Introduction
Functional dyspepsia (FD) is a functional syndrome
that originates in the gastroduodenal region (1). FD, one of the most prevalent
functional gastrointestinal disorders (FGIDs), has prevalence rates
of 11.0–29.2% worldwide (2) and
reduces quality of life significantly (3–5).
Although symptom criteria have been proposed for FD, there remain
cases where diagnosis is performed by a method of exclusion, owing
to a lack of diagnostic pathology (6,7).
Delayed gastric emptying, impaired proximal gastric accommodation
to a meal, gastric hypersensitivity to distension, abnormal
duodenojejunal motility, psychological disturbance and
Helicobactor pylori (H. pylori) infection have been
proposed to be associated with FD in several studies (8–12),
however, the pathogenesis of FD remains unclear to date.
Studies have demonstrated that the number of
duodenal mucosal eosinophils significantly increases in individuals
with FD compared with controls and the degree of eosinophilic
infiltration is also correlated with early satiety, which indicates
that changes in the number of duodenal eosinophils may be an
underlying feature of FD (13,14).
In addition, other studies identified that immune activation of
duodenal eosinophilia is closely associated with FD, while the
increase of gastric eosinophilic granulocytes is not associated
with FD (15,16). Eosinophils degranulate and release
a variety of substances following activation and help mast cells
release substances to stimulate neurons, which leads to contraction
of smooth muscle and results in symptoms, including abdominal pain
or meal-related symptoms (17).
The presence of increased numbers of gastrointestinal eosinophils
may serve as a useful biomarker of FD and these potent effector
cells may prove to be therapeutic targets in the treatment of these
diseases (13).
The role of H. pylori in FD remains a
controversy. One study indicated that the eradication of H.
pylori has modest but clear benefits for patients with FD
(18). Conversely, another
meta-analysis provided little support for the use of H.
pylori eradication therapy in patients with non-ulcer dyspepsia
(19).
Currently, treatment of FD remains challenging
(6,7). Observations from clinical practice
show that <60% of patients with FD had symptomatic improvement
following drug therapy, which is often incomplete (20). This is likely due to the fact that
FD is a heterogeneous disease. In general, the approach to treating
patients with FD based on their main symptom is practical and
effective. Proton-pump inhibitors (PPIs), prokinetics, H.
pylori eradication and antidepressant drugs are the common
choices for the treatment of FD (21).
Although previous studies have investigated the role
of H. pylori in FD, they did not focus on the correlation
between gastroduodenal eosinophil levels and H. pylori
clearance or drug therapy. The present study was designed to
evaluate H. pylori eradication treatment in the symptomatic
response of patients with FD and to determine whether H.
pylori eradication and drug therapy affect gastroduodenal
eosinophil numbers. In particular, we aimed to discover whether
there is a correlation between symptom improvement and the change
in gastroduodenal eosinophil numbers following treatment.
Patients and methods
Patients
Adult FD patients (aged 18–70 years) fulfilling Rome
III criteria were recruited into the study (22). Dyspepsia was defined as epigastric
pain, epigastric burning, postprandial fullness and early
satiation. All patients had no history of surgery and anaphylactic
disease and did not receive antacids, antibiotics, prokinetic drugs
or non-steroidal anti-inflammatory drugs during the 4 weeks before
the study. Liver and renal function tests, blood glucose,
electrolytes, abdominal B ultrasound and upper gastrointestinal
tract endoscopy examination were performed for all patients to
exclude metabolic and organic diseases. The subjects were required
to have no evidence of peptic ulcer disease or gastroesophageal
reflux disease with or without esophagitis, malignancy and
pancreaticobiliary disease. All subjects signed informed consent
forms prior to entering the study. Ethical approval for the study
was obtained from the Medical Ethics Committee of Qilu Hospital,
Shandong University.
Abdominal symptom questionnaire
The self-administered abdominal symptom
questionnaire assessed symptoms from the upper and lower part of
the abdomen over the preceding 3 months (13). A standardized procedure for the
administration of the questionnaire at three time points (week 0 as
baseline, initial diagnosis and gastroscopy; week 2, end of drug
therapy; and week 6, six weeks later after baseline) was conducted.
The questionnaire included the following abdominal symptoms:
epigastric pain, heartburn, early satiety, postprandial fullness,
belching, nausea, vomiting, retching, eructation, anorexia,
abdominal distension, epigastric discomfort (noisy), dysphagia and
retrosternal pain.
Patients were contacted by telephone and referral to
determine clinical symptoms at various time points and the clinical
symptoms were marked. In the 5-point Likert table (23), the degrees of the abdominal
symptoms (asymptomatic, mild, moderate, severe and very severe)
were recorded as 0, 1, 2, 3 and 4 points, respectively, and the
scores of four main symptoms (epigastric pain, epigastric burning,
postprandial fullness and early satiation) were accumulated for
each patient.
Gastroscopy, biopsy and the
14C-urea breath test
Gastroscopy and the 14C-urea breath test
(Young-heart Medical Appliance Equipment Co., Ltd., Tongcheng,
China) were performed for all patients at recruitment. Gastroscopy
of all patients was performed by two physicians who were unaware of
the symptoms of the subjects before and during endoscopy. At
endoscopy, biopsy specimens were collected from the following
sites: body (lesser curvature and middle of greater curvature),
antrum (lesser curvature and greater curvature), duodenal bulb (D1)
and descending part of the duodenum (D2); two specimens were
collected from each site. At week 6, certain patients were
reexamined by gastroscopy and biopsy and the H.
pylori-positive patients received the 14C-urea
breath test again.
