Introduction
Resistant starch (RS) is defined as the sum of
starch and starch-degradation products that are not absorbed in the
small intestine due to their resistance to digestive enzymes
(1). Based on structure and
physicochemical properties, RS may be subdivided into four
categories (2): RS1 is a
physically inaccessible starch in partially milled grains and
seeds; RS2 is a resistant granular starch, such as those in raw
potato and banana; RS3 is a retrograded starch, formed in processed
foods on cooling (cooled, cooked potato, bread and cornflakes); and
RS4 is chemically modified starch. Numerous studies in rats have
shown that RS escapes digestion in the small intestine and is
slowly fermented in the large intestine to produce short chain
fatty acids (SCFAs), lactate and gases
(CO2,CH4 and H2) (3–6). RS
is reported to have various physiological effects, including weight
control, prevention of diabetes, lipid level reduction, promotion
of inorganic salt absorption, altering microbial populations and
increasing SCFA production in the large intestine (7).
Constipation is defined medically as fewer than
three stools per week and severe constipation as less than one
stool per week. It occurs when the colon absorbs too much water
(8). In the current study,
activated carbon was orally administered to mice. Activated carbon
attaches to gastrointestinal (GI) mucosal surfaces and reduces the
drainage of the GI tract, causes GI fluid reduction and slows down
GI movement, resulting in weakness of the spleen and stomach, to
produce a model of constipation.
Previous studies have used the constipation model
induced by activated carbon to demonstrate the effects of drugs for
constipation treatment (9,10). One study demonstrated that a
megadose of activated carbon results in digestive tract obstruction
(11). Therefore, in the present
study, we examined the functional effects of RS in the alimentary
tract using an activated carbon-induced constipation mouse model.
We examined GI transit, time to the first black stool defecation,
histopathological sections and serum assay of motilin (MTL),
gastrin (Gas), endothelin (ET), somatostatin (SS),
acetylcholinesterase (AChE), substance P (SP) and vasoactive
intestinal peptide (VIP) levels. Bisacodyl was used as a positive
control. Bisacodyl is a laxative drug that acts as a stimulant of
intestinal peristalsis and acts directly on the colon to produce a
bowel movement. It is typically prescribed for the relief of
constipation and for the management of neurogenic bowel
dysfunction, as well as for bowel preparation prior to medical
examinations (12–14).
Materials and methods
RS preparations
Hylon VII (containing 53% RS2), Novelose 330
(containing 41% RS3) and Novelose 2480 (containing 80% RS4) were
supplied by the National Starch and Chemical Co. (Bridgewater, NJ,
USA). Casein was obtained from Zhengzhou TianTong Food Ingredients
Co., Ltd. (Zhengzhou, China). Soybean oil was purchased from
Chongqing Grain Group Co. Ltd. (Chongqing, China). Acetic acid and
crotonic acid (standard, purity >99.7%) were obtained from
Johnson Matthey (London, UK). Propionic acid, butyric acid and
isobutyric acid (standard, purity >99%) were purchased from
Tokyo Chemical Industry Co., Ltd. (Shanghai, China). All other
chemicals were of reagent grade.
Animals
Seven-week-old female ICR mice (n=120) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The mice were maintained in a
temperature- and humidity-controlled (temperature 25±2°C, relative
humidity 50±5%) facility with a 12-h light/dark cycle and free
access to a standard rat chow diet and water.
During the experiment, four groups of rats were fed
the RS-free basal diet (control group), or a diet containing 15%
RS2, 15% RS3 or 15% RS4, respectively. All rats were provided with
food and water ad libitum and were maintained on each diet
for a 4-week period. These experiments followed a protocol approved
by the Animal Ethics Committee of Chongqing Medical University
(Chongqing, China).
Induction of constipation in mice
To investigate the preventive effects of RS against
activated carbon-induced constipation, the animals were divided
into 6 groups with 20 mice in each. The experimental design was as
follows: the normal and control groups were fed normal diet for 9
days and the treatment groups were orally fed the RS-free basal
diet containing 15% RS2, 15% RS3 or 15% RS4 in their ration, or
were fed the RS-free basal diet and treated with a 100 mg/kg dose
of bisacodyl dissolved in water. The control and treatment groups
received an oral administration of activated carbon (0.2 ml 10%
activated carbon, w/w; activated carbon dissolved in 10% arabic
gum) at 6 p.m. from the sixth to ninth day to induce
constipation.
