Introduction
The future use of titanium alloys as implant
materials is predicted to increase due to their good
biocompatibility, mechanical properties and chemical stability
(1). Titanium alloys are also
lightweight, possess high toughness and tensile ductility, a high
corrosion resistance and the ability to withstand extreme
temperatures (2). Titanium alloys
are roughly classified into four categories depending on their
purity and crystal structures: commercially pure titanium (CP-Ti);
α alloy (body-centered cubic structure, BCC); β alloy (hexagonal
close-packed structure, HCP) and α + β dual-phase alloy (3). Compared with α and α + β types,
β-type titanium alloy offers a more advantageous material for
dental implants due to its improved mechanical properties, higher
corrosion resistance and a lower modulus of elasticity (4). Ti-6Al-4V, an α + β type titanium
alloy, is the most widely used titanium alloy for medical implants,
including orthopedic, dental and cardiovascular implants (5). However, the elastic modulus of
Ti-6Al-4V alloy is 4–10 times that of human bone, and an elastic
modulus mismatch between bone and implant material has often been
cited as a cause of implant failure (6). Furthermore, the elements Al and V of
Ti-6Al-4V alloy are capable of being released into tissue cells by
passive film dissolution, which may induce Alzheimer’s disease,
neuropathy, osteomalacia and allergic reactions. Therefore, novel
titanium alloys containing non-toxic and non-allergic elements,
including Nb, Ta, Zr, Mo and Sn, have been designed and constructed
for biomedical applications (7).
The novel β-type titanium alloy Ti-24Nb-4Zr-8Sn (Ti2448) has
attracted considerable attention due to its high strength, high
fatigue resistance and a low elastic modulus (8). An in vivo study of Ti2448 in
New Zealand white rabbits demonstrated that the low elastic modulus
of Ti2448 leads to significant improvements in new bone formation
following a tibial shaft fracture compared with a Ti-6Al-4V alloy
(9). These results suggest that
intramedullary nails constructed of low modulus Ti2448 alloy
improve new bone formation in the marrow cavity during the initial
stages of bone healing.
Since titanium is biologically inert and does not
bond directly nor immediately to the bone following implantation,
electrochemical anodic oxidation has been applied to improve a
number of the surface characteristics of titanium, including
corrosion resistance, cell proliferation, cell adhesion and cell
viability (10). Anodic oxidation
is generally considered as a useful method for modifying the
surface structure of titanium in several ways, including corrosion
protection, aspect improvement and bonding of polymers (11). The anodic oxidation technique is
capable of forming a nanoporous titanium oxide film of controllable
pore size, good uniformity and conformability over large areas at
low cost (12). Titanium oxide
films with nanoporous structures are desirable for these
applications due to their large surface areas and high reactivity
(13). It has also been reported
that the interactions between osteoblasts and titanium oxide films
involve chemical reactions (14).
Greater surface roughness and surface energy, more surface hydroxyl
groups and the presence of fibronectin or vitronectin are important
elements for the adhesion, spreading and proliferation of
osteoblasts on titanium surfaces (14,15).
However, the majority of studies have focused on osteoblast
adhesion using a sol-gel derived hydroxyapatite coating on
Ti-6Al-4V alloy (16–18). Few studies have been published with
regard to nanoporous titanium oxide films on Ti2448 alloy formed by
anodic oxidation with a typical pore size <100 nm. Therefore, we
hypothesize that nanostructured oxide films may enhance osteoblast
cell adhesion on Ti2448 alloys.
In the present study, the effects of the surface
characteristics of nanoporous titanium oxide films on the initial
adhesion of osteoblast-like MG-63 cells was investigated.
Nanoporous titanium oxide films with two different pore sizes (30
and 90 nm) were formed by anodization in NH4F solution
on Ti2448 alloy. Titanium surface roughness was examined using a
Surftest Formtracer and field emission scanning electron microscopy
(FESEM). Cells were evaluated for cell viability at different time
points using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. To investigate the regulatory mechanisms involved in the
focal adhesion of osteoblasts to Ti2448 alloys, the expression
levels of integrin β1 and paxillin mRNAs in response to the surface
structure of nanoporous titanium oxide films during the initial
osteoblast adhesion were quantified using real-time RT-PCR. The
present study may serve as a foundation for the development and
clinical application of Ti2448 alloy as a novel implant
material.
Materials and methods
Preparation of nanoporous titanium oxide
films on Ti2448 alloys
Ti2448 alloys were purchased from Shenyang National
Laboratory for Materials Science, Institute of Metal Research,
Chinese Academy of Sciences (Shenyang, China). Disks of 10-mm
diameter [used for scanning electron microscopy (SEM) analysis and
in the MTT assay] and 30-mm diameter (used for real-time RT-PCR
analysis) were cut from Ti2448 alloys using a diamond cut-off wheel
(Struers, Glasgow, UK). Prior to electrochemical anodization, the
sheets were ground using 2400-grit emery paper (Softflex, Bad
Säckingen, Germany) and polished with diamond paste (6 μm).
The samples were then degreased by sonication for 10 min in
acetone, isopropanol and methanol; following this, the samples were
rinsed with deionized water, dried in a nitrogen stream and stored
covered under UV light (19).
Anodic oxidation treatment of Ti2448 alloys was
performed at room temperature in a neutral electrolyte with 1 mol/l
(NH4)2SO4 and 0.15 mol/l
NH4F (pH 6.7) prepared from analytical grade chemicals
and deionized water. A direct current power supply was used to keep
the potential at a constant value. On several occasions, the
potential was swept from the open circuit potential to the desired
final potential with a sweep rate of 0.5 V/sec. A two-electrode
system, with stainless steel as a cathode and sample as an anode,
was used to form the nanoporous titanium oxide films under
non-stirred conditions. All samples were cleaned using deionized
water following the anodization process.
Anodic oxidation was performed at potentials of 10
and 25 V, and TiO2 nanotubes with diameters of 30 and 90
nm were formed in turn. The anodized samples were named as the 30-
and 90-nm groups, respectively. Samples without anodic oxidation
treatment were used as the control. All samples were cleaned
ultrasonically in acetone, alcohol and deionized water (each for 10
min), and sterilized by cobalt-60 irradiation prior to cell culture
(20,21).
