Introduction
Hepatocellular carcinoma is the most common solid
organ tumor worldwide. China has a high incidence rate, with
∼300,000 mortalities each year (1). Surgery is the method of choice, but
remains unsatisfactory due to the high rate of tumor recurrence.
Therefore, treatments of liver cancer with a reliable curative
effect and that maximize the protection of liver function are
sought.
The most commonly offered therapy is transcatheter
arterial chemoembolization (TACE) (2–5).
However, in patients with advanced cirrhosis and hepatic
decompensation, TACE is contraindicated since the ischemic damage
associated with embolization may lead to a rapid decline in liver
function with worsening encephalopathy, increased ascites and,
potentially, fatality (6–8).
Currently, percutanous microwave coagulation therapy
(PMCT) as a liver-directed therapy, offers the potential for
extended survival in patients with advanced hepatocellular
carcinoma (9–11). However, PMCT success may also be
limited by the presence of large portal or hepatic vein branches
adjacent to the tumor. The flowing blood may act as a heat sink and
limits the ability to heat the tissue to a sufficient temperature.
Therefore, PMCT occasionally does not completely kill tumor cells.
Another newly developed local treatment, 125I seed
brachytherapy (12–14), delivers low-dose brachytherapy to
the tumor. This treatment is also contraindicated since
radiotherapy does not completely kill hypoxic tumor cells.
The purpose of the current study was to evaluate the
efficacy and safety of PMCT followed by 125I seed
brachytherapy for VX2 liver cancer in rabbits, to overcome the
respective limitations of the two types of treatment and to explore
a novel combined method of treatment for liver cancer.
Materials and methods
Establishment of the animal models and
tumor growing techniques
Ninety-six New Zealand white rabbits (age, 3–4
months; weight, 3.1–3.6 kg) were used for the experiment. They were
provided by Animal Laboratory of the Chinese Air Force General
Hospital (Beijing, China). This study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health of
China. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of Qingdao
University (Qingdao, China).
In this study, the VX2 carcinoma was maintained via
serial transplantation into the hind limb muscle of the New Zealand
white rabbit. Following implantation, the tumor enlarged rapidly.
In order to reduce the potential difference of tumor growth, tumor
tissues were obtained from the same tumor donor rabbit and fresh
VX2 tumor tissue was obtained from the same lateral thigh muscle of
the New Zealand white rabbit.
For preparation of a VX2 tumor cell suspension, the
VX2 tumor was stripped aseptically, mechanically homogenized,
filtered through iron mesh with 0.08 mm2 pores and
centrifuged at 2,000 rpm for 10 min (Centrifuge 5702, Eppendorf,
Hong Kong, China). Finally, the viable cells were adjusted to a
concentration of 1×107 cells/ml.
Using computed tomography (CT)-guidance, 0.2 ml VX2
tumor cell suspension was percutaneously injected into the center
of the lobe of the liver of rabbits slowly using an 18G needle
under i.v. anesthesia. With B-ultrasound monitoring the liver of
each rabbit, we chose the rabbits with only one tumor implanted in
the left liver lobe and its diameter was measured as 2 cm within 14
to 26 days after tumor implantation. Contrast-enhanced CT and MRI
were performed to detect the necrotic region of the tumor.
Of the 96 rabbits, 80 (in which the tumor diameter
reached 2 cm) were randomly divided into 4 groups (each n=20). The
rabbits in group A were treated with CT-guided PMCT at 40 W for 120
sec. The rabbits in group B were treated with CT-guided
125I seed brachytherapy (0.5 mCi, range, 60 Gy). The
rabbits in group C (combination therapy) were treated with
percutaneous microwave coagulation therapy followed by
125I seed brachytherapy. The rabbits in the control
group (group D) were not treated. At 14 days after surgery, the
rabbits were sacrificed for pathological assessment.
Pretreatment tumor location
Spiral CT scanning conditions were as follows: 120
kV, 200 mA, FOV 14×14 cm, slice thickness 3 mm and spacing 3 mm.
The selection of Ultravist® was 300 mg/ml, the dose of
contrast was 7 ml, the speed of injection was 3 ml/sec. The animals
were anesthetized following the first liver CT scan delay of 10–12
sec, and an early arterial and portal venous phase enhanced scan
was conducted to determine the location of the tumor after 40–50
sec. It is extremely important to detect the necrosis of the tumor
prior to treatment in order to increase the accuracy of this
experiment. In each group prior to treatment, MRI diffusion imaging
was also used.