GI transit and defecation time
Mice were fasted for sixteen hours from the ninth
day at 6 p.m.; however, they were not deprived of water. After 16
h, the mice in the control and treatment groups received an oral
administration of 10% activated carbon while the mice in the normal
group eceived an oral administration of 10% arabic gum. Thirty
minutes later, mice were sacrificed by cervical dislocation under
anesthesia with diethyl ether. Ten mice in each group were
dissected and the small intestine from the pylorus to the blind
intestine was carefully removed. The GI transit of each mouse was
calculated as the percentage of the distance traveled by the
activated carbon meal relative to the total length of the small
intestine. The following equation was used to calculate GI transit:
GI transit (%) = distance traveled by the activated carbon/total
length of the small intestine ×100. The remaining 10 mice of each
group were used to measure the time to the first black stool
defecation following the oral administration of 10% activated
carbon.
Histological examination of intestinal
tissue
For histological investigations, intestinal tissues
were fixed in 10% (v/v) buffered formalin for 24 h, dehydrated in
ethanol and embedded in paraffin. Then, 4-μm thick sections
were prepared and stained with hematoxylin and eosin (H&E) for
observation under an Olympus BX41 microscope (Olympus, Tokyo,
Japan).
MTL, Gas, ET, SS, AChE, SP and VIP levels
in serum
MTL, Gas, ET, SS, AChE, SP and VIP levels in the
serum were determined using radioimmunoassay kits (Beijing Puer
Weiye Biotechnology Co., Ltd., Beijing, China). The serum of mice
were collected from heart following surgery.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Differences between the mean values for individual groups
were assessed with one-way analysis of variance (ANOVA) with
Duncan’s multiple range test. P<0.05 was considered to indicate
a statistically significant difference. SAS version 9.1 (SAS
Institute Inc., Cary, NC, USA) was used for statistical
analyses.
Results
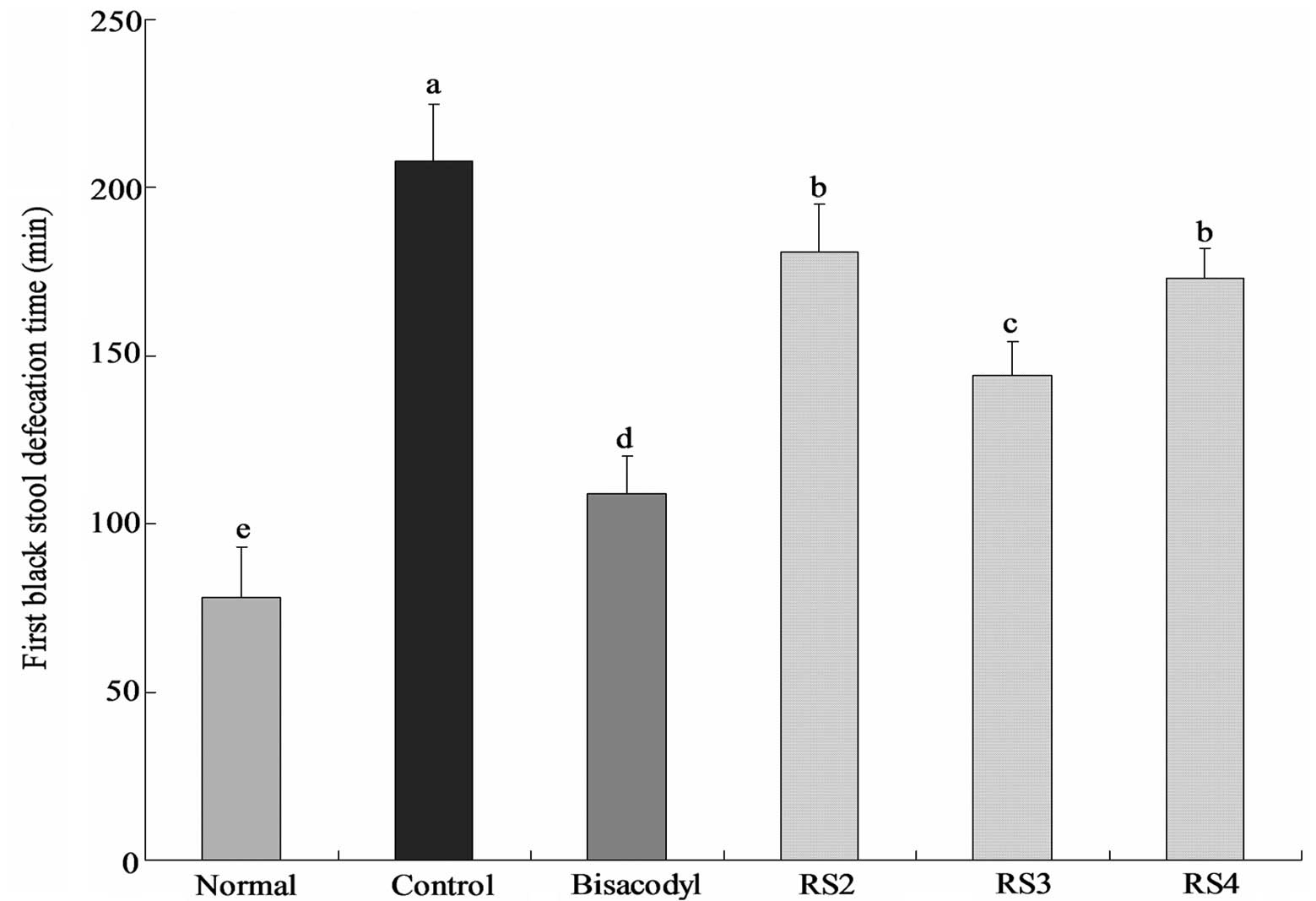
Time to the first black stool
defecation
The time to the first black stool defecation for
each group of mice following the administration of activated
carbon, which demonstrates the constipation-inhibiting effect of
different treatments, is shown in Fig.
