Introduction
The liver receives a dual blood supply from the
hepatic portal vein and the hepatic artery. Whilst 70–80% of the
supply is provided by the portal vein, which transports nutrients
and various other substances to the liver, the remaining 20–30% of
the blood supply arrives via the hepatic artery, which mainly
nourishes the biliary system (1).
The blood pressure of the arterial system is >100 mmHg, whereas
the pressure of the portal vein is considerably lower at 6–8 mmHg.
As a result of this pressure difference, when problems arise in the
liver or in other tissues supplied by these vessels, the portal
vein is usually the primary vessel affected, which leads to a
reduction in blood flow. A reduction in the total hepatic blood
supply of this nature appears to activate a compensatory mechanism
that increases the arterial blood flow (1–4).
Budd-Chiari syndrome occurs as a result of the
congestion of the liver, due to the chronic obstruction of hepatic
blood flow draining into the venous system. Long-lasting hepatic
congestion has been demonstrated to lead to necrosis of the
hepatocytes in the vicinity of the hepatic venules (central vein)
and sinusoidal enlargement, whilst congestive cirrhosis has been
indicated to develop as a result of the progressive fibrosis in the
proximity of the hepatic venules (5). This leads to a further reduction in
the speed and the volume of portal venous blood flow (6), eventually resulting in a cessation of
the blood flow. The reduction in hepatic blood flow, due to a
blockage in the portal vein, is compensated for by the hepatic
arterial blood flow and the portal vein subsequently functions as a
drainage vessel for the arterial blood. As a consequence, the
hepatic blood flow becomes hepatofugal, and this is the mechanism
for disease progression.
With the aim of gaining a better understanding the
pathology of liver disease, the focus of our group is the study of
hepatic hemodynamic changes. The present study concerns a case of
Budd-Chiari syndrome that may reveal, in part, the mechanism that
regulates the blood flow balance between the hepatic artery and the
hepatic portal vein.
Case report
Patient history and presentation
A 40-year-old male had been previously admitted to
The Toho University Omori Medical Center (Tokyo, Japan) for the
treatment of an esophageal varix rupture, at the age of 30 years.
At that time, as the physical examination indicated occlusion of
the inferior vena cava in the proximity of the liver, a liver
biopsy was performed. The biopsy revealed fibrosis in the vicinity
of the hepatic venules (zone III), and an enlargement of the
sinusoids and portal vein branches, which led to a diagnosis of
Budd-Chiari syndrome. The patient was subsequently monitored in the
hospital's outpatient clinic. The patient had no history of alcohol
consumption or tobacco use, but had suffered a single epileptic
seizure in 2004. The patient's family history revealed nothing of
note. In February, 2012, the patient underwent a routine abdominal
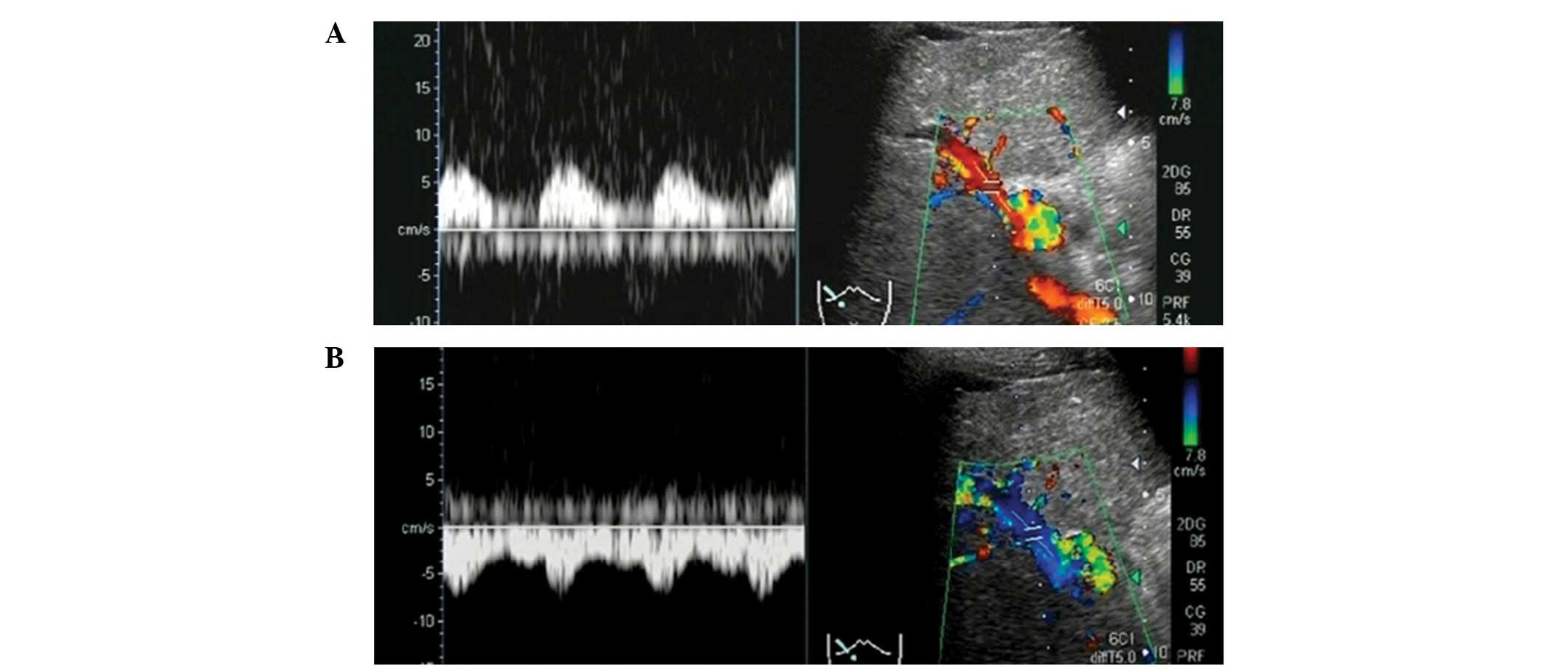