H&E staining and silver staining
Biopsy specimens were fixed in formalin and
routinely processed into paraffin wax sections. Sections were cut
at 3 μm and stained with hematoxylin and eosin (H&E) and
Warthin-Starry stains. H. pylori was detected as either
positive or negative according to whether the bacteria was observed
by Warthin-Starry staining.
The specimens were assessed by two pathologists
blinded to the case-control status independently. For each subject,
eosinophil counts were obtained from body, antrum, D1 and D2 in
five high-power fields (HPF) selected randomly (magnification,
×40). The sum of eosinophils over the 5-field counts were then
calculated in each subject. The non-overlapping HPF eosinophil
count ≥10 was set as eosinophil cluster positive (13).
Treatment for FD
The patients were divided into a H.
pylori-positive and H. pylori-negative group according
to the results of Warthin-Starry staining and the 14C
urea breath test. In general, patients in the H.
pylori-positive group were positive in the Warthin-Starry
staining assay and 14C urea breath test and patients in
the H. pylori-negative group were negative in the two
examinations. Patients in the H. pylori-positive group
received H. pylori eradication treatment (a quadruple
therapy, including 20 mg esomeprazole magnesium tablets, 1 g
amoxicillin tablets, 0.5 g clarithromycin dispersible tablets and
0.6 g bismuth potassium citrate, each twice a day for 2 weeks).
After 6 weeks, the patients were divided into group A (H.
pylori-eradicated group) and group B (H.
pylori-uneradicated group) according to the clearance of H.
pylori confirmed by the 14C urea breath test.
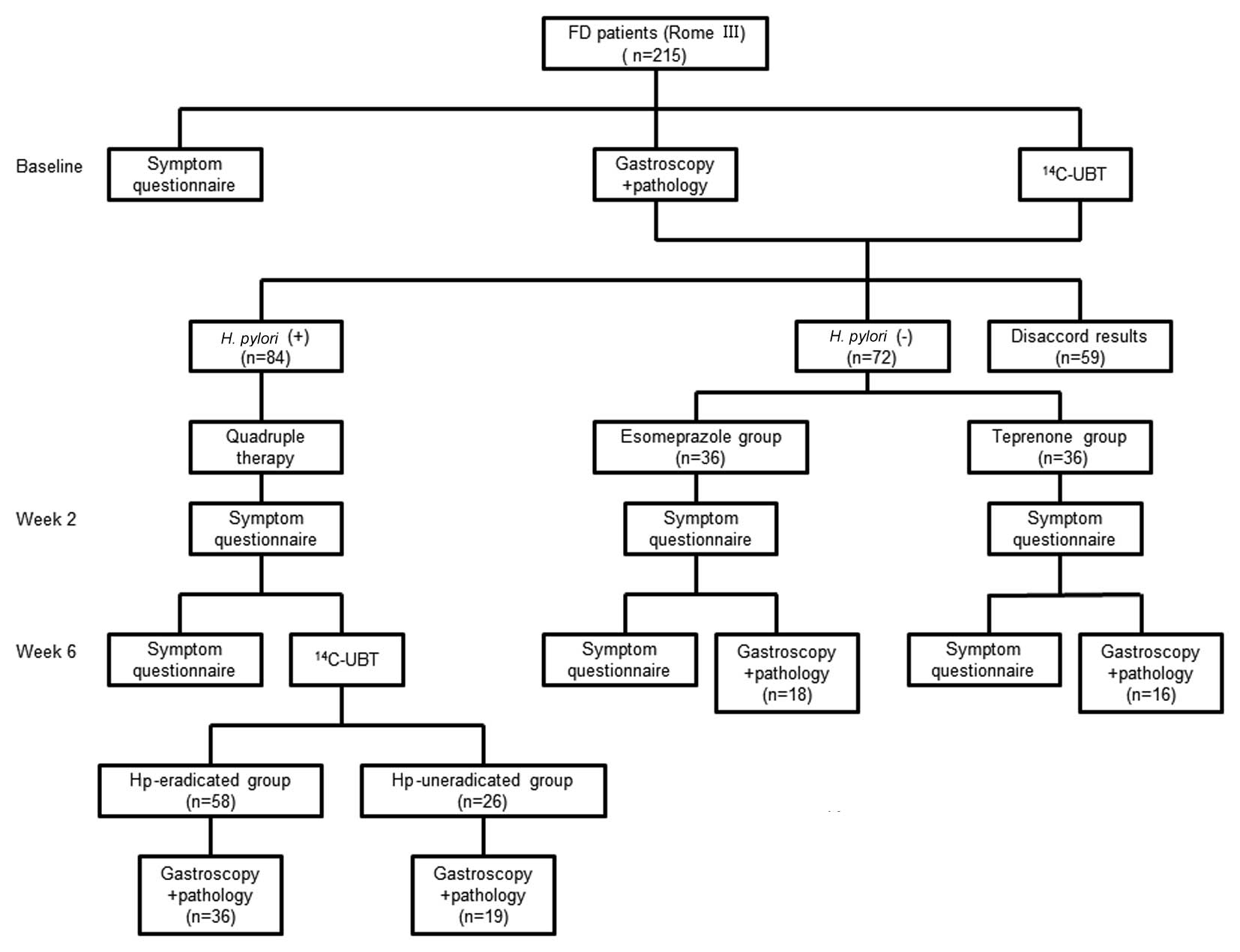
Subjects from the H. pylori-negative group
were randomly divided into two groups according to a random number
table. Group C received 20 mg esomeprazole twice a day
(esomeprazole group) and group D received 50 mg teprenone three
times a day (teprenone group) for two weeks (Fig. 1).