Microwave coagulation treatment
Group A (the microwave treatment group) consisted of
20 rabbits. Following routine skin preparation, local disinfection
and anesthesia, a CT scan was applied to determine the location of
the tumor. With a CT-guided microwave coagulation antenna (diameter
1.6 mm) implanted into the tumor center, PMCT was performed with an
output power of 40 W for 2 min. The microwave therapeutic
instrument and microwave radiation antenna were made by Nanjing
Microwave Electronics Research Institute and Nanjing Kia Microwave
Technology Ltd. (Nanjing, China).
125I seed brachytherapy
For group B (125I seed brachytherapy
group) we used a treatment planning system (TPS) system combined
with the classic formula design. The plan for 125I seed
implantation was designed with the guidance of three experienced
doctors from the Department of Radiation Oncology (Beijing Cancer
Hospital, Beijing, China). The skin surface of the rabbits was
placed on a particle locator (Locator plexiglass; Ningbo Jaco
Pharmaceuticals Co. Ltd., Zhejiang, China; hole spacing, 0.5
cm).
CT-guided seed implantation was conducted using a
percutaneous locator which pierced the liver around the tumor, to
allow the implantation of seeds according to the designed radiation
treatment plan. Each rabbit was implanted with 12 125I
seeds (three layers, each layer 4 seeds). The radiation dose of
each seed was 0.5 mCi. The tumor peripheral prescription dose was
60 Gy. Two days after treatment, plain film of the abdomen (KUB)
was administered to the rabbits to determine whether the
125I seeds had migrated or not. The 125I seed
implantation instrument and 125I seeds were provided by
Ningbo Jaco Pharmaceuticals Co. Ltd. (Ningbo, China).
Combination treatment (PMCT followed by
125I seed brachy-therapy)
Group C were treated with CT-guided percutaneous
microwave therapy of the VX2 carcinoma of the rabbits in the same
manner as in group A, and 125I seed implantation was
performed in the same manner as in group B.
Pathological assessment
At 21 days after treatment, the rabbits were
sacrificed by means of Sumianxin II overdose (produced by the
Military Veterinary Academy of Medical Sciences Institute,
Changchun, China) at 2 ml/kg body weight. The rabbits were fixed in
a supine position by abdominal longitudinal midline incision of the
upper abdominal skin and local disinfection was performed. Two
experienced pathologists (Ji Xiang Rui and Peng Zhao) conducted an
assessment with the naked eye on the extent of the tumor and
adjacent liver, stomach and intestine, and changes in the
gallbladder, diaphragm and skin tissue. Histopathological
examination included cross-sectional hematoxylin and eosin
(H&E) staining, with a slice thickness of 5 μm, where
the pathologist used a light microscope to assess the tumor
necrosis rate and the presence of intrahepatic metastasis.
The injuries to the adjacent tissues of the
therapeutic region were classified from grade 0 to grade III (grade
0, damage; grade I, mild injury to 1/3 the thickness of the tissue;
grade II, moderate injury to 2/3 the thickness of the tissue; grade
III, severe injury to the full thickness of the tissue).
Statistical analysis
Data are presented as the mean ± SD. Gross tumor
volumes at different time points were compared by Student’s t-test.
Statistical analyses were performed using SPSS 11.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically signifcant result.
Results
VX2 hepatic tumor in a rabbit model
Ninety-six New Zealand white rabbits were used to
establish the animal model. Eighty of the rabbits developed primary
tumors, a success rate of 83.33%.