ultrasound, and the color Doppler ultrasonography (US) images
revealed a change in the blood flow from hepatopetal (in red) to
hepatofugal (in blue) in the right portal vein branch during deep
inspiration (Figs. 1 and 2). We subsequently performed arrival time
parametric imaging (At-PI), using Sonazoid-enhanced US, to
investigate the balance between the blood supply from the hepatic
artery and the portal vein in hepatic parenchymal perfusion during
deep expiration. The patient was alert during the At-PI, and a
blood pressure of 124/80 mmHg, a heart rate of 70 bpm and a body
temperature of 36.0°C were recorded. The palpebral conjunctiva
exhibited no signs of anemia; however, the bulbar conjunctiva
demonstrated a mild yellowish discoloration. Further investigations
revealed pure heart and clear breath sounds; a flat and soft
abdomen with no tenderness; a palpable spleen and the absence of
edema in the lower extremities. Hematological tests revealed
pancytopenia and mild jaundice (Table
I).
 | Table I.Test results for a patient with
Budd-Chiari syndrome. |
Table I.
Test results for a patient with
Budd-Chiari syndrome.
| Type of test | Result |
|---|
| Biochemical | |
| CRP (mg/dl) | 1.1 |
| Na (mEq/l) | 139.0 |
| K (mEq/l) | 3.3 |
| Cl (mEq/l) | 103.0 |
| TP (g/dl) | 7.9 |
| Alb (g/dl) | 3.6 |
| T-Bil (mg/dl) | 2.8 |
| D-Bil (mg/dl) | 1.7 |
| AST (IU/l) | 28 |
| ALT (IU/l) | 14 |
| LDH (IU/l) | 189 |
| ALP (IU/l) | 574 |
| γ-GTP (IU/l) | 166 |
| BUN (mg/dl) | 7.00 |
| Cr (mg/dl) | 0.46 |
| BS (mg/dl) | 122.00 |
| PT (%) | 56 |
| PT-INR | 1.5 |
| NH3
(μg/dl) | 76 |
| Hematogical | |
| WBC (per
μl) |
1.9×103 |
| RBC (per
μl) |
4.66×106 |
| Hgb (mg/dl) | 9.5 |
| Hct (%) | 30.7 |
| PLT (per
μl) |
6.5×104 |
| Serological | |
| HCV-Ab (+/-) | (-) |
| HBs-Ag (+/-) | (-) |
| HBs-Ab (+/-) | (-) |
| ANA (+/-) | (-) |
| AMA (+/-) | (-) |
| P-ANCA (+/-) | (-) |
| IgG (mg/dl) | 981 |
| IgA (mg/dl) | 263 |
| IgM (mg/dl) | 97 |
Contrast-enhanced US and At-PI
US imaging was performed using a Toshiba Aplio XG
diagnostic ultrasound system (model SSA-790A; Toshiba Medical
Systems Corporation, Tochigi, Japan) with a 3.75 MHz convex array
probe (model PVT-375BT, Toshiba Medical Systems Corporation) at a
mechanical index of 0.21. The right main branch of the portal vein
was visualized from the right intercostal space, and images
displaying the liver parenchyma of the right hepatic lobe (segments
5–8) were selected for analysis. The focal depth was set at 3–10 cm
using the dual-focus mode. Subsequent to the selection of the
imaging parameters, the recommended dose of Sonazoid
(perfluorobutane; 0.015 ml/kg), obtained from GE Healthcare (Oslo,
Norway), was injected via the cubital vein. Following the Sonaziod
infusion, imaging was performed for ∼40 sec to visualize the
patient during a period of deep inspiration, and the images were
then stored as raw data in the system hardware. The images
corresponding with deep expiration were acquired in the same
manner. US was performed by the same operator each time to maintain
imaging consistency.