Surface morphology
The anodized specimens were uniformly coated with a
layer of gold for electric conductivity and the morphology of the
nanoporous titanium oxide films was observed using FESEM (LEO model
Supra 35, Oberkochen, Germany) (22). Roughness was measured using a
Surftest Formtracer (Surftest SV-402; Mitutoyo Instruments, Tokyo,
Japan) on three samples of each group. For each sample, five
profiles were recorded (23).
ANOVA was performed to test the homogeneity of the anodization
process of each sample and the reproducibility of this process
between samples of each group. ANOVA was then applied to the usual
roughness parameters (Ra, arithmetic mean deviation of
the roughness profile; Rz, mean peak-to-valley height;
Ry, maximum height of the roughness).
Cell culture
Osteoblast-like MG-63 cells (CRL-1427; American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) (Invitrogen Life Technologies,
Carlsbad, CA, USA), supplemented with 2 mM L-glutamine (Invitrogen
Life Technologies), 10% fetal calf serum (FCS; Eurobio, Paris,
France) and 50 μg/ml gentamicin sulfate (Sigma, St. Louis,
MO, USA). Cultures were maintained at 37°C in a humidified
incubator in the presence of 5% CO2 and subcultures were
performed using a 0.01% trypsin solution in phosphate-buffered
saline (PBS) at pH 7.4. The cultures were observed daily under an
inverted microscope (magnification, ×400). Media were changed every
other day. The cells were routinely passaged until they reached 80%
confluency (14).
The titanium disks were placed in a 24-well plate
with a cell density of 5,000/cm2 (for SEM analysis and
in the MTT assay) or a 6-well plate with a cell density of
50,000/cm2 (for real-time RT-PCR analysis). The cells
were cultured for different incubation periods according to the
study design.
Cell morphology
Following 48 h incubation, the cultured disks were
rinsed three times with PBS and fixed with 2.5% glutaraldehyde
diluted in 0.1 M PBS for 2 h at 4°C, postfixed in 1% osmium