CT and MRI findings
With B-ultrasound monitoring the liver of each
rabbit, we chose the rabbits with only one tumor implanted in the
left liver lobe and its diameter was measured as 2 cm within 14 to
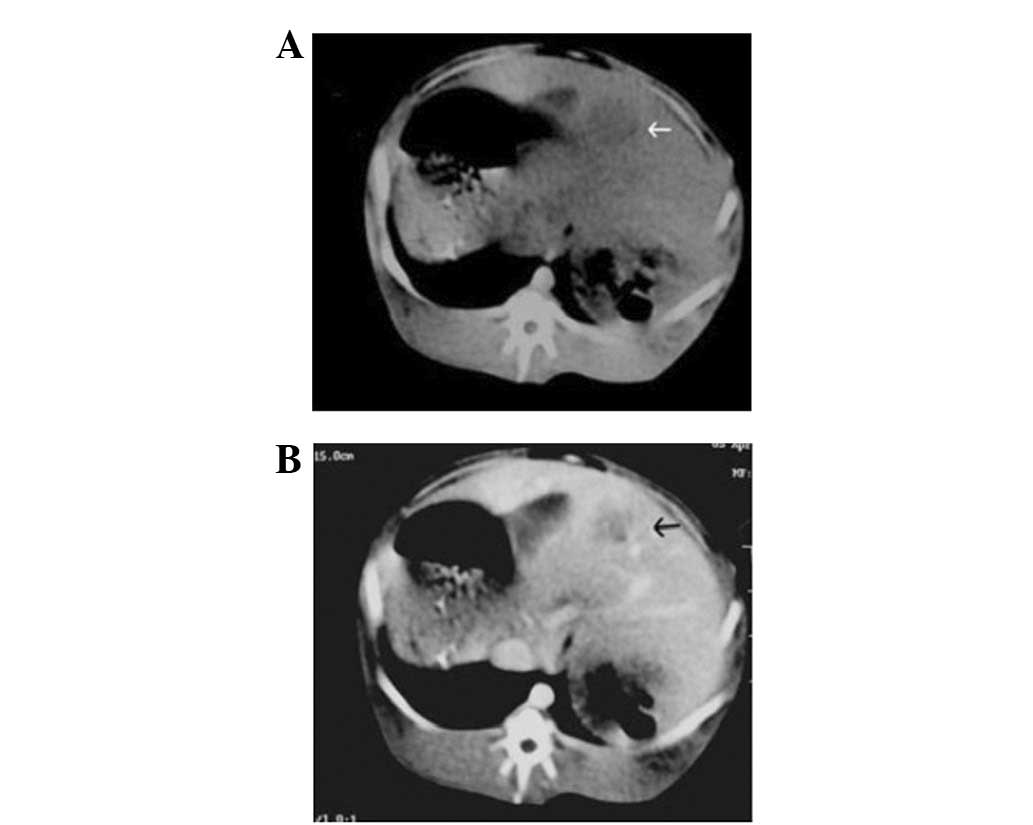
26 days after tumor implantation. CT plain scanning images revealed
a tumor (diameter 2 cm) with low density in the liver of an
experimental rabbit (Fig. 1A) and
CT enhancement scanning images (Fig.
1B) demonstrated that the tumor had a low-density central
region without intensification but the surrounding region of the
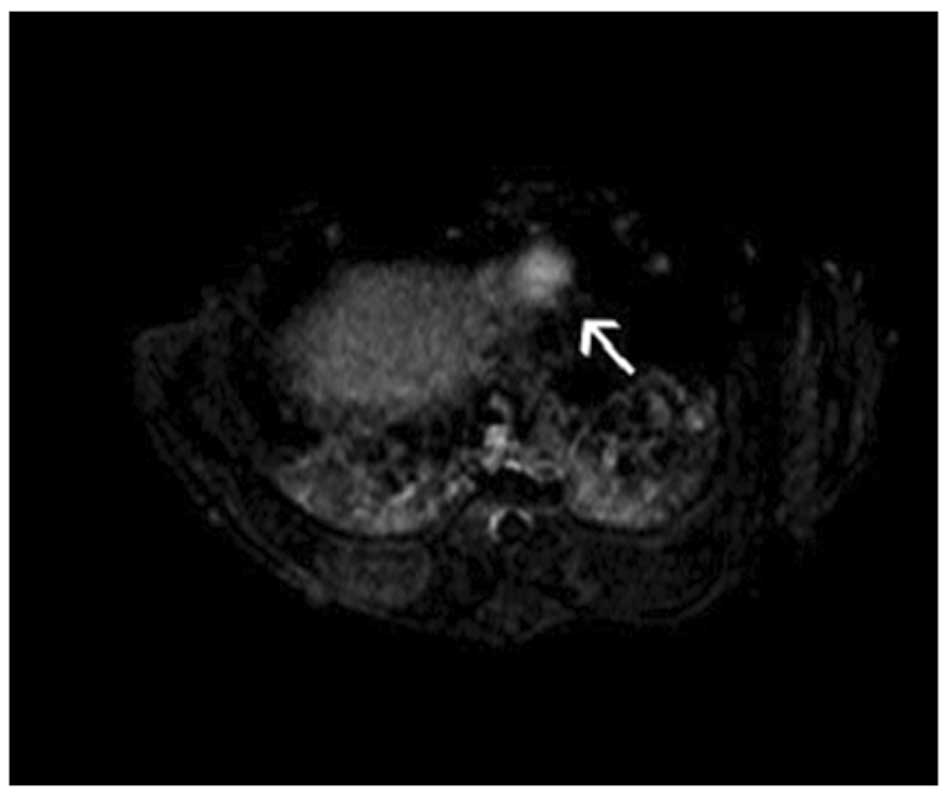
tumor intensification. MRI showed the VX2 tumor with a
high-intensity signal by diffusion imaging, allowing clear
visualization of the boundary. No clear signs of central necrosis
were shown on B-ultrasound when the tumor sizes were almost 20 mm.