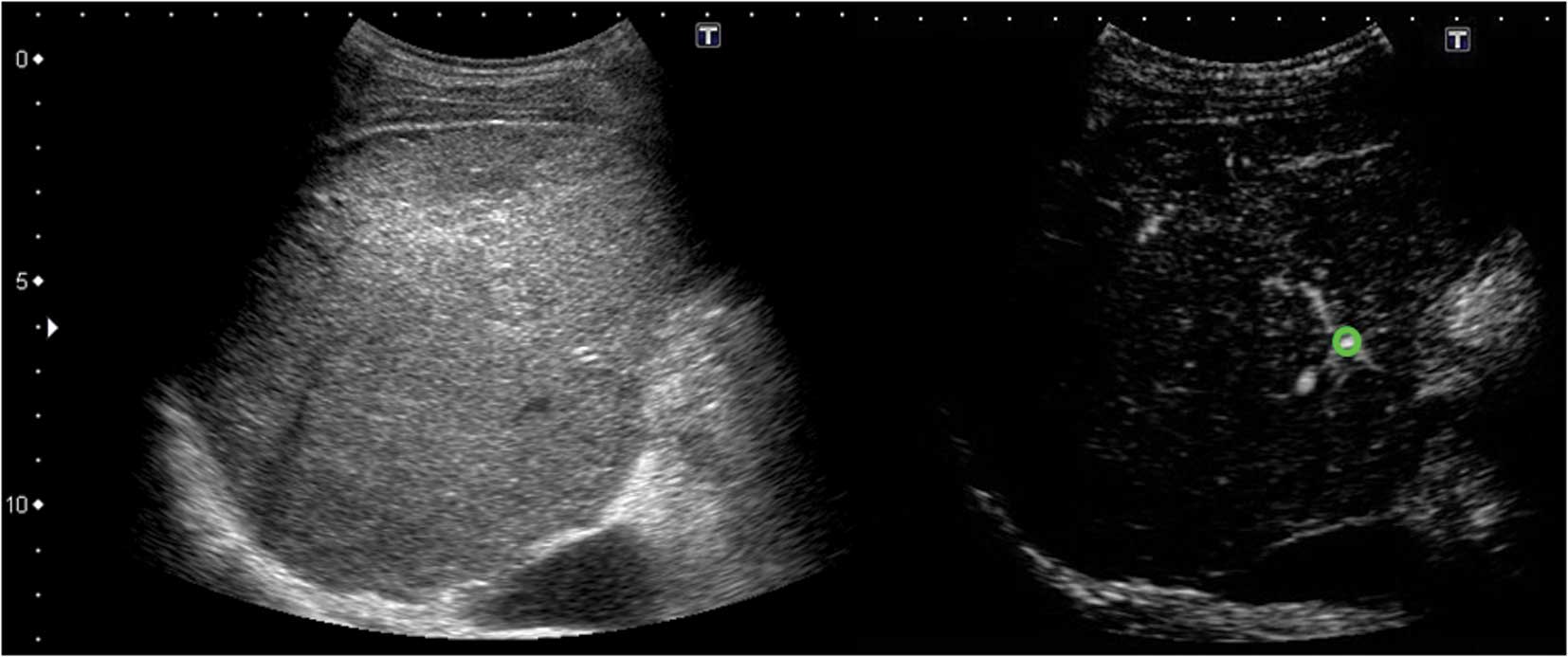
At-PI images were generated from the stored data
using the software interfaced with the ultrasound system. The right
branch of the hepatic artery was selected as the region of interest
(ROI); thus, the system set the moment at which the ROI was
contrasted as time 0, and sequentially calculated the arrival time
of individual pixels in the hepatic parenchyma. The system