tetroxide, dehydrated in graded ethanol series, treated with
hexamethyldisilazane (Sigma) and then subjected to critical point
drying. A thin layer of palladium-gold was sputter coated onto the
samples prior to examination by FESEM (24).
MTT assay
MTT is transformed by mitochondrial dehydrogenases
into formazan, enabling mitochondrial activity and cell viability
to be assessed. Following incubation, the samples were washed with
PBS and transferred to a new 24-well plate. Then, 300 μl
culture medium and 300 μl MTT reagent (5 mg/ml in PBS;
Sigma) were added to each well. Following 4 h incubation in a 5%
CO2 incubator at 37°C, the medium was replaced with 500
μl dimethyl sulfoxide to dissolve formazan. The plate was
shaken for 10 min and then the solution in each well was
transferred to a 96-well ELISA plate. The optical density (OD)
value of the dissolved solute was measured using an ELISA reader
(Tecan, Grödig, Austria) at 570 nm (n=9 for each group). The common
OD value of the blank group was subtracted from the OD value in
each group at each time point (n=9). The blank group was treated
with the same methods and incubated for the same time as those in
the above three groups (25).
Real-time RT-PCR assay
Following 24 and 48 h incubation, the samples were
washed with PBS and transferred to a new 6-well plate. Total RNA
was extracted from the cultured cells using the TRIzol™
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. First strand cDNA was synthesized from
10 μg total RNA using the PrimeScript™ first
strand cDNA synthesis kit (Takara Bio, Inc., Osaka, Japan). PCR
primers were designed using Primer Express 2.0 software (Applied
Biosystems, Foster City, CA, USA). The primer sequences and
products of selected genes for real-time PCR are summarized in
Table I. Real-time PCR was used to
quantify the gene expression levels of integrin β1 and paxillin
when the cells had been cultured for 24 and 48 h, and the standard
PCR cycle threshold (Ct) method was performed using the Roche