1. The defecation time was the shortest (78±15 min) in the
normal group and the longest (208±17 mins) in the control group;
the defecation time in the bisacodyl group was 109±11 min, higher
than that of the normal group. The times to the first black stool
defecation for the RS2, RS3 and RS4 group mice were 181±14, 144±10
and 173±9 min, respectively. According to the defecation time, RS3
has the strongest effect on inhibiting constipation.
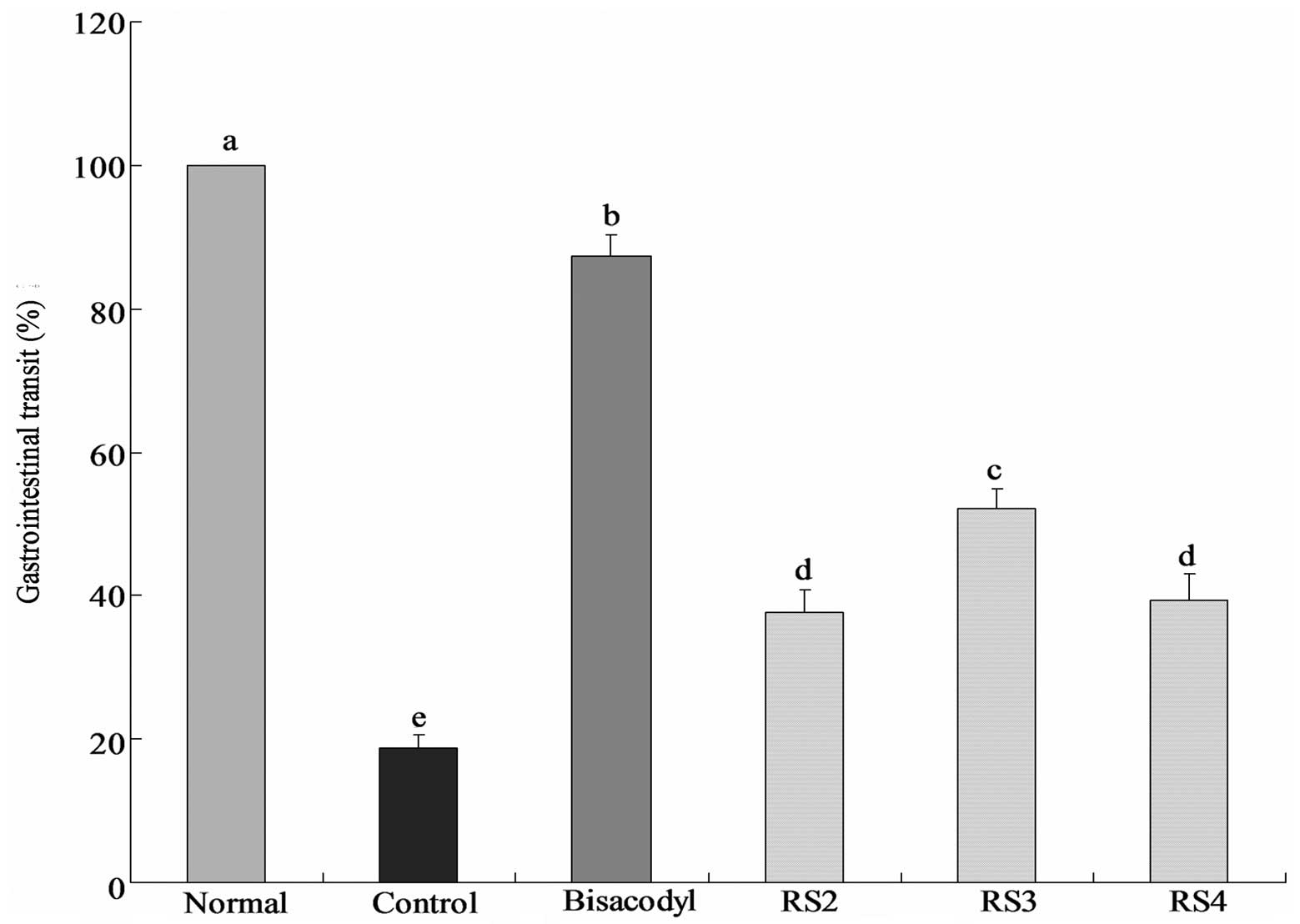
GI transit
The constipation inhibiting effects of the
treatments were determined by GI transit in mice following the
administration of activated carbon (0.2 ml/mouse, 10% activated
carbon). In the bisacodyl-treated group, the mean GI transit was
87.3±3.1%, which was higher than that of the control group
(18.8±1.8%; Fig. 2). The GI
transits of the RS2, RS3 and RS4 groups were 37.7±3.1, 52.1±2.6 and
39.3±3.7%, respectively. RS3 increases the GI transit compared with
the control, reduces constipation and increases the functional
effect.