Statistical analysis
All data were analyzed by SPSS 20.0 statistical
software package (SPSS, Inc., Chicago, IL, USA) and expressed as
mean ± standard deviation (SD). The counts of eosinophils and
symptom scores were analyzed using single sample t-test among the
groups and each time point. The eosinophil cluster rate was
examined using χ2 analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient population
From March 2011 to August 2012, a total of 215
patients (mean age, 54.2 years, 64% female) were enrolled in the
study. Of these, 84 patients were positive in the Warthin-Starry
stain and 14C urea breath test; 72 patients were
negative in the Warthin-Starry stain and 14C urea breath
test; 19 patients were positive in the Warthin-Starry stain but
negative in the 14C-urea breath test; and 17 patients
were negative in the Warthin-Starry stain but positive in the
14C-urea breath test. Twelve patients were lost to
follow-up and 11 patients were excluded owing to adverse reactions,
including unbearable diarrhea and stomach discomfort (6 in the
H. pylori-positive group, 3 in the teprenone group and 2 in
the esomeprazole group). As shown in Fig. 1, the numbers of patients in group
A, B, C and D were 58, 26, 36 and 36, respectively, and the numbers
of subjects who received gastroscopy at week 6 were 36, 19, 18 and
16, respectively, in the four groups. In the H.
pylori-positive group, the successful H. pylori
eradication rate was 69.0% (58/84).
Upper gastrointestinal pathology
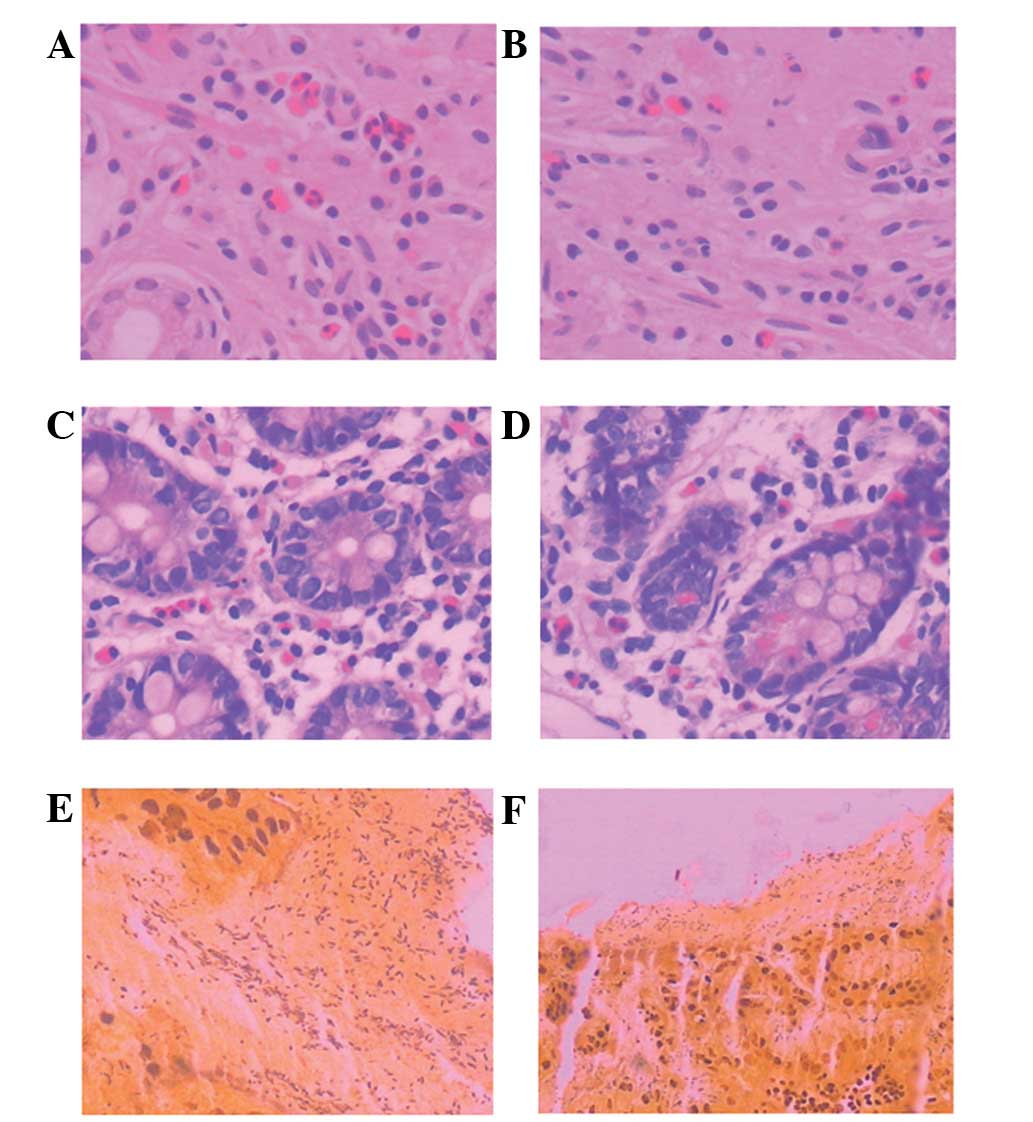
The pathology of the FD patients was analyzed by
H&E and Warthin-Starry staining of biopsy specimens. Histology
by H&E staining in the body and descending part of duodenum
(D2) of all patients revealed normal morphology or mild
inflammation (Fig. 2A–D). No
active duodenitis, visible intestinal parasites or cancer was
observed in all subjects. Gastric specimens from the H.