subsequently automatically created and superimposed a color map
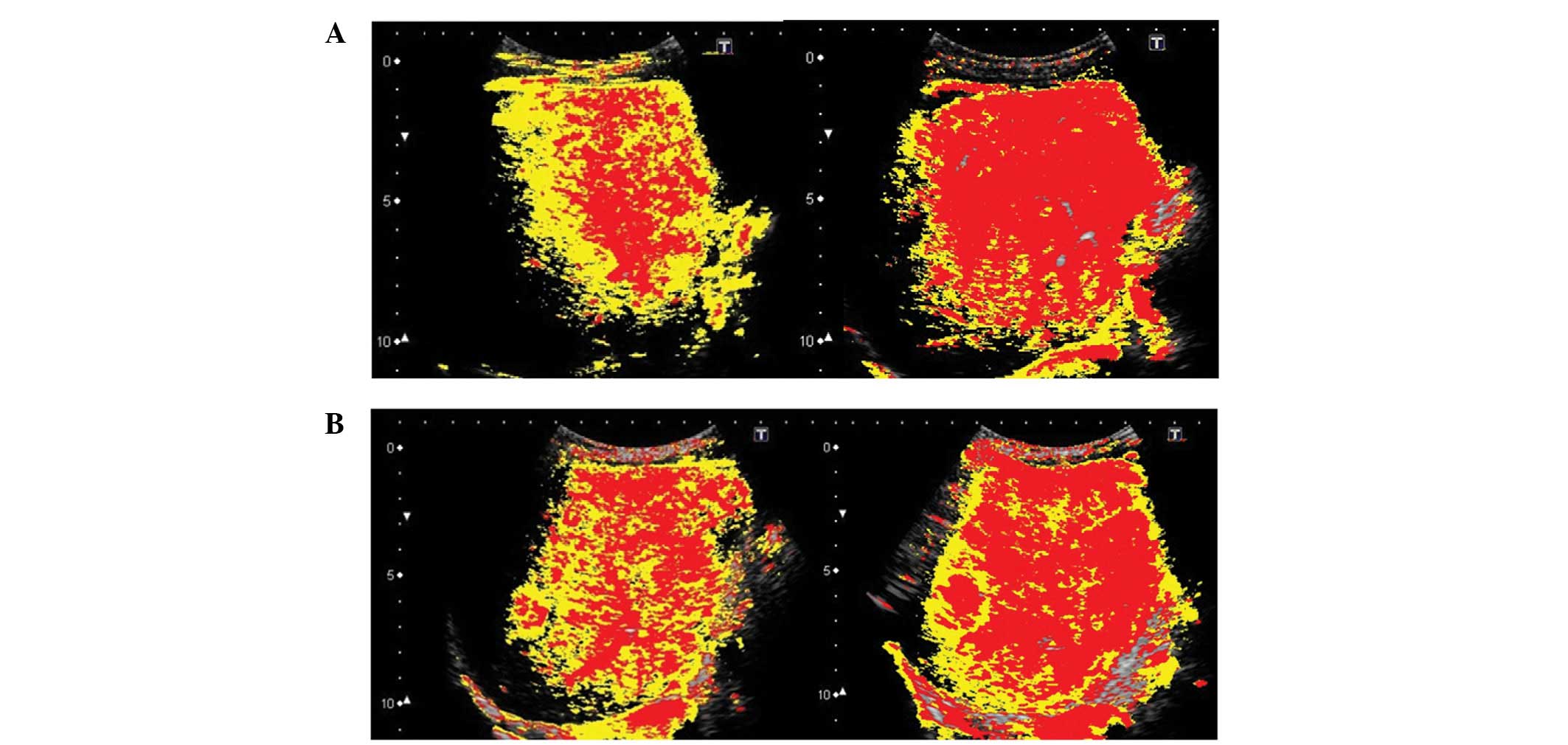
onto a B-mode image. In the present study, it took ∼10 sec for the
contrast agent to completely visualize the liver parenchyma,
following its arrival at the hepatic artery. We therefore displayed
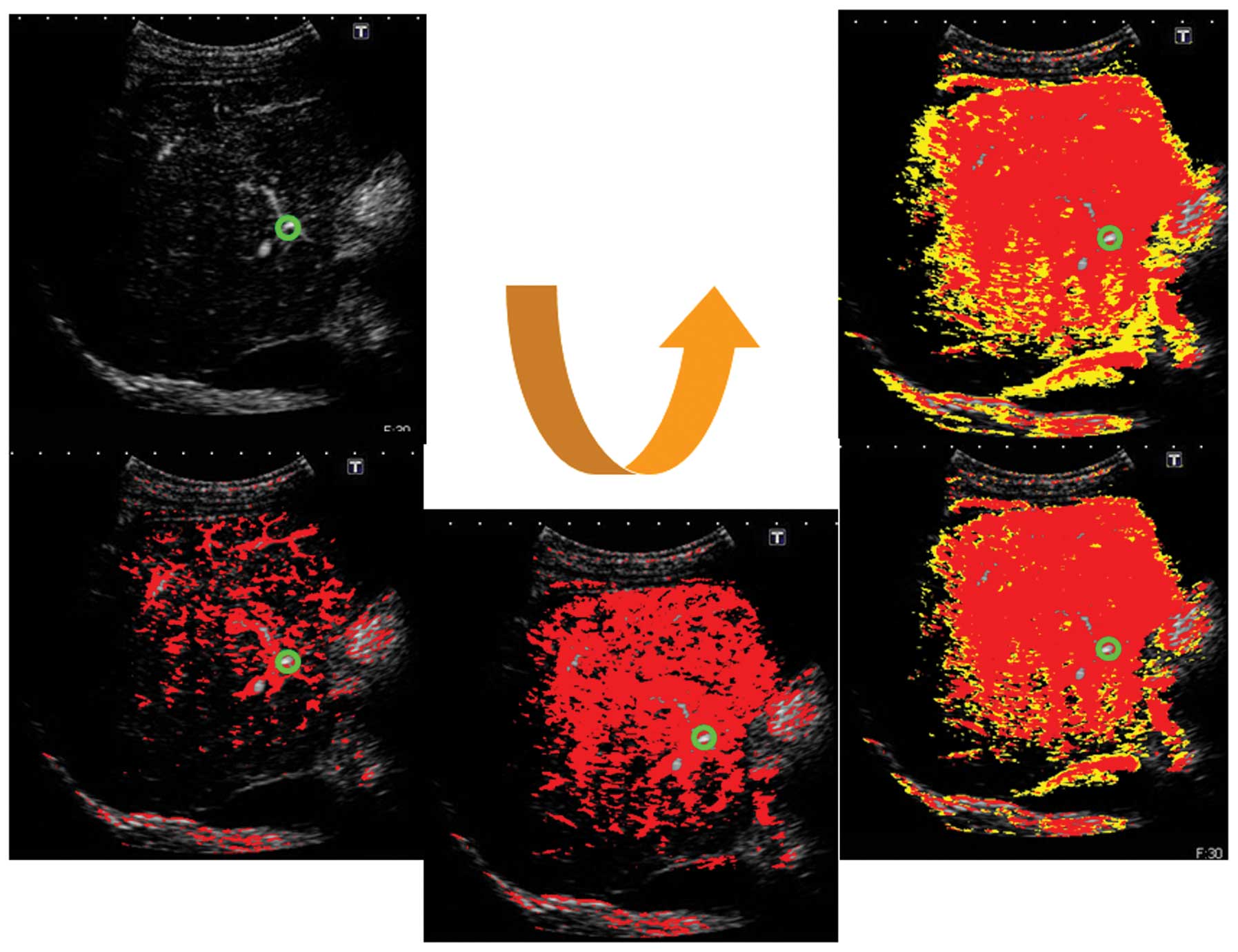
pixels arriving between 0–5 sec in red, and those arriving between
6–10 sec in yellow (Figs.