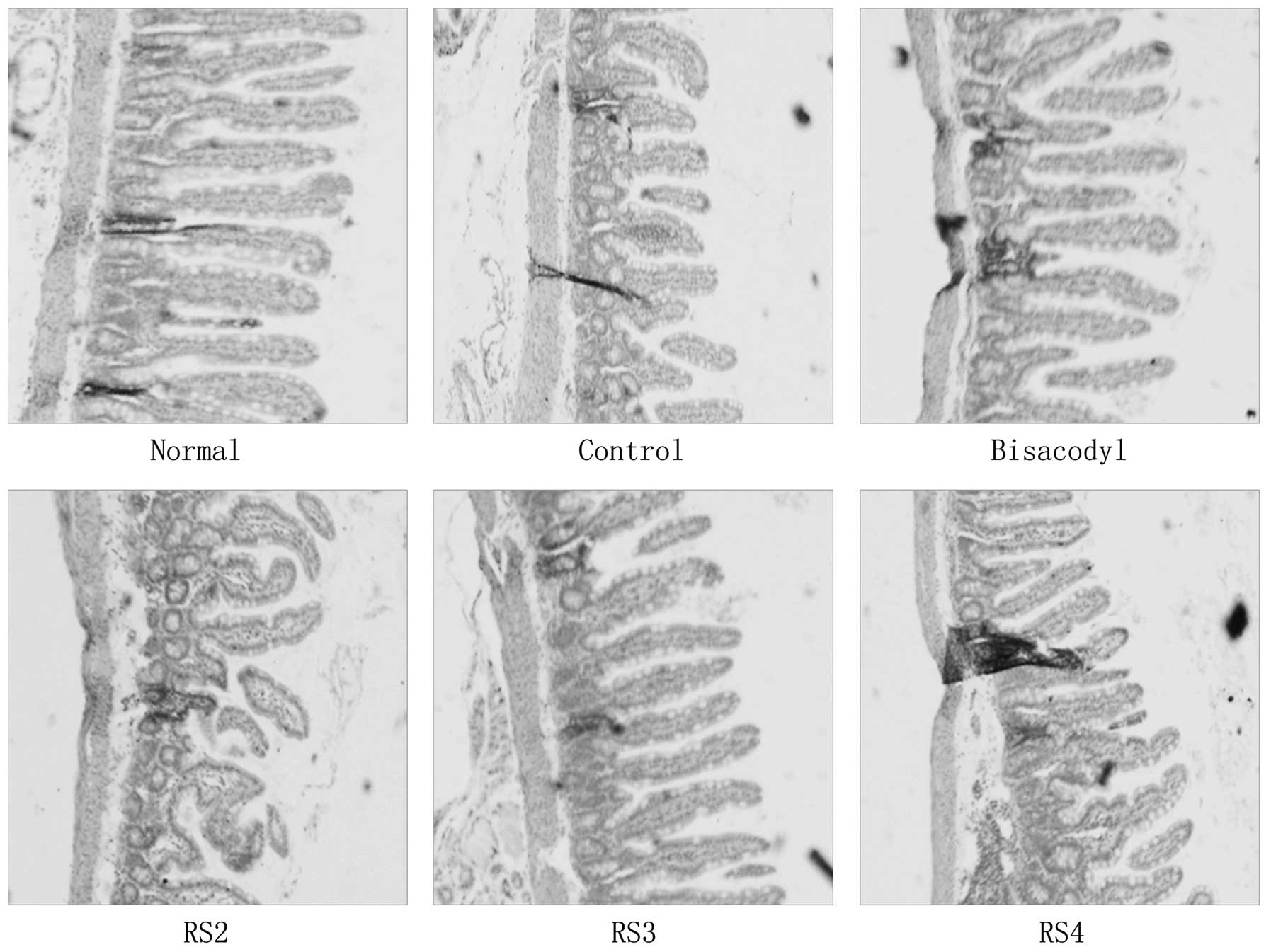
Histopathology of hepatic damage
H&E staining revealed activated carbon-induced
histopathological changes in the intestine; the small intestinal
villi became shorter (Fig. 3). The
small intestinal villi of the RS3-treated mice were shorter than
those of the normal and bisacodyl-treated mice; however, they were
longer than those of the RS2- and RS4-treated mice. The small
intestinal villi of all RS mice were longer than those of the
control mice.