pylori-positive group processed by Warthin-Starry staining are
shown in Fig. 2E and F; H.
pylori was stained black while the background was light
golden.
H. pylori infection and gastrointestinal
symptoms
There was no significant difference in symptom
scores between the H. pylori-positive group and H.
pylori-negative group at baseline (Table I). Moreover, in the H.
pylori-positive group before and after eradication treatment,
the symptom scores of the H. pylori-eradicated group were
overall not significantly different compared with those of the
H. pylori-uneradicated group at baseline, week 2 and week 6
(Table II). These data suggest
that H. pylori infection is not associated with the symptoms
of FD and H. pylori eradication is not necessary when
treating this disorder.
 | Table I.Symptom scores and eosinophil counts
in FD patients at baseline. |
Table I.
Symptom scores and eosinophil counts
in FD patients at baseline.
| Group | Symptom scores | Eosinophil counts
(mean ± SD)
| Cluster rate of
eosinophil (%)
|
|---|
| Body | Antrum | D1 | D2 | Body | Antrum | D1 | D2 |
|---|
| Hp-positive
(n=84) | 4.48±1.91 | 26.33±18.8 | 20.35±16.2 | 31.62±15.2 | 28.83±15.6 | 32.14 | 20.24 | 40.48 | 38.10 |
| Hp-negative
(n=72) | 4.69±1.81 | 10.92±11.2 | 9.63±9.60 | 33.06±19.3 | 31.31±15.9 | 11.11 | 5.56 | 37.5 | 36.11 |
| P-valuea | 0.48 | 0.00 | 0.00 | 0.60 | 0.33 | 0.00 | 0.01 | 0.69 | 0.79 |
 | Table II.Symptom scores of the four groups
before and after treatment (mean ± SD). |
Table II.
Symptom scores of the four groups
before and after treatment (mean ± SD).
| Groups | Baseline | Week 2 | Week 6 |
|---|
| Hp-eradicated group
(n=58) | 5.21±1.42 | 1.93±1.33a | 0.71±0.75b,c |
| Hp-uneradicated
group (n=26) | 4.65±1.97 | 1.73±1.33a | 0.77±0.96b |
| P-valued | 0.14 | 0.53 | 0.76 |
| Esomeprazole group
(n=36) | 4.92±1.57 | 1.33±0.57a | 0.67±0.62b |
| Teprenone group
(n=36) | 4.53±1.87 | 1.67±1.15a | 3.67±3.21 |
| P-valuee | 0.34 | 0.12 | 0.00 |
Effect of drug therapy on symptom
improvement
To investigate the improvement of infection
following various treatments, symptom scores at baseline, week 2
and week 6 after treatment were analyzed. The symptom scores of the
four groups were all improved at week 2 after treatment compared
with baseline (Table II). The same
result was also obtained in the H. pylori-eradicated group,
H. pylori-uneradicated group and esomeprazole group between
baseline and week 6, but not in the teprenone group (Table II). At baseline, the symptom scores
of the esomeprazole group and the teprenone group were almost the
same; however, the symptom scores of the esomeprazole group were
significantly improved compared with those of the teprenone group
at week 6 (Table II).
H. pylori infection and gastroduodenal
eosinophil counts
At the baseline level, the eosinophil counts in the
body and antrum were significantly increased in the H.
pylori-positive group compared with the H.
pylori-negative group (Table
I, Fig. 2). However, in the
duodenum (including the duodenal bulb and descending part of the
duodenum), the H. pylori-positive group did not demonstrate
a marked increase in eosinophil count compared with the H.
pylori-negative group (Table
I). These results demonstrate that H. pylori infection
upregulates gastric eosinophil counts but has no effect on duodenal
eosinophil counts in patients with FD.