Molecular Biochemicals LightCycler instrument (Roche Diagnostics
GmbH, Mannheim, Germany).
 | Table I.Real-time PCR primer sequences. |
Table I.
Real-time PCR primer sequences.
| Gene | Access number (Gene
Bank) | PCR primer
sequences | Length
(nucleotides) | Cycle number | PCR products
(bp) |
|---|
| Integrin β1 | X07979 |
5′-TTACGATGACGGTCTGGG-3′ | 18 | 46 | 122 |
|
3′-AAATGGCTTGTGCTTGTT-5′ | 18 | | |
| Paxillin | P49023 |
5′-CTGCTGGCGGACTT-3′ | 14 | 23 | 120 |
|
3′-TGGCACGGCAATCT-5′ | 14 | | |
| GAPDH | M32599 |
5′-GAGCCACATCGCTCAGACAC-3′ | 20 | 24 | 150 |
|
3′-CATGTAGTTGAGGTCAATGG-5′ | 20 | | |
Statistical analysis
Data are presented as mean ± SD, median with
interquartile ranges (IQR) or frequencies. A χ2 test was
used to compare frequencies. One-way ANOVA and the Student’s t-test
were used for normally distributed variables, and the Mann-Whitney
U test was used for non-normal distributed variables. Comparisons
between two groups for nominal variables were performed by Fisher’s
exact test. P<0.05 was considered to indicate a statistically
significant result. All statistical analyses were performed using
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Morphology and roughness
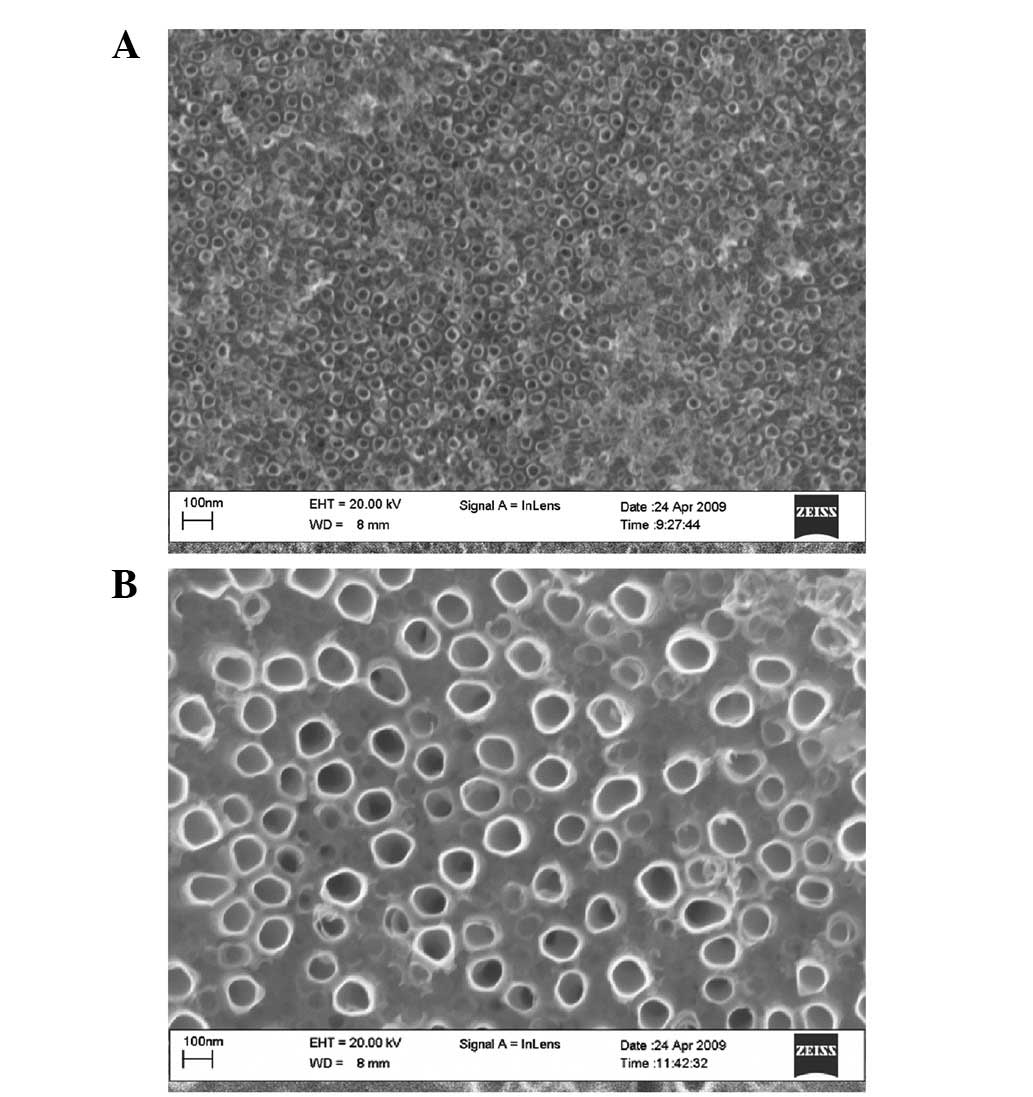
Representative FESEM micrographs of the 30- and
90-nm nanoporous titanium oxide films on Ti2448 alloys are shown in
Fig. 1. Samples with a 30-nm
nanoporous film exhibited a greater number of overlapping
microporous structures with microprojections than samples with a
90-nm nanoporous film. As shown in Table II, the anodized titanium surfaces
(30 and 90 nm) were rougher in comparison with the unanodized
control titanium surface (P<0.05). However, no significant
difference was observed in surface roughness between the 30- and
90-nm nanoporous films (P<0.05).
 | Table II.Surface roughness for each group. |
Table II.
Surface roughness for each group.
| Group | Roughness |
|---|
|
|---|
| Ra
(μm) | Rz
(μm) | Ry
(μm) |
|---|