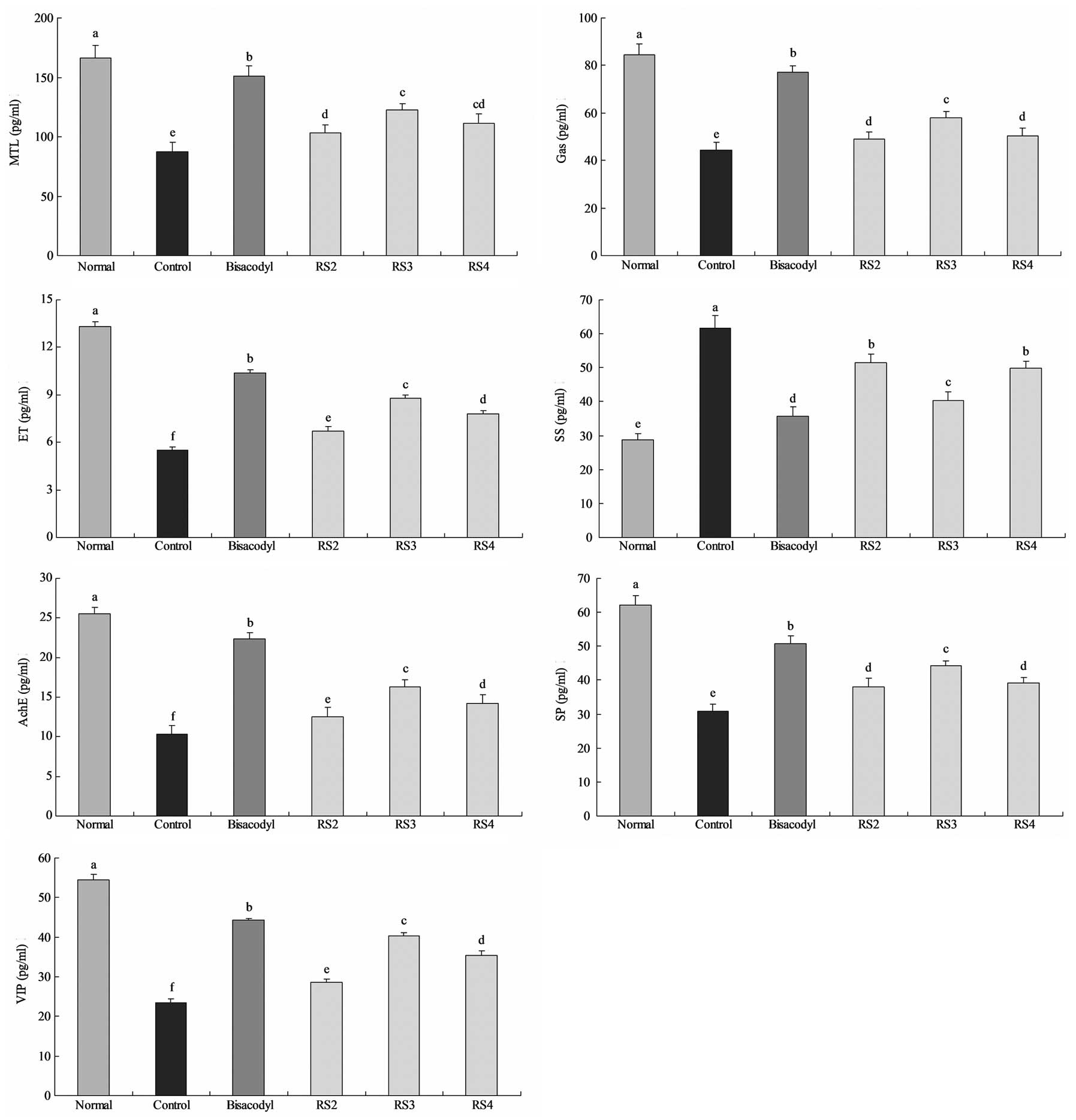
MTL, Gas, ET, SS, AChE, SP and VIP levels
in serum
The mean MTL level of normal mice was 166.3±10.2
pg/ml; whereas the MTL level in the activated carbon-induced
constipation control mice was reduced to 87.5±7.8 pg/ml (Fig. 4). The level of MTL in mice treated
with bisacodyl was 151.3±8.2 pg/ml. The levels of MTL in mice
treated with RS2, RS3 and RS4 were 103.4±6.7, 122.2±5.6 and
111.3±7.7 pg/ml, respectively. The Gas levels of the normal,
control, 100 mg/kg bisacodyl-, RS2-, RS3- and RS4-treated mice were
84.6±4.4, 44.5±3.1, 77.2±2.6, 48.9±3.1, 57.8±2.7 and 50.2±3.3
pg/ml, respectively. The levels of ET in the RS2, RS3 and RS4
groups were 6.7±0.3, 8.8±0.2 and 7.8±0.2 pg/ml, respectively, and
the levels in the normal, control and bisacodyl-treated mice were
13.3±0.3, 5.5±0.2 and 10.4±0.2 pg/ml, respectively. The SS levels
of normal and control mice were 28.7±2.0 and 61.6±3.8 pg/ml,
respectively, and the SS levels in the treated mice were 35.8±2.6
(100 mg/kg bisacodyl), 51.5±2.5 (RS2), 40.3±2.5 (RS3) and 49.9±2.1
(RS4) pg/ml. The AChE, SP and VIP levels of normal mice were
25.5±0.8, 62.2±2.8 and 54.4±1.4 pg/ml, respectively, and were
22.4±0.7, 50.8±2.2 and 44.3±0.5 pg/ml, respectively, for the
bisacodyl-treated mice. The AChE, SP and VIP levels in the RS2
(12.5±1.2, 38.1±2.4 and 28.7±0.8 pg/ml), RS3 (16.3±0.9, 44.2±1.5
and 40.3±0.8 pg/ml) and RS4 (14.2±1.1, 39.2±1.9 and 35.3±1.2
pg/ml)-treated mice were higher than those of the control mice
(10.3±1.1, 30.8±2.1 and 23.4±1.1 pg/ml, respectively).
Discussion
Anorexia is an important symptom in constipation
(15). The observation of dietary
and water intake in mice may determine the level of constipation
and the inhibitory effects of different substances on constipation.
The definition of constipation includes infrequent bowel movements
and difficulty during defecation (16,17).
Constipation most commonly occurs when the stool that forms after
food is digested moves too slowly (slow transit) as it passes
through the digestive tract. Dehydration, changes in diet and
activity, and certain drugs are frequently to blame for the slow
transit of stools. When stools move slowly, too much water is
absorbed from the stool and it becomes hard and dry (18). Defecation status, dietary intake,