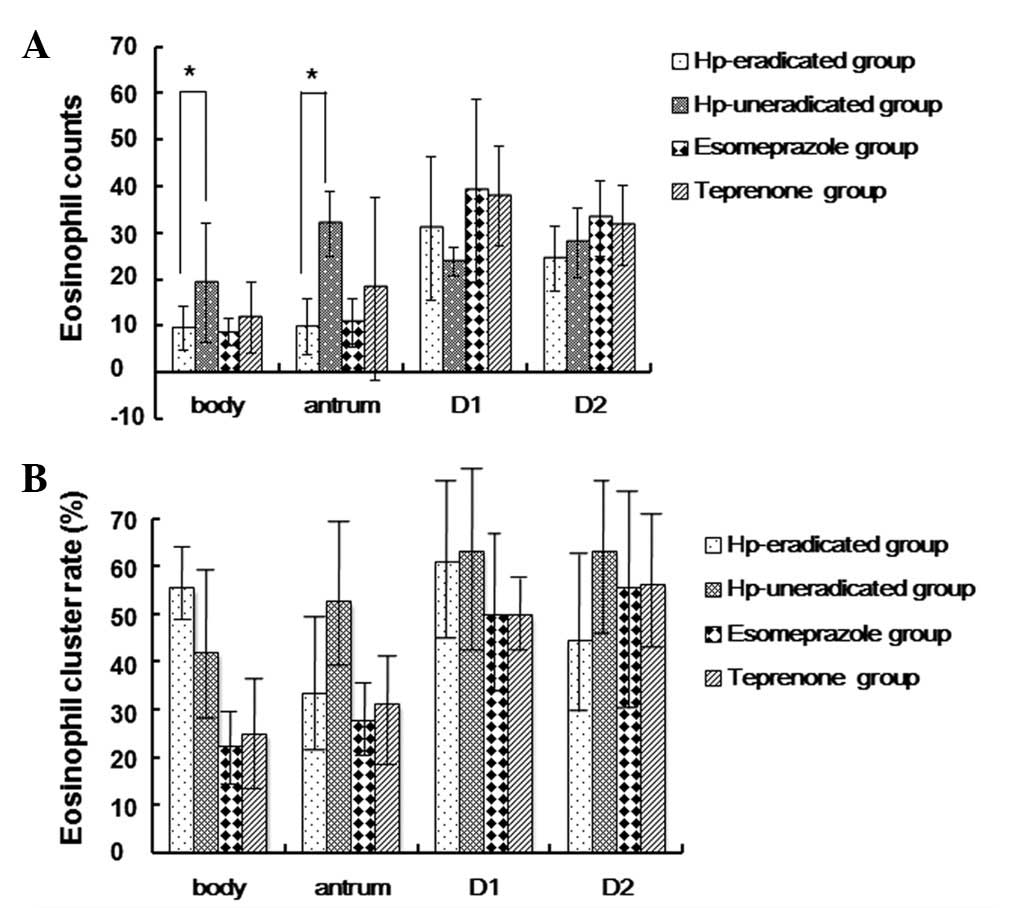
Effect of drug therapy on gastroduodenal
eosinophil counts
Next, we investigated whether the commonly used
drugs and H. pylori eradication treatment affect the
gastroduodenal eosinophil counts and whether there is a correlation
between symptom improvement and eosinophil counts. Compared with
the H. pylori-uneradicated group, eosinophil counts in the
antrum and body were significantly reduced in the H.
pylori-eradicated group at week 6 (Fig. 3). In the duodenal bulb and the
descending duodenum, the eosinophil counts of the H.
pylori-eradicated group were not significantly different from
those of the H. pylori-uneradicated groups at week 6.
The gastric and duodenal eosinophil counts of the
H. pylori-uneradicated group did not present a significant
reduction after week 6, while the gastric eosinophil counts of the
H. pylori-eradicated group were reduced at week 6 compared
with baseline; however, the duodenal eosinophil counts did not show
a marked change between the two time points (Table III).
 | Table III.Eosinophil counts and cluster rates
of the Hp-eradicated group (group A) and Hp-uneradicated group
(group B) at baseline and week 6. |
Table III.
Eosinophil counts and cluster rates
of the Hp-eradicated group (group A) and Hp-uneradicated group
(group B) at baseline and week 6.
| Eosinophil counts
(mean ± SD)
| Cluster rate of
eosinophils (%)
|
|---|
| Body | Antrum | D1 | D2 | Body | Antrum | D1 | D2 |
|---|
| Baseline of group A
(n=58) | 20.86±12.11 | 14.36±13.65 | 27.60±11.12 | 27.07±13.99 | 39.66 | 20.70 | 43.10 | 31.03 |
| Week 6 of group A
(n=36) | 9.36±4.78 | 9.78±6.02 | 30.92±15.47 | 24.36±7.08 | 55.56 | 33.33 | 61.11 | 44.44 |
| P-valuea | 0.00 | 0.06 | 0.23 | 0.28 | 0.13 | 0.17 | 0.09 | 0.19 |
| Baseline of group B
(n=26) | 25.00±19.57 | 23.65±16.63 | 28.65±14.03 | 29.08±9.03 | 34.62 | 38.46 | 57.69 | 57.69 |
| Week 6 of group B
(n=19) | 19.26±12.77 | 31.74±7.01 | 23.74±3.11 | 27.74±7.55 | 42.10 | 52.63 | 63.16 | 63.16 |
| P-valuea | 0.27 | 0.053 | 0.14 | 0.60 | 0.49 | 0.34 | 0.71 | 0.71 |
The eosinophil counts of the four gastroduodenal
sites in the esomeprazole group were not statistically different
from those of the teprenone group at week 6 (Fig. 3). Moreover, the gastric and
duodenal eosinophil counts of the esomeprazole and teprenone groups
were unchanged, respectively, between baseline and week 6 of
treatment (Table IV), indicating
that esomeprazole and teprenone are not effective for the treatment
of eosinophilia.
 | Table IV.Eosinophil counts and cluster rate of
the esomeprazole (group C) and teprenone groups (group D) at
baseline and week 6. |
Table IV.