3–5).
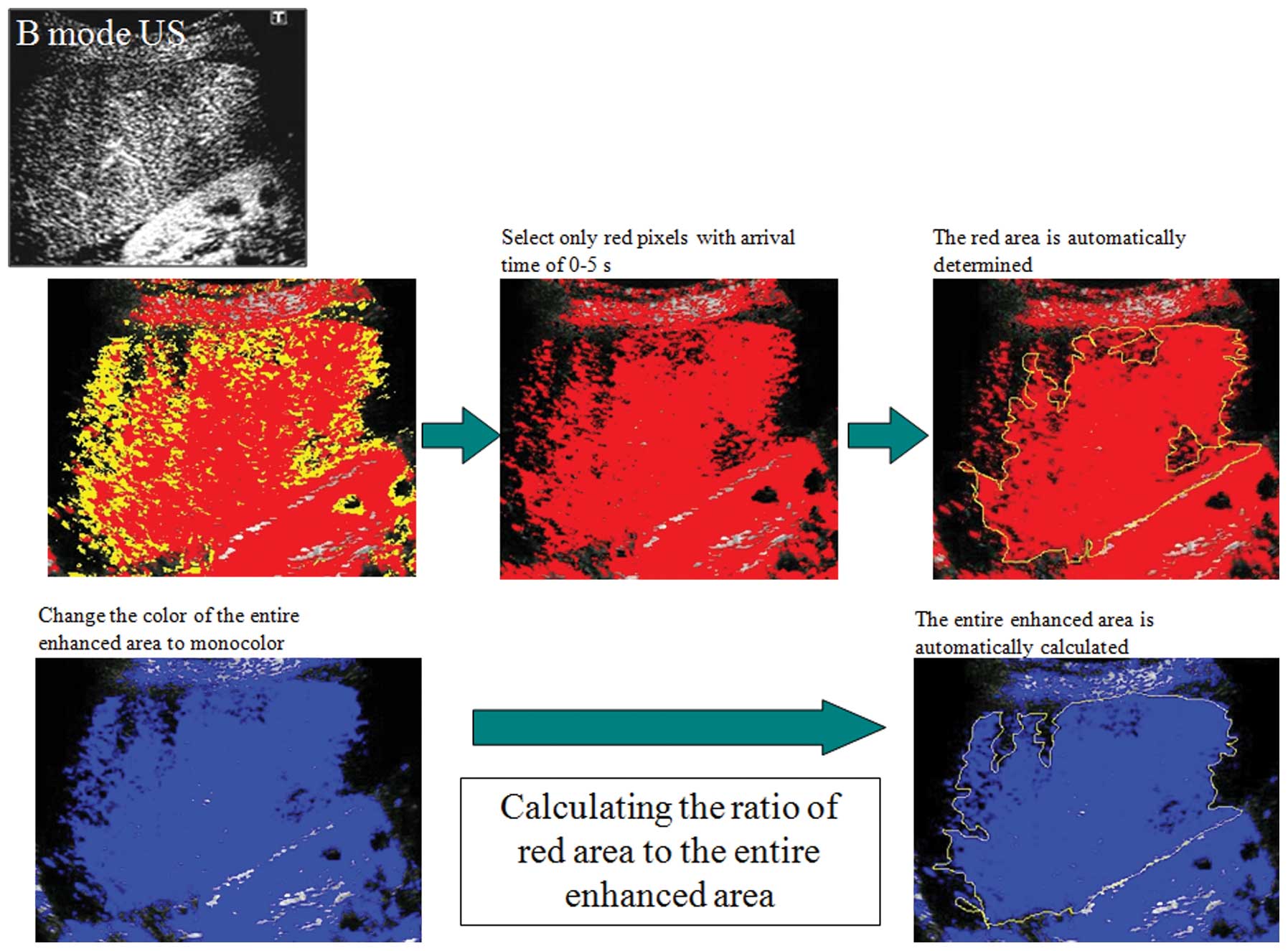
Calculation of the ratio of the area of
red pixels to the entire contrast-enhanced area
For the quantitative evaluation of the At-PI data
obtained, the ratio of the area of red pixels, i.e. the pixels with
shorter arrival times, to the entire contrast-enhanced area was
calculated as the ‘ratio of red’ (ROR), using the image analysis
software ImageJ (version 1.42; Rasband WS, US National Institutes
of Health, Bethseda, MD, USA; Fig.
6).
Imaging and calculation results
In the first US imaging, conducted in February 2012,
the ROR for deep inspiration was 77.1% and that of deep expiration
was 44.5%. In the second imaging procedure, conducted in March
2012, the ROR for deep inspiration was 76.9% and that of deep
expiration was 56.3%. These results demonstrated that the ROR was
higher for deep inspiration than expiration (Fig. 7).
Discussion
At-PI, a US image analysis tool that was introduced
into the Toshiba Aplio XG diagnostic ultrasound system (Toshiba
Medical Systems Corporation) in October 2010, traces and color
codes temporal changes in contrast-enhanced US images. We have
previously used At-PI to investigate the influence of the hepatic
portal vein and the hepatic artery on hepatic parenchymal
enhancement, and have reported its use in the clinical assessment
of type C chronic liver disease (7) and alcohol-induced liver disease
(8). We have also utilized At-PI
to investigate the effects of portal vein thrombosis on hepatic
parenchymal enhancement (9),
demonstrating its efficacy in the differential diagnosis of benign
recurrent intrahepatic and familial intrahepatic cholestasis
(10).