water consumption, stool defecation time and GI transit are
important standards when investigating constipation.
Histopathology is an important clinical standard in
the diagnosis of intestinal function (19). The intestinal villi together
increase the intestinal absorptive surface area, providing
exceptionally efficient absorption of nutrients in the lumen
(20). Intestinal villi also help
the intestines to move food along the digestive pathway (21). From our results, we determined that
RS3 reduced the damage to intestinal villi in mice treated with
activated carbon.
The serum levels of MTL, Gas, ET, AChE, SP and VIP
in patients with constipation are lower than those in healthy
individuals while the SS levels are higher (22–24).
The main function of MTL is to increase the migrating myoelectric
complex component of GI motility and stimulate the production of
pepsin. It is one of the intestinal hormones responsible for the
proper filling and emptying of the GI system in response to intake
of food, as well as hunger stimuli and responses (25). Gas is a polypeptide hormone
secreted by certain cells of the pyloric glands, which strongly
stimulates the secretion of gastric acid and pepsin, and weakly
stimulates the secretion of pancreatic enzymes and gallbladder
contraction (22). Gas produces
effects throughout the GI tract, including promoting GI secretion,
increasing GI movement and promoting pyloric sphincter relaxation.
ET plays an important role in the stability of vascular tension and
maintains the basic cardiovascular system. Constipation not only
causes disease, including intestinal obstruction and other serious
diseases, but it also induces or aggravates cardiocerebrovascular
diseases in the elderly (26). An
SS analog, octreotide, has been reported to stimulate intestinal
motor complexes and this agent has been used to treat
sclerodermatous pseudo-obstruction (27). Stools are formed from the
non-digestible components of food after water is either absorbed or
secreted in the large intestine. Mucous is also produced in the
large intestine to provide viscosity. Thin segments of muscle line
the intestinal tract and contract and relax in concert to propel
the stool forward. Muscle contraction and mucous secretion are
regulated by acetylcholine (28).