This was also verified by CT contrast imaging (Fig. 1B) and MRI diffusion imaging
(Fig. 2).
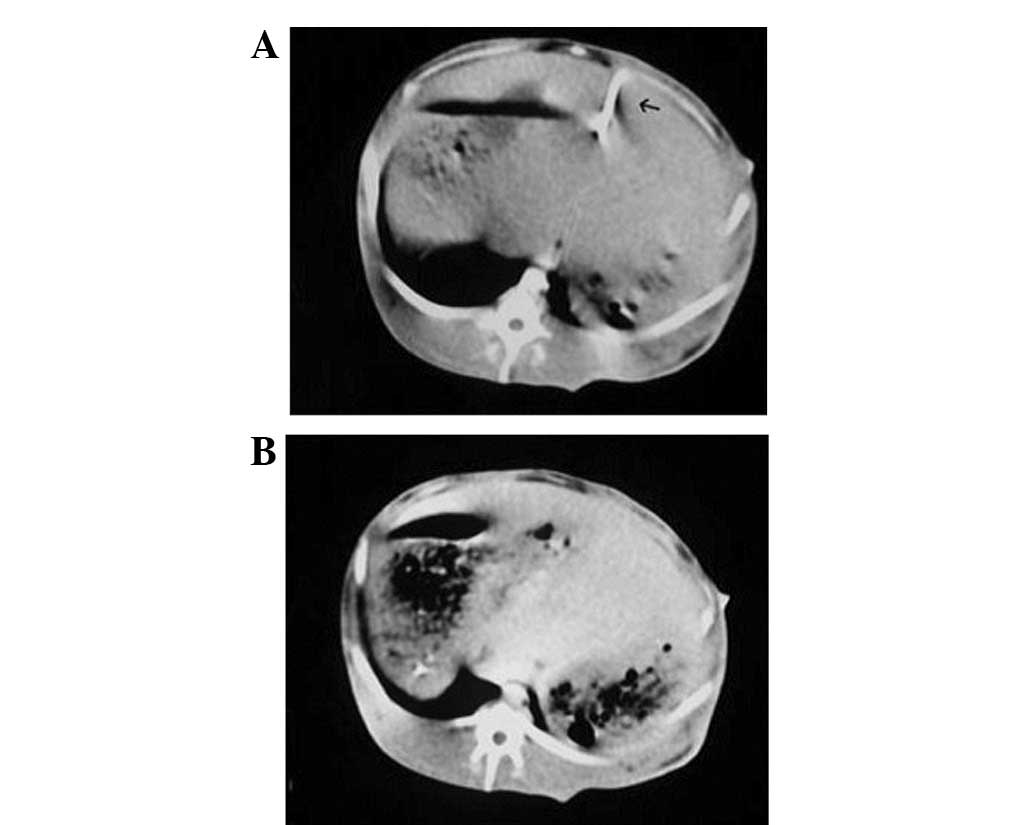
CT plain scanning images revealed the microwave
ablation needle in the center of the tumor during the CT-guided
PMCT at 40 W for 120 sec (Fig.
3A). Small bubbles were observed in the region of the tumor
five minutes after the ablation on CT plain scanning images
(Fig. 3B).