In the present study, At-PI technology was used to
reveal changes in hepatic parenchymal enhancement in a patient
whose blood flow balance between the hepatic artery and portal vein
was affected by deep breathing. The arrival time of Sonazoid in the
liver was the primary element that was considered in the study of
this blood flow balance. The contrast agent, injected via the
cubital vein, first arrived at the liver via the hepatic artery,
having passed through the heart. It was also carried through the
venous system to arrive at the liver via the portal vein, once the
blood had first nourished the stomach, intestines and spleen. The
contrast agent has been demonstrated to arrive at the liver via
these two routes ∼5 sec apart, with the hepatic arterial blood flow
arriving more rapidly (11). In
At-PI, blood flow arriving 0–5 sec following the contrast agent
injection is displayed in red, and is from the hepatic artery,
whereas blood flow arriving 6–10 sec subsequent to the injection is
displayed in yellow, and is from the portal vein. The system
therefore enables an objective study of the blood flow balance
between the hepatic artery and the portal vein.
Budd-Chiari syndrome occurs as a result of the
congestion of the liver, due to the chronic obstruction of hepatic
blood flow draining into the venous system. Long-lasting hepatic
congestion has been demonstrated to lead to necrosis of the
hepatocytes in the vicinity of the hepatic venules (central vein),
and sinusoidal enlargement, whilst congestive cirrhosis has been
indicated to develop as a result of the progressive fibrosis in the
proximity of the hepatic venules (5). This leads to a further reduction in
the speed and the volume of portal venous blood flow (6), eventually resulting in a cessation of
the blood flow. The reduction in hepatic blood flow, due to a
blockage in the portal vein, is compensated for by the hepatic
arterial blood flow, and the portal vein subsequently functions as
a drainage vessel for the arterial blood. As a consequence, the
hepatic blood flow becomes hepatofugal, and this is the mechanism
for disease progression. Several studies have investigated the
effect of respiration on hepatic blood flow, and have demonstrated
that portal venous blood flow is reduced by inspiration and
enhanced by expiration (12–15),
due to an increase in the intra-abdominal pressure. This occurs
since inspiration compresses the blood vessels in the liver, and
increases the resistance to portal venous blood flow (12). In the present study, the change in
portal venous blood flow occurred as a result of deep inspiration,
due to a slight increase in the intra-abdominal pressure, which
indicated that at this time the portal venous blood flow of the
patient was changing from hepatopetal to hepatofugal.
As observed in the present case, when the portal
venous blood flow is reduced, the hepatic vascular system has a
mechanism to compensate for the loss in hepatic parenchymal
perfusion (1–4). However, prior to this case, it had
not been determined whether the hepatic artery is able to fully
respond to acute changes in portal venous blood flow. Therefore,
this was investigated in the present study, using At-PI. In deep
inspiration, when the portal venous blood flow was hepatofugal, the
hepatic parenchymal perfusion was displayed by early-arriving red
pixels, indicating that hepatic arterial blood flow was the major
source of blood flow. However, during deep expiration, when the
portal venous blood flow was hepatopetal, there was a reduction in
the proportion of red pixels in the hepatic parenchymal perfusion,
indicating an increase in portal venous blood flow. These results
appeared to indicate that the hepatic artery successfully
compensated for the acute reduction in the portal venous blood
flow.
Blood flow in the liver, particularly intrahepatic
micro-circulation, is important in the study of changes in hepatic
parenchymal perfusion. Sonazoid, injected via the cubital vein,
arrives at the liver via arterial blood flow. In healthy
individuals, the hepatic artery is the blood vessel that feeds the
biliary system. Arterial blood flow nourishes large and small bile
ducts, while traveling through the liver toward the periphery. The
peribiliary capillary plexus is formed around the bile ducts, and a
number of the capillaries merge into the terminal portal venules
and sinusoids. However, certain branches of the hepatic artery
merge directly into the sinusoids, without passing through the
peribiliary capillary plexus, or nourish the portal vein by
extending into the wall (16–18).
In healthy individuals, the ratio of blood inflow
via the hepatic portal vein and the hepatic artery is 7–8:2–3, and
therefore the portal vein is the predominant blood supply to the
liver (1). This is considered to
be due to the fact that blood flowing through the portal vein
contains nutrients from the stomach and the intestine, which
results in the portal vein acting as a nutrient vessel for the
liver. A previous study, which investigated the pressure difference
between the two vascular systems by micropuncturing the hepatic
microvasculature under a biomicroscope, determined that blood
pressure at the distal end branches of the hepatic artery was
6–8-fold greater than that at the portal vein branches (300–400 vs.
50 mm H2O, respectively) (19). The ability of the portal vein to
carry large volumes of blood into the sinusoids at this low
pressure is, in part, due to the precapillary sphincter. It has
been demonstrated that the distal end branches of the hepatic
artery and the peribiliary capillary plexus around the bile ducts
exist in the form of capillaries, and that each capillary is
encircled by a precapillary sphincter. This sphincter adjusts the
blood flow from the arterioles into the capillary (20), thus controlling the volume of blood
entering the capillary from the high-pressure arterial system. The
pumping of the blood into the sinusoids by the low-pressure portal
vein system is therefore facilitated by the precapillary
sphincter.