| Control group | 0.28±0.11 | 1.37±0.26 | 1.88±0.17 |
| 30-nm group | 0.76±0.03a | 5.13±0.41a | 6.14±0.29a |
| 90-nm group | 0.71±0.04a | 4.97±0.51a | 5.79±0.56a |
Cell adhesion and morphology
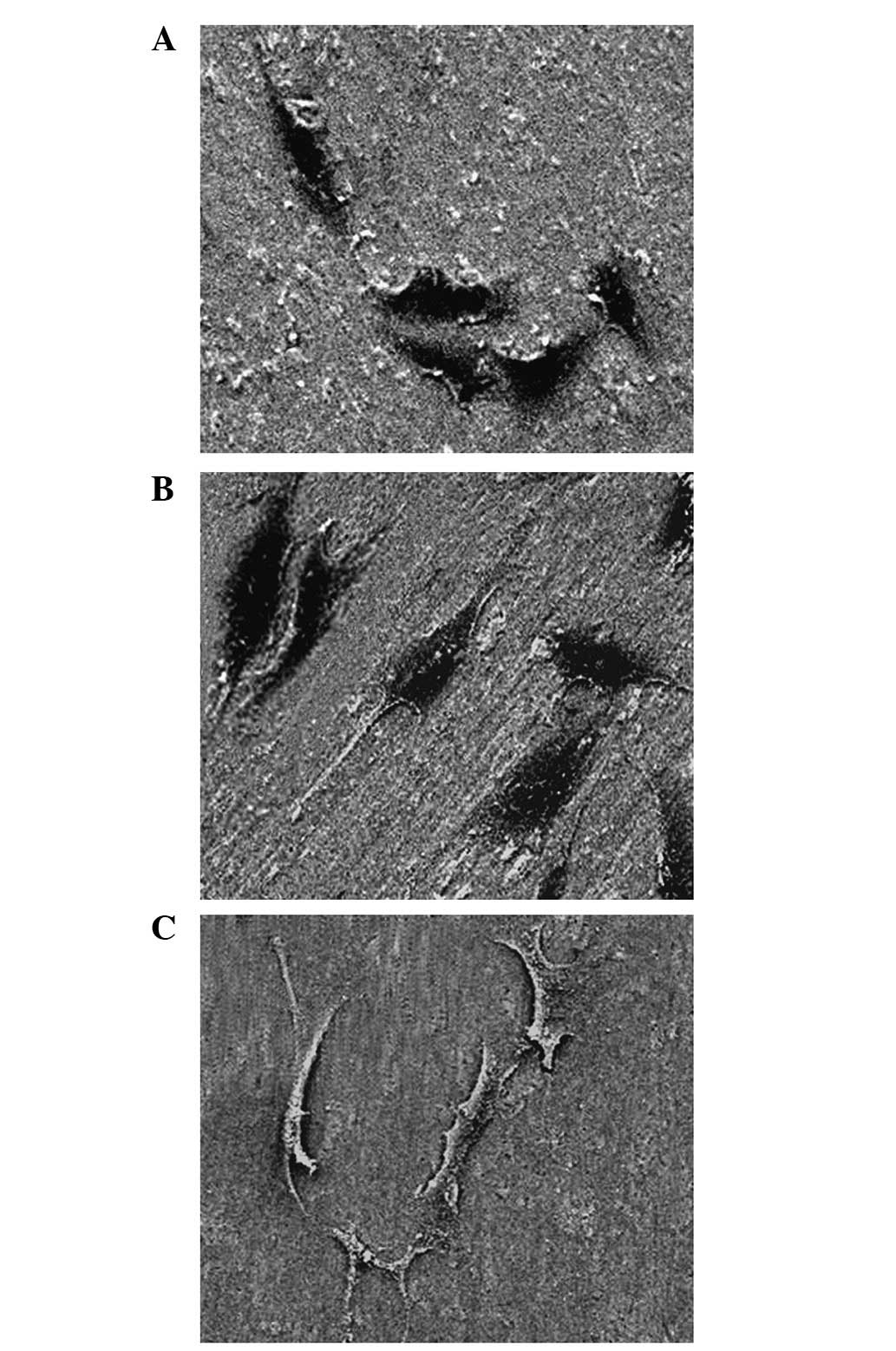
The morphology of the osteoblasts following cell
culture in each group for 48 h is shown in Fig. 2. On the 30-nm nanoporous surface,
the cells were densest on the overlapping structures and formed a
polygonal shape without an evident wrapped edge. On the 90-nm
nanoporous surface, cell adhesion was weaker than that observed on
the unanodized control and 30-nm nanoporous surfaces, and a wrapped
edge was evident. In addition, the cells exhibited a distorted and
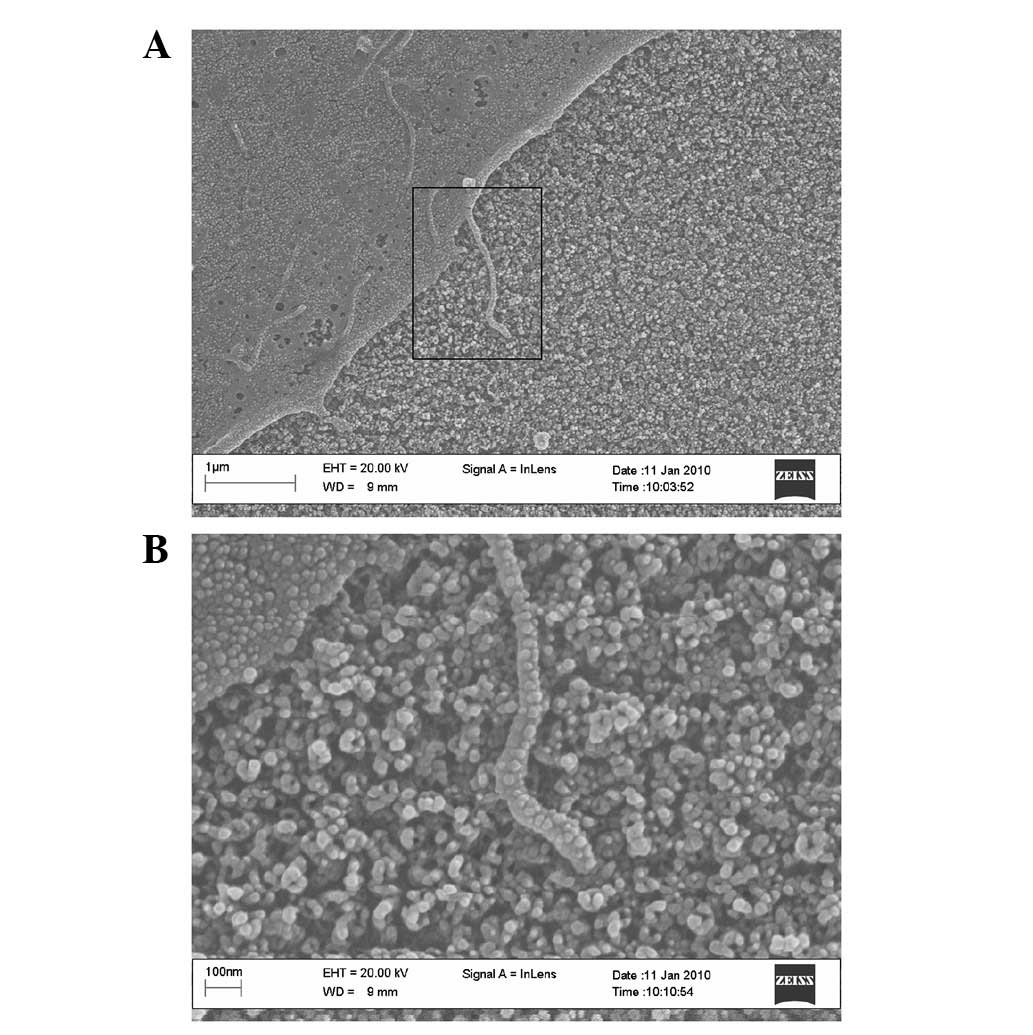
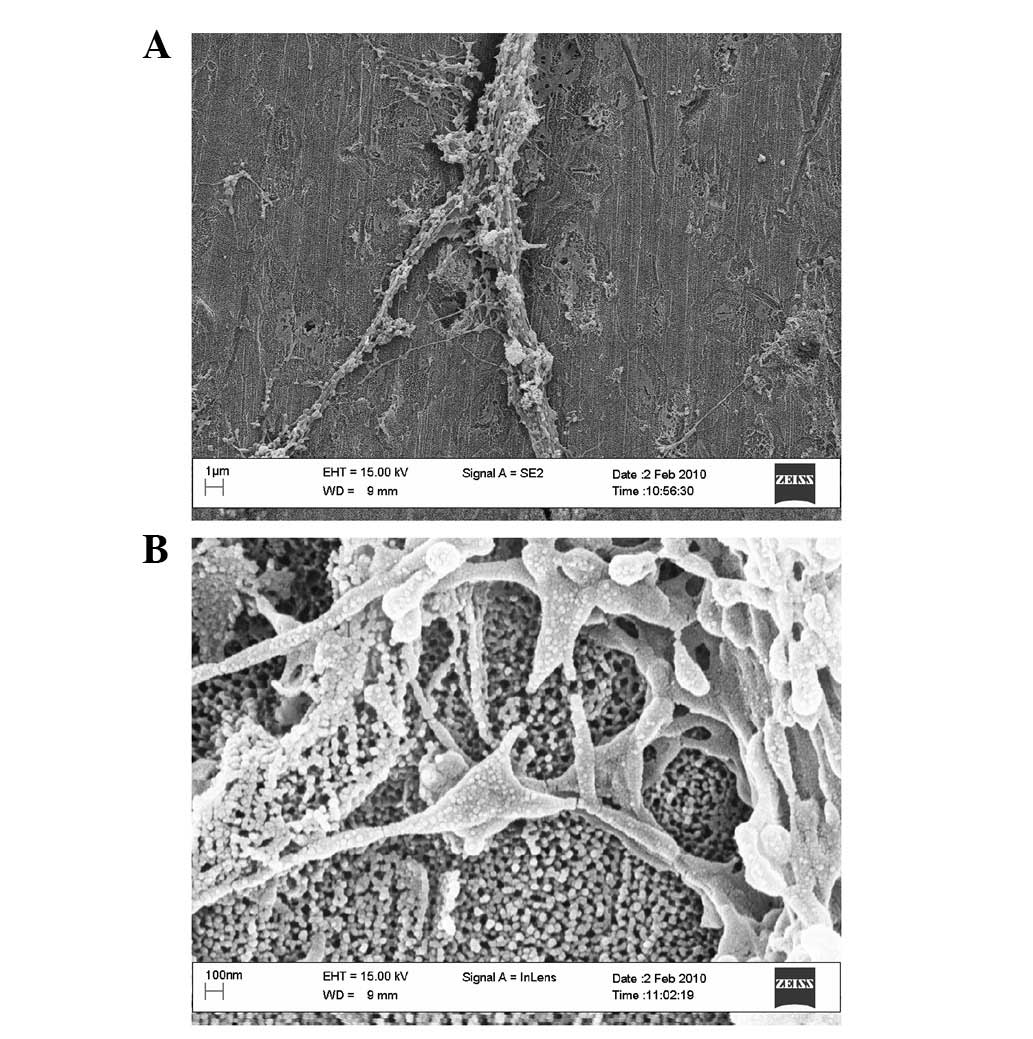
irregular shape. Under a high-magnification microscope, we observed
that the extracellular matrix (ECM) protein adhered densely to the
top wall surface of the 30-nm nanoporous surface (Fig. 3) and adhered sparsely to the 90-nm
nanoporous surface (Fig. 4). We
also observed the extension of filopodias from cells adhered to the
nanoporous surface, which was mediated by ECM proteins, and that
filopodias deviated around the nanoporous openings which were not
covered by ECM proteins.
MTT analysis
As shown in Table
III, the MTT assay indicated that the viabilities of the cells
on the 30-nm nanoporous surface were higher than those on the
unanodized control and 90-nm nanoporous surfaces following 24 and
48 h of culture (P<0.05). The cell viabilities on the 90-nm
nanoporous surface were lower than those on the control unanodized
surface following 48 h of culture (P<0.05), while there was no
significant difference between the cell viabilities of the 90-nm
and control groups following 24 h of culture (P>0.05).
 | Table III.Optical density of each group at
different time points examined using the MTT assay. |
Table III.
Optical density of each group at
different time points examined using the MTT assay.