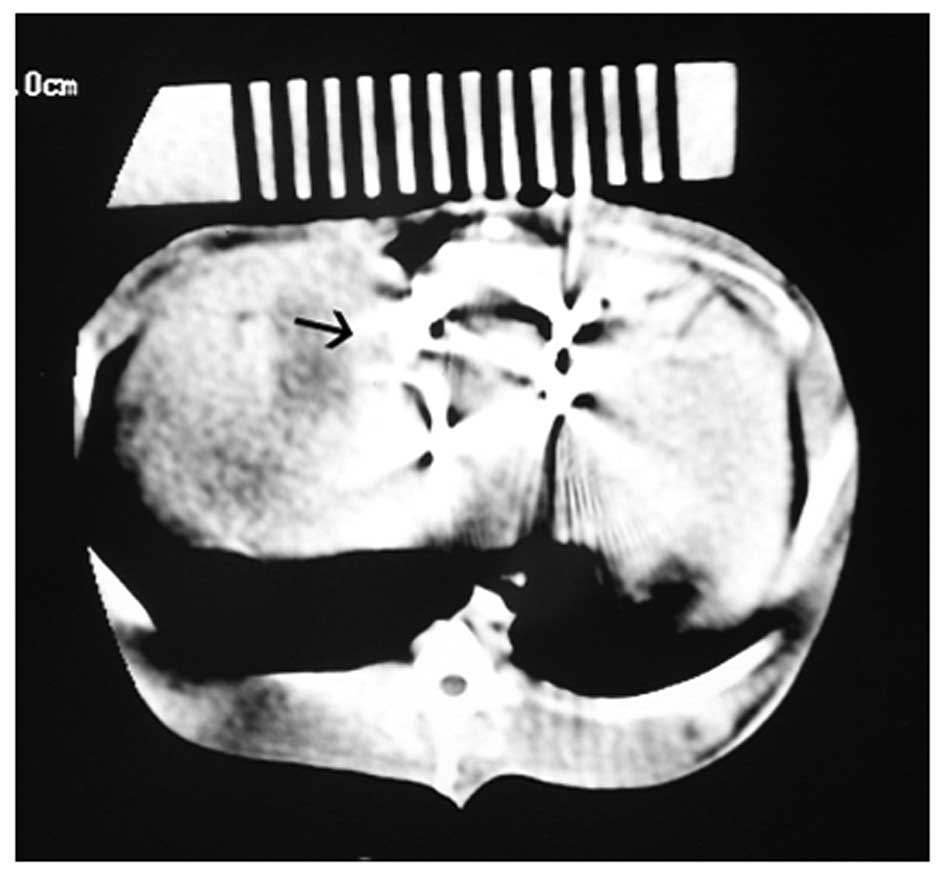
CT plain scanning images demonstrated that the
125I seeds were implanted around the tumor during the
CT-guided 125I seed brachytherapy (0.5 mCi, range dose,
60 Gy; Fig. 4).
Histopathological findings
Gross specimens show that the tumors treated with
combined PMCT following 125I seed brachytherapy (group
C) were the smallest tumors of the four groups, although tumor size
is not an assessment criterion in this experiment, with completely
necrosis and no liver metastasis compared with 125I seed
brachytherapy (group B) and PMCT (group A). The tumors in the
control group (group D) were the largest in size and liver
metastasis was also present in this group (Fig. 5).
PMCT followed by 125I seed brachytherapy
(group C) resulted in complete necrosis in 19 of 20 (95%) tumors,
compared with 6 of 20 (30%) tumors treated with PMCT alone (group
A) and 0 of 20 (0%) tumors treated with 125I seed
brachytherapy alone (group B; P<0.01).
No liver metastasis occurred in the rabbits which
received the combined treatment (group C), whereas metastasis was
observed in 7 of 20 (35%) rabbits treated with PMCT alone (group
A), 2 of 20 (10%) rabbits treated with 125I seed
brachytherapy alone (group B) and all 20 rabbits (100%) in the
control group (group D).
In the microwave treatment group (group A), there
were two cases (2/20) in which the treatment area was adjacent to
the gallbladder and grade I mild injury was present. No severe
necrotic changes were observed in the adjacent tissues of the
therapeutic region on the cutis, stomach, bowel, gallbladder or
diaphragm of the rabbits in groups A, B and C.