Patients with slow-transit constipation have abnormal
neurotransmitters in the muscular layer of their intestinal walls.
These abnormalities include a deficiency of a peptide known as SP,
which is thought to contribute to peristalsis (29). Disturbances in the normal neural
content of VIP in the bowel wall in idiopathic constipation and
diverticular disease may initiate or contribute to the functional
changes observed in these disorders (30).
In our previous study (unpublished data), RS3 was
demonstrated to produce more SCFAs compared with RS2 and RS4. RS3
demonstrated preventive effects in constipation due to its ability
to increase SCFA levels. SCFAs increase the levels of probiotics in
the stomach and intestine. SCFA and probiotics have a number of
physiological and biological functions, including enhancement of
intestinal immunity (31).
The aim of the current study was to investigate
whether RS has a preventive effect against activated carbon-induced
constipation in mice. In the mice fed with RS3, the results
demonstrated that the time to the first black stool defecation was
only a little longer than that in mice treated with bisacodyl. The
GI transit was longer than that in the control mice and was similar
to that in the bisacodyl group. Various serum levels, including
MTL, Gas, ET, AChE, SP and VIP in the RS-treated mice were higher
compared with those in the control mice and the SS levels
demonstrated the opposite tendency. These results suggest that RS
has a significant preventive effect on activated carbon-induced
constipation in mice and RS3 demonstrated the most potent
activity.
References
|
1.
|
No authors listed. Carbohydrates in human
nutrition. Report of a Joint FAO/WHO Expert Consultation. FAO Food
Nutr Pap. 66:1–140. 1998.PubMed/NCBI
|
|
2.
|
Englyst HN, Kingman SM and Cummings JH:
Classification and measurement of nutritionally important starch
fractions. Eur J Clin Nutr. 46(Suppl 2): S33–S50. 1992.PubMed/NCBI
|
|
3.
|
Gordon DT, Topp K, Shi YC, Zallie J and
Jeffcoat R: Resistant starch: physical and physiological
properties. New Technologies For Healthy Foods &
Nutraceuticals. Yalpani M: ATL Press Inc; Shrewsbury, MA: pp.
157–178. 1997
|
|
4.
|
McIntyre A, Albert V, Folino M, Muir J,
Gibson PR and Young GP: Resistant starch from corn behaves like
soluble and not insoluble fibre in a rat model of large bowel
cancer. Proc Nutr Soc Aust. 18:611994.
|
|
5.
|
Younes C, Levrat MA, Demigné C and Rémésy
C: Resistant starch is more effective than cholestyramine as a
lipid-lowering agent in the rat. Lipids. 30:847–853. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Macfarlane GT and Englyst HN: Starch
utilization by the human large intestinal microflora. J Appl
Microbiol. 60:195–201. 1986.PubMed/NCBI
|
|
7.
|
Baghurst PA, Baghurst KI and Record SJ:
Dietary fiber, non-starch polysaccharides and resistant starch - a
review. Food Australia. 48:S1–S36. 1996.
|
|
8.
|
Ueki A and Otsuka M: Life style risks of
Parkinson’s disease: Association between decreased water intake and
constipation. J Neurol. 251(Suppl 7): S18–S23. 2004.
|
|
9.
|
Wexner SD, Beck DE, Baron TH, Fanelli RD,
Hyman N, Shen B and Wasco KE: A consensus document on bowel
preparation before colonoscopy: prepared by a task force from the
American Society of Colon and Rectal Surgeons (ASCRS), the American
Society for Gastrointestinal Endoscopy (ASGE), and the Society of
American Gastrointestinal and Endoscopic Surgeons (SAGES).
Gastrointest Endosc. 63:894–909. 2006.
|
|
10.
|
Farrugia G, Miller SM, Rich A, Liu X,
Maines MD, Rae JL and Szurszewski JH: Distribution of heme
oxygenase and effects of active carbon monoxide in canine jejunum.
Am J Physiol. 274:G350–G358. 1998.PubMed/NCBI
|
|
11.
|
Farrugia G, Lei S, Lin X, Miller SM, Nath
KA, Ferris CD, Levitt M and Szurszewski JH: A major role for carbon
monoxide as an endogenous hyperpolarizing factor in the
gastrointestinal tract. Proc Natl Acad Sci USA. 100:8567–8570.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Miller SM, Reed D, Sarr MG, Farrugia G and
Szurszewski JH: Haem oxygenase in enteric nervous system of human
stomach and jejunum and co-localization with nitric oxide synthase.