| Group | Time points |
|---|
|
|---|
| 12 h | 24 h | 48 h |
|---|
| Control group | 0.015 | 0.062 | 0.093 |
| 30-nm group | 0.017 | 0.133a,b | 0.184a,b |
| 90-nm group | 0.014 | 0.053 | 0.068a |
Quantitative real-time RT-PCR
analysis
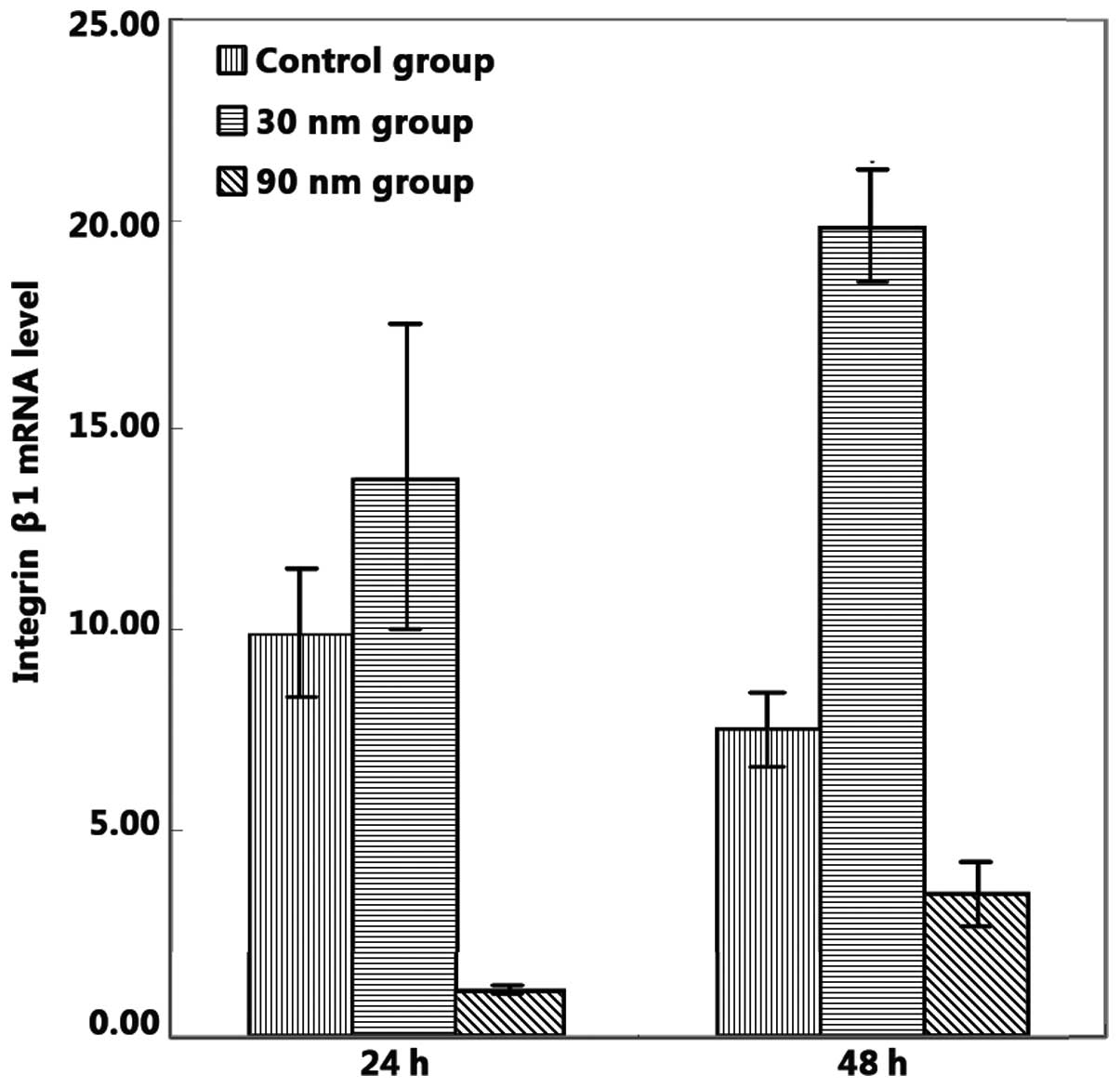
Integrin β1 mRNA expression levels on the 30-nm
nanoporous surface were significantly higher than those observed on
the unanodized control and 90-nm nanoporous surfaces, following 48
h of cell culture (P<0.05; Fig.
5); however, no significant difference in the integrin β1 mRNA
expression levels between the unanodized control and 30-nm
nanoporous surfaces were observed following 24 h of cell culture
(P>0.05). However, integrin β1 expression levels on the 90-nm
nanoporous surface were evidently lower than those on the
unanodized control surface following 24 and 48 h of cell culture
(P<0.05). No statistically significant differences were observed
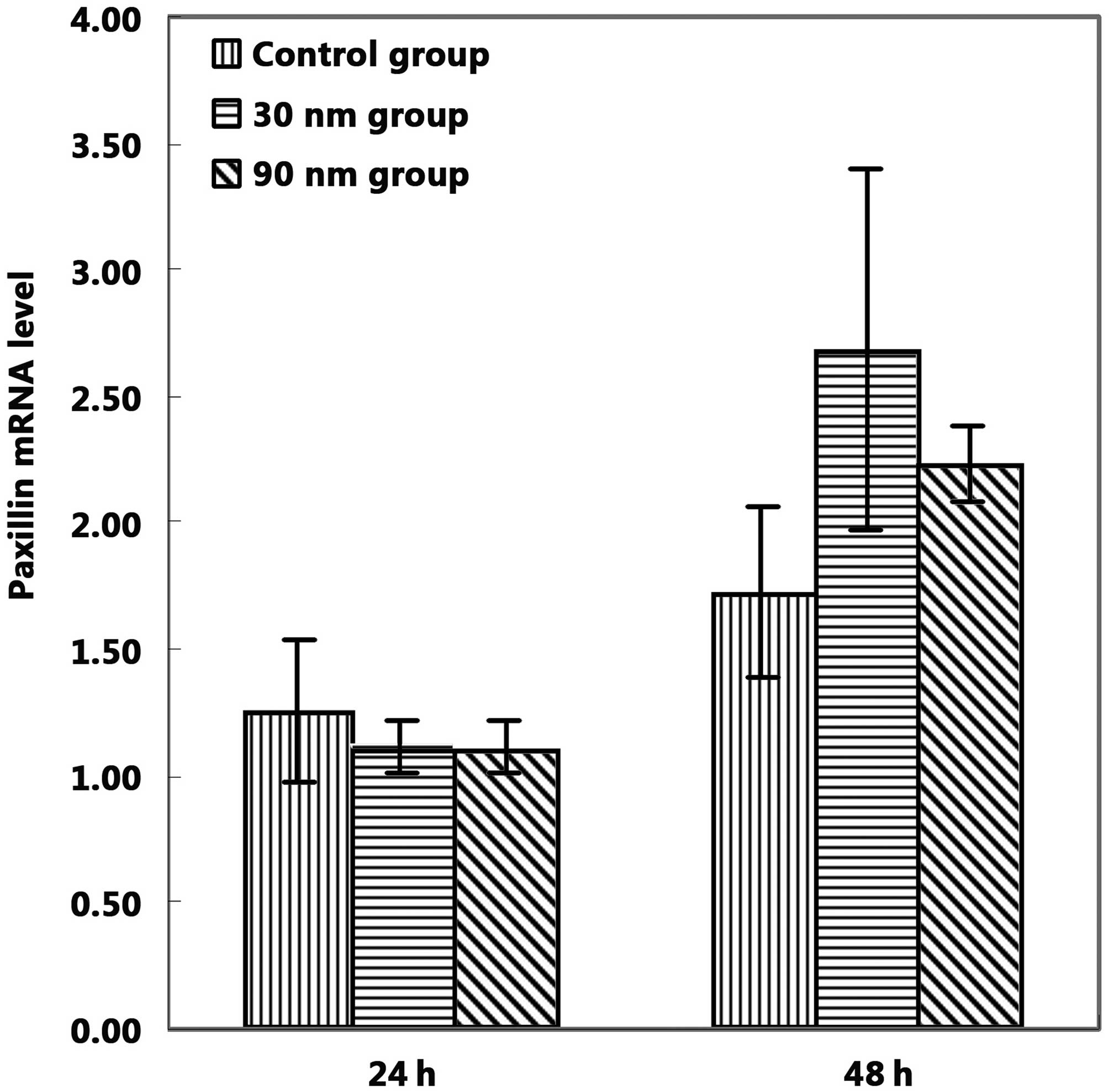
in the expression levels of paxillin mRNA between the unanodized
and anodized groups following 24 and 48 h of cell culture (Fig. 6).