The results showed that the tumor necrosis rate of
group C was significantly higher than those of groups A and B,
which indicates that combination therapy increases the tumor
necrosis rate. PMCT followed by 125I seed brachytherapy
is a safe, effective and minimally invasive therapeutic option for
liver cancer.
Discussion
PMCT followed by 125I seed brachytherapy
is a safe, minimally invasive and promising therapeutic option for
liver cancer due to the eradication of local tumor cells.
PMCT appears to be a useful and safe treatment,
particularly for cases of superficial hepatocellular carcinoma.
Microwave irradiation creates an ablation area around the needle in
a columnar or round shape, depending on the type of needle used and
the generating power. However, similar to other thermal methods,
such as radio frequency (RF) ablation for local tumor treatment
(15,16), tumor size and the presence of large
(≥3 mm) abutting vessels significantly affects the outcome of the
procedure. Another limitation of microwave ablation is the lesion
location. The treatment of lesions located along the liver surface,
particularly in proximity to the gastrointestinal tract, or
adjacent to the porta hepatis or the gallbladder, are at risk of
major complications (17).
CT-guided permanent brachytherapy was initially used
for treating liver malignancies (18,19),
recurrent rectal carcinoma and spinal metastatic and primary
paraspinal malignancies (20).
This novel technique ensures protracted cell killing over a period
of several months via targeted delivery of high-dose radiation. The
advantages of this technique are as follows; i) it is minimally
invasive, ii) dose distribution may be accurately predicted, iii)
continuous irradiation increases the likelihood of damaging
malignant cells in a vulnerable phase of the cell cycle and iv) the
incidence rate of acute adverse effects is low. However,
radiotherapy does not completely kill hypoxic tumor cells, but does
have cytoreductive effects.
In the present study, complete tumor necrosis was
achieved in 19 of 20 (95%) tumors and no intraheptic metastasis was
present in the rabbits in the combined PMCT and 125I
seed brachytherapy group. The results show that combined PMCT
followed by 125I seed brachytherapy to the liver cancer
has certain advantages. Although PMCT is a viable method for the
treatment of cancer, in the majority of cases, the tumor blood flow
removes part of the heat from the microwaves. Since the shape of
the microwave ablation region is not round, residual tumor tissue
is often observed at the tumor margin. To eliminate these
unfavorable treatment factors, combined PMCT should be followed by
125I seed brachytherapy, which may provide improved
local control of the tumor. To the best of our knowledge, combined
PMCT followed by 125I seed brachytherapy to the VX2
rabbit liver cancer has never previously been documented.
The VX2 tumor is a squamous carcinoma from a
virus-induced papilloma. However, the behavior of VX2 tumors is
similar to that of the primary tumor. The biological
characteristics of VX2 tumors and liver cancer cells are similar,
so VX2 tumors are widely used to study human hepatocellular
carcinoma.
Although PMCT and 125I seed brachytherapy
have a low likelihood of causing complications (19–22),
care should be taken to avoid possible complications following
125I seed brachytherapy and microwave combination
therapy. We studied irreversible injury changes of the tissues
adjacent to the therapeutic region, not reversible injury.
Therefore, tissue congestion, edema and reversible tissue damage,
such as the two cases of gallbladder grade I mild injury, may be
ignored in this experiment.
The main purpose of this experiment was to verify if
the actions of PMCT followed by 125I seed brachytherapy
for VX2 hepatic tumors in a rabbit model are synergistic.
Therefore, in this experiment, the elevated intrahepatic metastasis
rate of the microwave group and the reduced complete tumor necrosis
rate of the 125I seed brachytherapy group do not have
universal significance. The complete tumor necrosis rate of PMCT
followed by 125I seed brachytherapy is up to 95%,
however, the limitations of the animal models require
consideration. For clinical applications, further study
required.
For example, how to combine the thermal field of the
PMCT with the radiation field of 125I Seeds effectively
in some liver cancer patients with Child-Pugh liver function of
score C (23). It is beneficial to
combine ablation with radiation for the treatment of tumors
(24). PMCT followed by
125I seed brachytherapy is a safe, effective and
promising minimally invasive therapeutic option for liver
cancer.