Neurogastroenterol Motil. 13:121–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xue L, Farrugia G, Miller SM, Ferris CD,
Snyder SH and Szurszewski JH: Carbon monoxide and nitric oxide as
coneurotransmitters in the enteric nervous system: evidence from
genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA.
97:1851–1855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Battish R, Cao GY, Lynn RB, Chakder S and
Rattan S: Heme oxygenase-2 distribution in anorectum:
colocalization with neuronal nitric oxide synthase. Am J Physiol
Gastrointest Liver Physiol. 278:G148–G155. 2000.PubMed/NCBI
|
|
15.
|
Dolk A, Brodén G, Holmström B, Johansson C
and Schultzberg M: Slow transit chronic constipation (Arbuthnot
Lane’s disease). An immunohistochemical study of
neuropeptide-containing nerves in resected specimens from the large
bowel. Int J Colorectal Dis. 5:181–187. 1990.
|
|
16.
|
Walia R, Mahajan L and Steffen R: Recent
advances in chronic constipation. Curr Opin Pediatr. 21:661–666.
2009. View Article : Google Scholar
|
|
17.
|
Emmanuel AV, Tack J, Quigley EM and Talley
NJ: Pharmacological management of constipation. Neurogastroenterol
Motil. 21(Suppl 2): S41–S54. 2009. View Article : Google Scholar
|
|
18.
|
Lubowski DZ, Chen FC, Kennedy ML and King
DW: Results of colectomy for severe slow transit constipation. Dis
Colon Rectum. 39:23–29. 1996. View Article : Google Scholar
|
|
19.
|
Ludvigsson JF, Montgomery SM, Ekbom A,
Brandt L and Granath F: Small-intestinal histopathology and
mortality risk in celiac disease. JAMA. 302:1171–1178. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhao Y, Zhang B, Qin G, Wang T, Wang J and
Han R: Distribution of glycinin in the gastrointestinal tissue of
pigs at different growth stages. Food Agric Immunol. 1–8. 2012.
|
|
21.
|
Palay SL and Karlin LJ: An electron
microscopic study of the intestinal villus II. The pathway of fat
absorption. J Biophys Biochem Cytol. 5:373–384. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sjölund K, Ekman R, Akre F and Lindner P:
Motilin in chronic idiopathic constipation. Scand J Gastroenterol.
21:914–918. 1986.
|
|
23.
|
el-Salhy M and Norrgård O: Colonic
neuroendocrine peptide levels in patients with chronic idiopathic
slow transit constipation. Ups J Med Sci. 103:223–230. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Silkoff P, Karmeli F, Goldin E, Ewenson A,
Gilon C, Chorev M, Laufer R, Selinger Z and Rachmilewitz D: Effect
of substance P on rat gastrointestinal transit. Digest Dis Sci.
33:74–77. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Feighner SD, Tan CP, McKee KK, Palyha OC,
Hreniuk DL, Pong SS, Austin CP, Figueroa D, MacNeil D, Cascieri MA,
Nargund R, Bakshi R, Abramovitz M, Stocco R, Kargman S, O’Neill G,
Van Der Ploeg LH, Evans J, Patchett AA, Smith RG and Howard AD:
Receptor for motilin identified in the human gastrointestinal
system. Science. 284:2184–2188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Preston DM, Adrian TE, Christofides ND,
Lennard-Jones JE and Bloom SR: Positive correlation between
symptoms and circulating motilin, pancreatic polypeptide and
gastrin concentrations in functional bowel disorders. Gut.
26:1059–1064. 1985. View Article : Google Scholar
|
|
27.
|
Soudah HC, Hasler WL and Owyang C: Effect
of octreotide on intestinal motility and bacterial overgrowth in
scleroderma. N Engl J Med. 325:1461–1467. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tzavella K, Riepl RL, Klauser AG,
Voderholzer WA, Schindlbeck NE and Müller-Lissner SA: Decreased
substance P levels in rectal biopsies from patients with slow
transit constipation. Eur J Gastroen Hepat. 8:1207–1211. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Milner P, Crowe R, Kamm MA, Lennard-Jones
JE and Burnstock G: Vasoactive intestinal polypeptide levels in
sigmoid colon in idiopathic constipation and diverticular disease.
Gastroenterology. 99:666–675. 1990.PubMed/NCBI
|
|
31.
|
Roy CC, Kien CL, Bouthillier L and Levy E:
Short-chain fatty acids: ready for prime time? Nutr Clin Pract.
21:351–366. 2006. View Article : Google Scholar : PubMed/NCBI
|