Eosinophil counts and cluster rate of
the esomeprazole (group C) and teprenone groups (group D) at
baseline and week 6.
| Eosinophil counts
(mean ± SD)
| Cluster rate of
eosinophils (%)
|
|---|
| Body | Antrum | D1 | D2 | Body | Antrum | D1 | D2 |
|---|
| Baseline of group C
(n=36) | 7.78±7.66 | 7.61±5.00 | 30.83±12.56 | 31.47±14.21 | 19.44 | 33.33 | 50.00 | 63.89 |
| Week 6 of group C
(n=18) | 8.50±2.89 | 10.5±5.20 | 39.00±19.63 | 33.00±8.04 | 22.22 | 27.77 | 50.00 | 55.56 |
| P-valuea | 0.70 | 0.053 | 0.07 | 0.67 | 0.81 | 0.68 | 1.00 | 0.13 |
| Baseline of group D
(n=36) | 9.56±5.62 | 12.14±4.07 | 35.56±24.05 | 31.11±17.89 | 22.22 | 27.78 | 55.56 | 58.33 |
| Week 6 of group D
(n=16) | 11.69±7.63 | 18.00±19.64 | 37.81±10.74 | 31.50±8.60 | 25.00 | 31.25 | 50.00 | 56.25 |
| P-valuea | 0.27 | 0.09 | 0.72 | 0.99 | 0.83 | 0.80 | 0.71 | 0.89 |
Eosinophil clusters
At baseline, compared with the H.
pylori-negative group, the gastric eosinophil cluster rate was
significantly increased in the H. pylori-positive group,
whereas the duodenal eosinophil cluster rate of the H.
pylori-positive group was not significantly different from that
of the H. pylori-negative group (Table I). At week 6, the gastroduodenal
eosinophil cluster rates were not significantly different between
the H. pylori-eradicated and H. pylori-uneradicated
groups (Fig. 3), or between the
esomeprazole and teprenone groups (Table IV). The gastroduodenal eosinophil
cluster rates did not show significant differences before and after
treatment in the four groups (Tables
III and IV). These results
suggest that H. pylori infection upregulates gastroduodenal
eosinophil clustering and that the eosinophil clusters are not
reduced immediately after H. pylori eradication. Moreover,
gastroduodenal eosinophil clusters are not affected by esomeprazole
or teprenone treatment.
Discussion
FD is a heterogeneous disorder characterized by the
presence of recurrent or persistent symptoms originating in the
gastroduodenal region without any organic, systemic or metabolic
disease that is likely to explain the symptoms (24). The treatment of FD is based on the
relief of symptoms rather than the treatment of abnormal
pathophysiology (24–26).
In 2006, Rome III reformulated the FD classification
method, which, to date, is the most recognized definition of FD.
According to the Rome III criteria, FD must include one or more of
the following symptoms: bothersome postprandial fullness, early
satiation, epigastric pain and epigastric burning, with no evidence
of structural disease for at least 3 months, with symptom onset at
least 6 months before.
The role of H. pylori in FD is not fully
understood. Currently, eradication treatment of H. pylori in
FD continues to be controversial, even though a certain number of
patients with FD appear to benefit from H. pylori
eradication treatment (27). One
study identified a tendency toward greater symptomatic benefit with
H. pylori eradication therapy when compared with control
treatment in patients with FD, particularly in an area with a high
prevalence of infection (28). A
study assessed the clinical course of FD during a long follow-up
period of 7 years in a homogeneous sample of H.
pylori-eradicated patients, which demonstrated that only a
proportion of patients (10–50%) were symptom-free following
eradication and at each 12-month evaluation, whereas other patients
became symptomatic at different time points. FD symptoms were
slightly improved following H. pylori eradication over a
long period of time; however, a large percentage of these improved
patients may experience FD symptoms again, even after a number of
years of well-being after H. pylori eradication (29).
Our study identified that overall the symptom scores
were not significantly different between the H.
pylori-positive group and the H. pylori-negative group
and the symptom scores of the H. pylori-eradicated group
were not significantly different from those of the H.
pylori-uneradicated group at baseline, week 2 and week 6 of
treatment. These data suggest that H. pylori infection is
not associated with the symptoms of FD and H. pylori
eradication is not necessary when treating this disorder.
