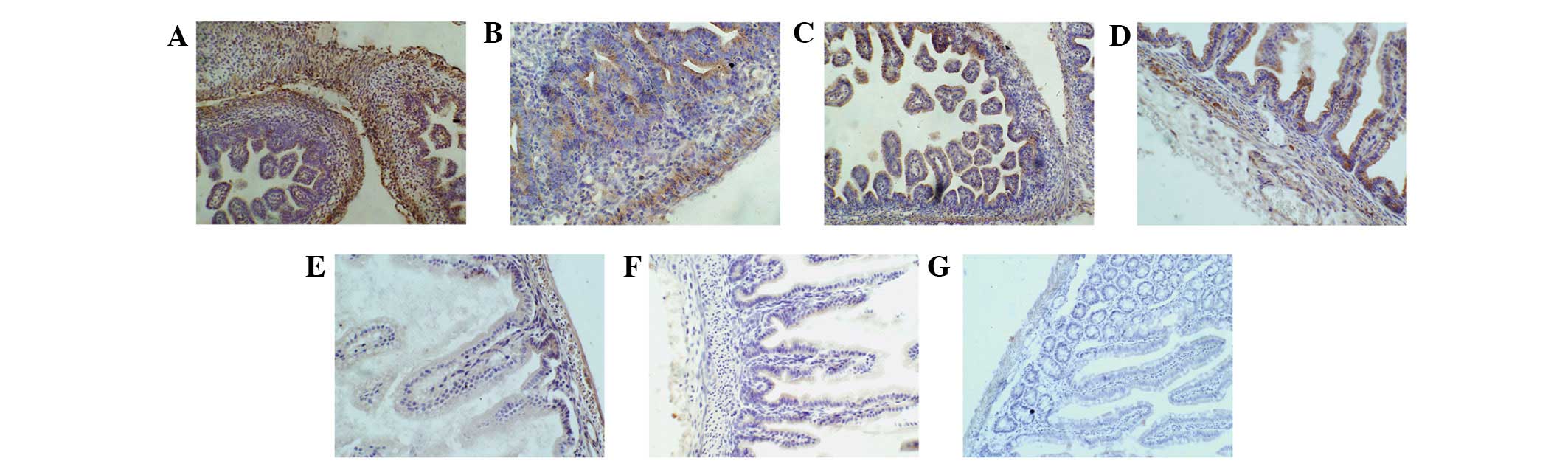
(including the gastric lamina propia and gland, shown in Fig. 1G). Similarly, in the primitive rat
gut at day 13 of gestation, shown in Fig. 3A–G, the brown stains were not
evident in the mucousal and submucousal layers; these stains were
clearly visible in the muscular layer and adventitial cells, which
showed that β-catenin had accumulated in the cytoplasm. The brown
stains were observed in the cytoplasm and nuclei of rat intestinal
cells at days 18 and 21 of gestation and days 1, 3 and 7 of age. At
day 21 of gestation and days 1 and 3 of age, the positive rates of
these samples gradually increased to a peak, but the stain
intensity decreased at day 3 of age. These data suggest that
β-catenin moved from the cytoplasm into the nuclei at day 18 of
gestation and that the β-catenin signaling pathway was most
strongly activated at day 21 of gestation and days 1 and 3 of age
but then decreased after day 3 of age. The brown-stain positive
rates and stain intensity were markedly lower in the cytoplasm and
nuclei of the rat intestinal cells at day 7 of age, and the brown
stains disappeared from the nuclei at day 18 of age. This finding
indicated that a small amount of β-catenin existed in the cytoplasm
of the rat intestinal cells at day 28 of age. However, Fig. 3G showed the intestinal structure,
including the intestinal villi and glands.
In summary, we examined the development and
differentiation of gastrointestinal tissues in the middle and late
embryonic periods and the early postnatal period. We observed that
the expression of β-catenin in the gastrointestinal tissues was
present at day 13 of gestation, activated from day 18 of gestation
to day 3 of age, and began to decrease at day 7 of age. These data
suggest that β-catenin plays an important role by promoting the
development of gastrointestinal tissues in the middle and late
embryonic periods and the early postnatal period. However, after
tissues and organs mature, β-catenin is maintained at a low level
in the cytoplasm so it is not able to promote development. The
results of these experiments suggest that β-catenin plays an
important role in gastrointestinal tissue development during the
middle and late embryonic periods and the early postnatal period,
which provides the necessary experimental evidence for further
studies concerning the mechanism of this signaling pathway,
tumorigenesis and tumor treatment.