The investigations in the present study demonstrated
that, during deep inspiration, when portal venous blood flow was
obstructed by an increase in the intra-abdominal pressure, the
reduction in hepatic parenchymal perfusion was immediately
counterbalanced by the hepatic artery. As a result of this
compensatory mechanism, the portal vein appeared to serve as a
drainage vessel for the hepatic artery, and changed the blood flow
to hepatofugal. The reduction in the portal venous blood flow may
have generated signals to stimulate the precapillary sphincter,
which then regulated hepatic parenchymal perfusion by activating an
alternative mechanism that enhanced hepatic arterial blood flow
into the sinusoids. During expiration, when intra-abdominal
pressure was reduced, blood flow through the low-pressure portal
vein into the liver parenchyma was facilitated. Consequently, the
precapillary sphincter regulated the hepatic arterial blood flow to
reestablish the dominance of the portal vein in the hepatic
hemodynamics. In the present study, it was possible to establish
how hepatic parenchymal perfusion was affected by acute changes in
the portal venous blood flow by examining a patient with
Budd-Chiari syndrome, who exhibited an inspiration-induced change
in the portal venous blood flow, from hepatopetal to hepatofugal.
At-PI was utilized as a simple and effective imaging tool for the
visualization of these changes in hepatic hemodynamics.
Due to the lack of valves, blood flow in the hepatic
portal vein is easily converted to hepatofugal blood flow, as the
pressure gradient of hepatic parenchymal perfusion is reversed by a
change in the hemodynamics. Comprehensive studies of hepatofugal
blood flow in the portal venous system, particularly in the main
portal vein, have demonstrated that the reversal of portal venous
blood flow is rare when the portosystemic shunt is naturally
occurring. It is possible that this is due to the fact that hepatic
blood flow is maintained by a mechanism that prevents portal venous
pressure from decreasing below sinusoidal pressure (21–29).
In the case of hepatofugal portal venous blood flow, the mechanism
that maintains hepatopetal blood flow may be impaired due to
obstructed hepatic outflow, a characteristic of Budd-Chiari
syndrome. This may be in addition to the increased sinusoidal
pressure that occurs as a result of a hepatocellular
carcinoma-induced hepatic arterial-portal venous shunt (23,27).
However, a study by Nishida et al indicated that the
conversion of hepatic blood flow from hepatofugal to hepatopetal
occurred following transcatheter arterial embolization therapy
(TAE) for hepatocellular carcinoma (30). This may have been a result of the
closure of the hepatic arterial-portal venous shunt due to the TAE,
leading to a reduction in the arterial blood flow and the
reestablishment of hepatopetal blood flow in the portal vein.
To the best of our knowledge, the present study is
the first in which respiration-induced reversible changes in
hepatic blood flow, from hepatopetal to hepatofugal, have been
investigated with the use of the At-PI technology. The results have
provided an insight into the effect of respiration on the
hemodynamic changes between the hepatic artery and portal vein.
We have previously used At-PI to investigate the
hepatic arterial dominance that occurs as a result of disease
progression in patients with chronic hepatitis C (7). The peribiliary capillary plexus has
been demonstrated to be important in the compensatory mechanism of
the hepatic artery that counteracts any reduction in portal venous
blood flow. In the present study, we revealed that the peribiliary
capillary plexus also regulated the acute reduction in portal
venous blood flow, in addition to the chronically progressive
reduction that is apparent in patients with hepatitis C.
Abbreviations:
|
At-PI
|
arrival time parametric imaging;
|
|
US
|
ultrasonography;
|
|
ROI
|
region of interest;
|
|
ROR
|
ratio of red
|
References
|
1.
|
Kleber G, Steudel N, Behrmann C, et al:
Hepatic arterial flow volume and reserve in patients with
cirrhosis: use of intra-arterial Doppler and adenosine infusion.
Gastroenterology. 116:906–914. 1999.PubMed/NCBI
|
|
2.
|
Rocheleau B, Ethier C, Houle R, Huet PM
and Bilodeau M: Hepatic artery buffer response following left
portal vein ligation: its role in liver tissue homeostasis. Am J
Physiol. 277:G1000–G1007. 1999.PubMed/NCBI
|
|
3.
|
Leen E, Goldberg JA, Anderson JR, et al:
Hepatic perfusion changes in patients with liver metastases:
comparison with those patients with cirrhosis. Gut. 34:554–557.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lautt WW: Mechanism and role of intrinsic
regulation of hepatic arterial blood flow: hepatic arteial buffer
response. Am J Physiol. 249:G549–G556. 1985.PubMed/NCBI
|
|
5.
|
Menon KV, Shah V and Kamath PS: The
Budd-Chiari syndrome. N Engl J Med. 350:578–585. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Iwao T, Toyonaga A, Oho K, et al: Value of
Doppler ultrasound parameters of portal vein and hepatic artery in
the diagnosis of cirrhosis and portal hypertension. Am J
Gastroenterol. 92:1012–1017. 1997.PubMed/NCBI
|
|
7.
|
Wakui N, Takayama R, Kanekawa T, et al:
Usefulness of arrival time parametric imaging in evaluating the
degree of liver disease progression in chronic hepatitis C
infection. J Ultrasound Med. 31:373–382. 2012.PubMed/NCBI
|
|
8.
|
Wakui N, Takayama R, Mimura T, et al:
Drinking status of heavy drinkers detected by arrival time
parametric imaging using Sonazoid-enhanced ultrasonography: study
of two cases. Case Rep Gastroenterol. 5:100–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wakui N, Takayama R, Matsukiyo Y, et al:
Visualization of segmental arterialization with arrival time
parametric imaging using Sonazoid-enhanced ultrasonography in
portal vein thrombosis: A case report. Exp Ther Med. 5:673–677.