Discussion
In the last few decades, titanium metal and its
alloys have been widely used as orthopedic and dental implants due
to their good biocompatibility, mechanical properties and neutral
interference with modern imaging techniques (26–28).
To accelerate the initial rate of osseointegration, the topographic
and chemical properties of implant surfaces should be considered
and modified. The surface structure of the titanium implants is
responsible for a good healing process. Therefore, numerous methods
have been used to produce a rough implant surface, including
titanium plasma spraying, blasting with aluminum oxide or other
ceramic particulate materials, strong acid etching, and the
potentiostatic or galvanostatic anodization of titanium (29). The majority of methods aim to form
a micro/nanoporous structure, which may promote bone bonding or
apposition of the implant surface (30).
In the present study, nanoporous titanium oxide
films with two different pore sizes (30 and 90 nm) on Ti2448 alloy
were formed by anodization in NH4F solution. The effects
of the surface characteristics of different nanoporous titanium
oxide films on the initial adhesion of osteoblasts was
investigated. Following anodization, the surface morphology was
examined using a Surftest Formtracer and FESEM. The roughness of
the anodized titanium surfaces (30 and 90 nm) was significantly
higher that of the unanodized titanium control surface. FESEM
images demonstrated that the number of adhered cells on the 30-nm
nanoporous surface was higher than those on the unanodized control
and 90-nm nanoporous surfaces following 48 h of cell culture. In
addition, cell adhesion on the 90-nm nanoporous surface was weaker
than that on the unanodized control surface, indicating that the
effects of surface micromorphology on initial cell adhesion may be
more significant than the surface roughness. Our data provide
strong evidence that the roughened surface of the 30-nm nanoporous
film was more favorable for the initial adhesion of osteoblasts.
Furthermore, we observed that the overlapped cells on the 30-nm
nanoporous surface were the densest, suggesting that the surface
demonstrated the characteristics of a typical bioactive surface
with high surface energy. Results of the MTT assay indicated that
cell viability on the 30-nm nanoporous surface following 24 and 48
h of cell culture was higher than those on the unanodized control
and 90-nm nanoporous surfaces, which may have resulted from greater
numbers of adhered osteoblasts and higher cell activities. This is
in agreement with previous studies (7,9).
In vivo studies have demonstrated an improved bone fixation
on implants with a rough surface structure compared with implants
with a smooth surface (27). A
previous study has shown that integrin β1 is an important bridge
for osteoblast adhesion on biomaterials (31). Zreiqat et al also
demonstrated that higher expression levels of integrin β1 may
contribute to the initial adhesion of osteoblastic cells to implant
surfaces (31). Our results are in
agreement with previously published studies with regard to the
influence of integrin β1 on cell adhesion properties. Using
real-time RT-PCR, we identified that integrin β1 mRNA expression
levels on the 30-nm nanoporous surface following 24 h of cell
culture were significantly higher than those on the control
unanodized and 90-nm nanoporous surfaces. However, the 90-nm
nanoporous surface demonstrated lower integrin β1 mRNA expression
levels compared with the unanodized control surface following 24
and 48 h of cell culture. These results provide support for our
hypothesis that nanoporous oxide films on Ti2448 alloy,
particularly 30-nm nanoporous films, have a significant influence
on osteoblast cell adhesion.
In conclusion, the results of the current study
indicate that 30-nm nanoporous titanium oxide films on Ti2448
alloys may provide the optimum bioactive implant surface for the
initial adhesion of osteoblasts. The 30-nm nanoporous film
exhibited the characteristics of a typical bioactive surface. Thus,
a Ti2448 alloy with a 30-nm nanoporous film is a promising
implant-coating material for the promotion of bone formation, as
well as for meeting the immediate implantation and early clinical
load requirements. Further studies are required to determine
whether the 30-nm nanoporous film on Ti2448 alloy has a strong
effect on osteoblast cell proliferation, migration and
differentiation.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 51071152 and no.
50901080) and by the Ministry of Science and Technology of China
(no. 2011AA030106).
References
|
1.
|
Biesiekierski A, Wang J, Gepreel MA and
Wen C: A new look at biomedical Ti-based shape memory alloys. Acta
Biomater. 8:1661–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fragou S and Eliades T: Effect of topical
fluoride application on titanium alloys: a review of effects and
clinical implications. Pediatr Dent. 32:99–105. 2010.PubMed/NCBI
|
|
3.
|
Lu X, Hiraki T, Nakajima K, et al:
Thermodynamic analysis of separation of alloying elements in
recycling of end-of-life titanium products. Sep Purif Technol.
89:135–141. 2012. View Article : Google Scholar
|
|
4.
|
Özcan M and Hämmerle C: Titanium as a
reconstruction and implant material in dentistry: Advantages and
pitfalls. Materials. 5:1528–1545. 2012.
|
|
5.
|
Gomes CC, Moreira LM, Santos VJ, et al:
Assessment of the genetic risks of a metallic alloy used in medical
implants. Genet Mol Biol. 34:116–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Barbas A, Bonnet AS, Lipinski P, Pesci R
and Dubois G: Development and mechanical characterization of porous
titanium bone substitutes. J Mech Behav Biomed Mater. 9:34–44.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bai Y, Li SJ, Prima F, Hao YL and Yang R:
Electrochemical corrosion behavior of Ti-24Nb-4Zr-8Sn alloy in a
simulated physiological environment. Appl Surf Sci. 258:4035–4040.