Acknowledgements
This study was financially supported
by the National Natural Science Foundation of China (81172356) and
the Natural Science Foundation of Tianjin Municipal Science and
Technology Commission (10JCZDJC18500).
References
|
1.
|
Kohn AD and Moon RT: Wnt and calcium
signaling: beta-catenin-independent pathways. Cell Calcium.
38:439–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Moon RT: Wnt/beta-catenin pathway. Sci
STKE. 15:2712005.
|
|
4.
|
Choi SC and Sokol SY: The involvement of
lethal giant larvae and Wnt signaling in bottle cell formation in
Xenopus embryos. Dev Biol. 336:68–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sawyer JM, Harrell JR, Shemer G,
Sullivan-Brown J, Roh-Johnson M and Goldstein B: Apical
constriction: a cell shape change that can drive morphogenesis. Dev
Biol. 341:5–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kumburegama S, Wijesena N, Xu R and
Wikramanayake AH: Strabismus-mediated primary archenteron
invagination is uncoupled from Wnt/β-catenin-dependent endoderm
cell fate specification in Nematostella vectensis (Anthozoa,
Cnidaria): Implications for the evolution of gastrulation. Evodevo.
2:22011.PubMed/NCBI
|
|
7.
|
Kapasa M, Arhondakis S and Kossida S:
Phylogenetic and regulatory region analysis of Wnt5 genes reveals
conservation of a regulatory module with putative implication in
pancreas development. Biol Direct. 5:492010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okubo T and Hogan BL: Hyperactive Wnt
signaling changes the developmental potential of embryonic lung
endoderm. J Biol. 3:112004. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Königshoff M and Eickelberg O: WNT
signaling in lung disease: a failure or are generation signal? Am J
Respir Cell Mol Biol. 42:21–31. 2010.
|
|
10.
|
Awasaki T, Tatsumi R, Takahashi K, Arai K,
Nakanishi Y, Ueda R and Ito K: Essential role of the apoptotic cell
engulfment genes draper and ced-6 in programmed axon pruning during
Drosophila metamorphosis. Neuron. 50:855–867. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Dugas JC, Tai YC, Speed TP, Ngai J and
Barres BA: Functional genomic analysis of oligodendrocyte
differentiation. J Neurosci. 26:10967–10983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Duncan JL, LaVail MM, Yasumura D, et al:
An RCS-like retinal dystrophy phenotype in mer knockout mice.
Invest Ophthalmol Vis Sci. 44:826–838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wodarz A and Nusse R: Mechanisms of Wnt
signaling in development. Annu Rev Cell Dev Biol. 14:59–88. 1998.
View Article : Google Scholar
|
|
15.
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Akiyama T: Wnt/beta-catenin signaling.
Cytokine Growth Factor Rev. 11:273–282. 2000. View Article : Google Scholar
|
|
17.
|
Mlodzik M: Planar cell polarization: do
the same mechanisms regulate Drosophila tissue polarity and
vertebrate gastrulation? Trends Genet. 18:564–571. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kühl M, Sheldahl LC, Park M, Miller JR and
Moon RT: The Wnt/Ca2+ pathway: a new vertebrate Wnt
signaling pathway takes shape. Trends Genet. 16:279–283. 2000.
|
|
19.
|
Thisse C, Thisse B, Schilling TF and
Postlethwait JH: Structure of the zebrafish snail1 gene and its
expression in wild-type, spadetail and no tail mutant embryos.
Development. 119:1203–1215. 1993.PubMed/NCBI
|
|
20.
|
Pelegri F: Maternal factors in zebrafish
development. Dev Dyn. 228:535–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Uthoff SM, Eichenberger MR, McAuliffe TL,
Hamilton CJ and Galandiuk S: Wingless-type frizzled protein
receptor signaling and its putative role in human colon cancer. Mol
Carcinog. 31:56–62. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
van Amerongen R, Fuerer C, Mizutani M and
Nusse R: Wnt5a can both activate and repress Wnt/β-catenin
signaling during mouse embryonic development. Dev Biol.
369:101–114. 2012.PubMed/NCBI
|