2013.PubMed/NCBI
|
|
10.
|
Wakui N, Fujita M, Oba N, et al:
Endoscopic nasobiliary drainage improves jaundice attack symptoms
in benign recurrent intrahepatic cholestasis: A case report. Exp
Ther Med. 5:389–394. 2013.PubMed/NCBI
|
|
11.
|
Shunichi S, Hiroko I, Fuminori M and Waki
H: Definition of contrast enhancement phases of the liver using a
perfluoro-based microbubble agent, perflubutane microbubbles.
Ultrasound Med Biol. 35:1819–1827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Smith HJ, Grøttum P and Simonsen S:
Ultrasonic assessment of abdominal venous return. I Effect of
cardiac action and respiration on mean velocity pattern,
cross-sectional area and flow in the inferior vena cava and portal
vein. Acta Radiol Diagn (Stockh). 26:581–588. 1985.PubMed/NCBI
|
|
13.
|
Moreno AH, Burchell AR, Van der Woude R
and Burke JH: Respiratory regulation of splanchnic and systemic
venous return. Am J Physiol. 213:455–465. 1967.PubMed/NCBI
|
|
14.
|
Rabinovici N and Navot N: The relationship
between respiration, pressure and flow distribution in the vena
cava and portal and hepatic veins. Surg Gynecol Obstet.
151:753–763. 1980.PubMed/NCBI
|
|
15.
|
Sugano S, Yamamoto K, Sasao K and Watanabe
M: Portal venous blood flow while breath-holding after inspiration
or expiration and during normal respiration in controls and
cirrhotics. J Gastroenterol. 34:613–618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Rappaport AM, Black RG, Lucas CC, Ridout
JH and Best CH: Normal and pathologic microcirculation of the
living mammalian liver. Rev Int Hepatol. 16:813–828.
1966.PubMed/NCBI
|
|
17.
|
Rappaport AM: Hepatic blood flow:
morphologic aspects and physiologic regulation. Int Rev Physiol.
21:1–63. 1980.PubMed/NCBI
|
|
18.
|
Ekataksin W and Kaneda K: Liver
microvascular architecture: an insight into the pathophysiology of
portal hypertension. Semin Liver Dis. 19:359–382. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nakata K, Leong GF and Brauer RW: Direct
measurement of blood pressures in minute vessels of the liver. Am J
Physiol. 199:1181–1188. 1960.PubMed/NCBI
|
|
20.
|
Rhodin JA: The ultrastructure of mammalian
arterioles and precapillary sphincters. J Ultrastruct Res.
18:181–223. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tochio H, Kudo M, Nishiuma S and Okabe Y:
Intrahepatic spontaneous retrograde portal flow in patients with
cirrhosis of the liver: reversal by food intake. AJR Am J
Roentgenol. 177:1109–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kessler RE, Tice DA and Zimmon DS:
Retrograde flow of portal vein blood in patients with cirrhosis.
Radiology. 92:1038–1042. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Okuda K, Moriyama M, Yasumoto M, Jinnouchi
S and Shimokawa Y: Roentgenologic demonstration of spontaneous
reversal of portal blood flow in cirrhosis and primary carcinoma of
the liver. Am J Roentgenol Radium Ther Nucl Med. 119:419–428. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Foster DN, Herlinger H, Miloszewski KJ and
Losowsky MS: Hepatofugal portal blood flow in hepatic cirrhosis.
Ann Surg. 187:179–182. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Takayasu K, Takashi M, Musha H, et al:
Spontaneous reversal of portal blood flow demonstrated by
percutaneous transhepatic catheterization: report of two cases.
Gastroenterology. 82:753–757. 1982.
|
|
26.
|
Sarfeh IJ, Rypins EB, Conroy RM and Mason
GR: Portacaval H-graft: relationships of shunt diameter, portal
flow patterns and encephalopathy. Ann Surg. 197:422–426. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tanabe T, Tobe K, Koide N, et al: Blood
flow dynamics of the portal venous system in liver diseases studied
by the ultrasonic pulsed doppler method. Kanzo. 26:65–73. 1985.(In
Japanese).
|
|
28.
|
Ohnishi K, Saito M, Sato S, et al:
Direction of splenic venous flow assessed by pulsed Doppler
flowmetry in patients with a large splenorenal shunt. Relation to
spontaneous hepatic encephalopathy. Gastroenterology. 89:180–185.
1985.
|
|
29.
|
Kawasaki T, Moriyasu F, Nishida O, et al:
Analysis of hepatofugal flow in portal venous system using
ultrasonic Doppler duplex system. Nihon Shokakibyo Gakkai Zasshi.
84:1655–1660. 1987.(In Japanese).
|
|
30.
|
Nishida O, Nishikawa K, Seko S, et al: Two
cases of reverse portal blood flow: clinical course and changes in
hemodynamics. Journal of Medical Ultrasonics. 24:1663–1670.
1997.
|