2012. View Article : Google Scholar
|
|
8.
|
Zheng K, Li X, Fu J, et al: Effects of
Ti2448 half-pin with low elastic modulus on pin loosening in
unilateral external fixation. Journal of materials science J Mater
Sci Mater Med. 22:1579–1588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Guo Z, Fu J, Zhang YQ, et al: Early effect
of Ti-24Nb-4Zr-7.9Sn intramedullary nails on fractured bone. Mat
Sci Eng C Mater Biol Appl. 29:963–968. 2009. View Article : Google Scholar
|
|
10.
|
Gordin DM, Gloriant T, Chane-Pane V, et
al: Surface characterization and biocompatibility of titanium
alloys implanted with nitrogen by Hardion+ technology. J Mater Sci
Mater Med. 23:2953–2966. 2012.PubMed/NCBI
|
|
11.
|
Yu XF, Li YX, Ge WY, Yang QB, Zhu NF and
Kalantar-Zadeh K: Formation of nanoporous titanium oxide films on
silicon substrates using an anodization process. Nanotechnology.
17:808–814. 2006. View Article : Google Scholar
|
|
12.
|
Zhuang HF, Lin CJ, Lai YK, Sun L and Li J:
Some critical structure factors of titanium oxide nanotube array in
its photo-catalytic activity. Environ Sci Technol. 41:4735–4740.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gong D, Grimes CA, Varghese OK, et al:
Titanium oxide nanotube arrays prepared by anodic oxidation. J
Mater Res. 16:3331–3334. 2001. View Article : Google Scholar
|
|
14.
|
Feng B, Weng J, Yang BC, Qu SX and Zhang
XD: Characterization of surface oxide films on titanium and
adhesion of osteoblast. Biomaterials. 24:4663–4670. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhu X, Chen J, Scheideler L, Reichl R and
Geis-Gerstorfer J: Effects of topography and composition of
titanium surface oxides on osteoblast responses. Biomaterials.
25:4087–4103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Neoh KG, Hu X, Zheng D and Kang ET:
Balancing osteoblast functions and bacterial adhesion on
functionalized titanium surfaces. Biomaterials. 33:2813–2822. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shtansky DV, Zhitnyak IY, Bashkova IA,
Pogozhev YS, Sheveiko AN and Gloushankova NA: The influence of
elemental composition and surface topography on adhesion,
proliferation and differentiation of osteoblasts. English Biochem
(Mosc) Suppl Ser A Membr Cell Biol. 4:272–276. 2010.
|
|
18.
|
Rosales-Leal JI, Rodríguez-Valverde MA,
Mazzaglia G, et al: Effect of roughness, wettability and morphology
of engineered titanium surfaces on osteoblast-like cell adhesion.
Colloid Surface A. 365:222–229. 2010. View Article : Google Scholar
|
|
19.
|
Macak JM, Tsuchiya H, Taveira L, Ghicov A
and Schmuki P: Self-organized nanotubular oxide layers on
Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F
solutions. J Biomed Mater Res A. 75:928–933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yasuda K and Schmuki P: Formation of
self-organized zirconium titanate nanotube layers by alloy
anodization. Adv Mater. 19:1757–1760. 2007. View Article : Google Scholar
|
|
21.
|
Taveira LV, Macak JM, Tsuchiya H, Dick LFP
and Schmuki P: Initiation and growth of self-organized
TiO2 nanotubes anodically formed in
NH4F/(NH4)2SO4
electrolytes. J Electrochem Soc. 152:B405–B410. 2005.
|
|
22.
|
Zhu X, Kim KH and Jeong YS: Anodic oxide
films containing Ca and P of titanium biomaterial. Biomaterials.
22:2199–2206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhu XL, Son DW, Ong JL and Kim K:
Characterization of hydrothermally treated anodic oxides containing
Ca and P on titanium. J Mater Sci Mater Med. 14:629–634.
2003.PubMed/NCBI
|
|
24.
|
van den Dolder J, Spauwen PH and Jansen
JA: Evaluation of various seeding techniques for culturing
osteogenic cells on titanium fiber mesh. Tissue Eng. 9:315–325.
2003.PubMed/NCBI
|
|
25.
|
Suh JY, Jang BC, Zhu XL, Ong JL and Kim K:
Effect of hydrothermally treated anodic oxide films on osteoblast
attachment and proliferation. Biomaterials. 24:347–355. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Langhoff JD, Voelter K, Scharnweber D, et
al: Comparison of chemically and pharmaceutically modified titanium
and zirconia implant surfaces in dentistry: a study in sheep. Int J
Oral Maxillofac Surg. 37:1125–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ohkubo C, Shimura I, Aoki T, Hanatani S,
Hosoi T and Okabe T: In vitro wear assessment of titanium alloy
teeth. J Prosthodont. 11:263–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Deligianni DD, Katsala N, Ladas S,
Sotiropoulou D, Amedee J and Missirlis YF: Effect of surface
roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell
response and on protein adsorption. Biomaterials. 22:1241–1251.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Le Guéhennec L, Soueidan A, Layrolle P and
Amouriq Y: Surface treatments of titanium dental implants for rapid
osseointegration. Dent Mater. 23:844–854. 2007.PubMed/NCBI
|
|
30.
|
Gittens RA, Olivares-Navarrete R,
McLachlan T, et al: Differential responses of osteoblast lineage
cells to nanotopographically-modified, microroughened
titanium-aluminum-vanadium alloy surfaces. Biomaterials.
33:8986–8994. 2012. View Article : Google Scholar
|
|
31.
|
Zreiqat H, Howlett CR, Zannettino A, et
al: Mechanisms of magnesium-stimulated adhesion of osteoblastic
cells to commonly used orthopaedic implants. J Biomed Mater Res.
62:175–184. 2002. View Article : Google Scholar : PubMed/NCBI
|