Eosinophils are effector cells that have
immunomodulatory effects by releasing cytokines and presenting
antigens. In animal models, eosinophils have been suggested to
cause gastrointestinal dysmotility and impaired gastric relaxation,
which lead to gastrointestinal symptoms, including abdominal pain
and bloating. However, its role in human gastrointestinal diseases
remains unclear (30). A striking,
statistically significant increase of eosinophils was identified in
H. pylori-infected gastric mucosa compared with H.
pylori-negative gastritis with similar activity (31). Talley et al identified that
gastric eosinophil numbers are greater in H. pylori-positive
subjects, implicating that the infection may upregulate eosinophils
(13). The concentration of
‘regulated on activation, normal T cell expressed and secreted’
(RANTES), a potent chemoattractant peptide for eosinophils, and the
numbers of RANTES-positive cells were evaluated in the gastric
mucosa from patients with H. pylori-positive chronic
gastritis before and after H. pylori eradication and from
H. pylori-negative healthy volunteers. The results revealed
that RANTES protein concentration and RANTES-positive cells were
significantly elevated in H. pylori-positive cases and
remained high immediately after H. pylori eradication. The
levels tended to decrease following H. pylori eradication
but did not reach the level of H. pylori-negative cases,
even at 24 months after H. pylori eradication. Therefore it
appears to be an important mechanism of prolonged gastric mucosal
immune response against H. pylori infection, even after
H. pylori eradication (32).
Studies focusing on the correlation between H.
pylori infection and duodenal eosinophilia are scarce. Talley
et al identified that H. pylori infection has no
significant relevance to duodenal eosinophilia (13). The current study identified that
eosinophil counts in the gastric body and antrum were significantly
increased in the H. pylori-positive group compared with the
H. pylori-negative group, while eosinophil numbers in the
duodenum were unchanged. The number of gastric eosinophil clusters
was affected in the same manner. A similar tendency was observed
between the H. pylori-eradicated and H.
pylori-uneradicated groups. The results demonstrated that H.
pylori infection upregulates gastric eosinophil counts but has
no effect on duodenal eosinophil counts in patients with FD.
Moreover, the pathophysiology of FD is considered to
be associated with duodenal motility disorders; however, its
pathogenesis has not been fully elucidated. A previous study
identified that the increased number eosinophils may be involved in
the pathogenesis of FD. Talley et al reported that
eosinophils in the upper gastrointestinal tract may be biomarkers
for non-ulcer dyspepsia. Increased duodenal eosinophils may be the
characteristic of a certain type of FD (13). It may be the case that eosinophils
are a key link of neuroimmunoregulation in FD rather than the
consequence.
The current therapy for FD is symptomatic and
largely ineffective (24–26). Empiric acid suppression with PPIs
is normally used in the treatment of FD. Generally, acid
suppressive therapy using PPIs appears effective in patients whose
predominant symptoms are epigastric pain or burning sensation
(33,34). The role of acid and the presence of
duodenal hypersensitivity to acid in FD patients remains
controversial. Samsom et al reported that duodenal acid
infusion induced nausea in a subset of FD patients but not in
healthy controls, suggesting the presence of duodenal
hypersensitivity to acid in FD patients (35). Duodenal acid perfusion (0.2 mol/l,
5 ml/min) for 15 min significantly exacerbated symptoms, including
discomfort, bloating, nausea and epigastric burning in healthy
subjects (36). In another study,
no significant differences in dyspeptic symptom scores were
observed before and after duodenal acid infusion and between
duodenal saline and acid infusion (37).
Increased spontaneous duodenal acid exposure has
also been identified in FD patients (38). One study demonstrated that reduced
duodenal acid clearance plays a role in increasing duodenal acid
exposure in FD patients (39).
However, the mechanism remains unknown. The prolonged duodenal
exposure to acid appears to be responsible for the generation of
dyspeptic symptoms through the induction of gastric motor and
sensory dysfunction (30).
H. pylori eradication in FD benefits a
minority of cases but is worthwhile as the response may be
maintained. Certain prokinetics may also be effective in the
treatment of FD; patients with meal-related symptoms have the best
response. Antidepressant therapy may also have a place in the
management of difficult cases; however, adequate randomized
controlled trials are unavailable (21). According to the study by Talley
et al, targeted drugs that inhibit eosinophil production or
eosinophil-derived products, including leukotriene-receptor
antagonists, humanized monoclonal antibody against inter-leukin-5
and histamine 1 and 2 antagonists, may be effective for the
treatment of FD (13).
In the current study, we identified that H.
pylori infection increases gastric eosinophil counts, which may
be induced by the upregulation of RANTES. The symptoms of FD
improved following treatment with either esomeprazole or teprenone;
however, no corresponding changes in duodenal eosinophil counts
were observed. Moreover, our results demonstrated that symptom
improvement resulted from treatment with esomeprazole but was not
related to the clearance of H. pylori. In the duodenal bulb
and the descending duodenum, the eosinophil counts of the H.
pylori-eradicated group were not significantly different from
those of the H. pylori-uneradicated group at week 6,
suggesting that symptom improvement was also not associated with
gastroduodenal eosinophil numbers.
In our study, esomeprazole, an acid suppressive
drug, provided significant relief of symptoms in FD patients. The
effect of esomeprazole on patients with FD may result from reduced
duodenal acid exposure and corresponding reductions in sensitivity
to gastric distension, impaired fundic accommodation to a meal and
slow gastric emptying.
The commonly used therapies for gastrointestinal
disorders in China are PPIs, domperidone and teprenone. Teprenone
is often used for gastritis and gastric ulcers and its
pharmacological effects include anti-ulcer effects, increase of
gastric mucus, improvement of gastric mucosal blood flow,
protection of the gastric mucosa and induction of heat shock
protein genesis. Studies on the effect of teprenone on FD are rare.