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lencioni R: Management of hepatocellular
carcinoma with transarterial chemoembolization in the era of
systemic targeted therapy. Crit Rev Oncol Hematol. 83:216–224.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ferenci P, Fried M, Labrecque D, et al:
Hepatocellular carcinoma (HCC): a global perspective. J Clin
Gastroenterol. 44:239–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
5.
|
Cormier JN, Thomas KT, Chari RS and Pinson
CW: Management of hepatocellular carcinoma. J Gastrointest Surg.
10:761–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Marelli L, Stigliano R, Triantos C, et al:
Treatment outcomes for hepatocellular carcinoma using
chemoembolization in combination with other therapies. Cancer Treat
Rev. 32:594–606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Takayasu K: Chemoembolization for
unresectable hepatocellular carcinoma in Japan. Oncology. 78(Suppl
1): 135–141. 2010. View Article : Google Scholar
|
|
8.
|
Liapi E and Geschwind JF:
Chemoembolization for primary and metastatic liver cancer. Cancer
J. 16:156–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Itoh S, Ikeda Y, Kawanaka H, et al:
Efficacy of surgical microwave therapy in patients with
unresectable hepatocellular carcinoma. Ann Surg Oncol.
18:3650–3656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Carrafiello G, Laganà D, Mangini M, et al:
Microwave tumors ablation: principles, clinical applications and
review of preliminary experiences. Int J Surg. 6(Suppl 1): S65–S69.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liang P, Dong BW, Yu XL, et al:
Ultrasound-guided percutaneous microwave coagulation therapy for
hepatic metastases. Zhonghua Zhong Liu Za Zhi. 26:301–304. 2004.(In
Chinese).
|
|
12.
|
Lee W, Daly BD, DiPetrillo TA, et al:
Limited resection for non-small cell lung cancer: observed local
control with implantation of I-125 brachytherapy seeds. Ann Thorac
Surg. 75:237–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Trombetta MG, Colonias A, Makishi D, et
al: Tolerance of the aorta using intraoperative iodine-125
interstitial brachytherapy in cancer of the lung. Brachytherapy.
7:50–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL
and Cheng WY: Bystander effects induced by continuous low-dose-rate
125I seeds potentiate the killing action of irradiation
on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys.
72:1560–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Lencioni R and Crocetti L: Radiofrequency
ablation of liver cancer. Tech Vasc Interv Radiol. 10:38–46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lencioni R, Cioni D, Crocetti L, Franchini
C, Pina CD, Lera J and Bartolozzi C: Early-stage hepatocellular
carcinoma in patients with cirrhosis: long-term results of
percutaneous image-guided radiofrequency ablation. Radiology.
234:961–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shibata T, Iimuro Y, Yamamoto Y, et al:
Small hepatocellular carcinoma: comparison of radio-frequency
ablation and percutaneous microwave coagulation therapy. Radiology.
223:331–337. 2002. View Article : Google Scholar
|
|
18.
|
Ricke J, Wust P, Stohlmann A, et al:
CT-guided brachytherapy of liver malignancies alone or in
combination with thermal ablation: phase I-II results of a novel
technique. Int J Radiat Oncol Biol Phys. 58:1496–1505. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang JJ, Yuan HS, Li JN, Jiang WJ, Jiang
YL and Tian SQ: Interstitial permanent implantation of
125I seeds as salvage therapy for re-recurrent rectal
carcinoma. Int J Colorectal Dis. 24:391–399. 2009.PubMed/NCBI
|
|
20.
|
Wang J, Yuan H, Ma Q, et al: Interstitial
125I seeds implantation to treat spinal metastatic and primary
paraspinal malignancies. Med Oncol. 27:319–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Livraghi T, Solbiati L, Meloni MF, Gazelle
GS, Halpern EF and Goldberg SN: Treatment of focal liver tumors
with percutaneous radio-frequency ablation: complications
encountered in a multi-center study. Radiology. 226:441–451. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bowles BJ, Machi J, Limm WM, et al: Safety
and efficacy of radiofrequency thermal ablation in advanced liver
tumors. Arch Surg. 136:864–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Durand F and Valla D: Assessment of the
prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol.
42(Suppl1): S100–S107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Dupuy DE and Goldberg SN: Image-guided
radiofrequency tumor ablation: challenges and opportunities-part
II. J Vasc Interv Radiol. 12:1135–1148. 2001. View Article : Google Scholar : PubMed/NCBI
|