Previously published data revealed that teprenone tended to improve
only gastric stasis (GSS) and that only 52% of patients treated
with teprenone favored their medication (40).
In the current study, teprenone was used for the
treatment of H. pylori-negative patients with FD and their
symptoms improved at week 2 compared with those at baseline, but
not at week 6. The mechanism remains unknown, however, we
hypothesize that the improvement of gastric mucosal blood flow and
increase of gastric mucus may help to reduce the gastroduodenal
sensitivity to stimulus. The protective effect is, however, limited
and temporary. Esomeprazole was shown to be more effective than
teprenone in the treatment of FD.
As the follow-up period of the present study was
relatively short and the FD patients were not divided into subtypes
of postprandial distress syndrome (PDS) and epigastric pain
syndrome (EPS), further studies are required to investigate the
mechanisms involved. The results demonstrated that duodenal
eosinophil number is not associated with H. pylori infection
or the esomeprazole or teprenone treatment of FD. Moreover,
esomeprazole appears to be more effective than teprenone and H.
pylori eradication is not necessary in the treatment of FD. The
effect of a PPI on FD observed in the current study suggest that
hypersensitivity to acid and/or increased duodenal acid exposure
are associated with the pathophysiology of FD.
References
|
1.
|
Geeraerts B and Tack J: Functional
dyspepsia: past, present, and future. J Gastroenterol. 43:251–255.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mahadeva S and Goh KL: Epidemiology of
functional dyspepsia: a global perspective. World J Gastroenterol.
12:2661–2666. 2006.PubMed/NCBI
|
|
3.
|
Talley NJ, Weaver AL and Zinsmeister AR:
Impact of functional dyspepsia on quality of life. Dig Dis Sci.
40:584–589. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chang L: Review article: epidemiology and
quality of life in functional gastrointestinal disorders. Aliment
Pharmacol Ther. 7:31–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Talley NJ: Functional gastrointestinal
disorders as a public health problem. Neurogastroenterol Motil.
1:121–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Powell N, Walker MM and Talley NJ:
Gastrointestinal eosinophils in health, disease and functional
disorders. Nat Rev Gastroenterol Hepatol. 7:146–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Speirs RS, Speirs EE and Ponzio NM: A role
for eosinophils in adaptive humoral immunity. Open Immunol J.
2:168–186. 2009. View Article : Google Scholar
|
|
8.
|
Stanghellini V, Tosetti C, Paternico A,
Barbara G, Morselli-Labate AM, Monetti N, Marengo M and Corinaldesi
R: Risk indicators of delayed gastric emptying of solids in
patients with functional dyspepsia. Gastroenterology.
110:1036–1042. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Sarnelli G, Caenepeel P, Geypens B,
Janssens J and Tack J: Symptoms associated with impaired gastric
emptying of solids and liquids in functional dyspepsia. Am J
Gastroenterol. 98:783–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Tack J, Piessevaux H, Coulie B, Caenepeel
P and Janssens J: Role of impaired gastric accommodation to a meal
in functional dyspepsia. Gastroenterology. 115:1346–1352. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tack J, Caenepeel P, Fischler B,
Piessevaux H and Janssens J: Symptoms associated with
hypersensitivity to gastric distention in functional dyspepsia.
Gastroenterology. 121:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Haug TT, Svebak S, Wilhelmsen I, Berstad A
and Ursin H: Psychological factors and somatic symptoms in
functional dyspepsia. A comparison with duodenal ulcer and healthy
controls. J Psychosom Res. 38:281–291. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Talley NJ, Walker MM, Aro P, Ronkainen J,
Storskrubb T, Hindley LA, Harmsen WS, Zinsmeister AR and Agréus L:
Non-ulcer dyspepsia and duodenal eosinophilia. An adult endoscopic
population-based case-control study. Clin Gastroenterol Hepatol.
5:1175–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Rothenberg ME and Cohen MB: An eosinophil
hypothesis for functional dyspepsia. Clin Gastroenterol Hepatol.
5:1147–1148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gargala G, Lecleire S, François A, et al:
Duodenal intraepithelial T lymphocytes in patients with functional
dyspepsia. World J Gastroenterol. 13:2333–2338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Walker MM, Talley NJ, Prabhakar M,
Pennaneac’h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS,
Zinsmeister AR and Agreus L: Duodenal mastocytosis, eosinophilia
and intraepithelial lymphocytosis as possible disease markers in
the irritable bowel syndrome and functional dyspepsia. Aliment
Pharmacol Ther. 29:765–773. 2009. View Article : Google Scholar
|
|
17.
|
Bischoff SC: Physiological and
pathophysiological functions of intestinal mast cells. Semin
Immunopathol. 31:185–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Moayyedi P, Soo S, Deeks J, et al:
Eradication of Helicobacter pylori for non-ulcer dyspepsia.
Cochrane Database Sys Rev. 1:CD 0020962006.PubMed/NCBI
|
|
19.
|
Laine L, Schoenfeld P and Fennerty MB:
Therapy for Helicobacter pylori in patients with nonulcer
dyspepsia. A meta-analysis of randomized, controlled trials. Ann
Intern Med. 134:361–369. 2001.
|
|
20.
|
Mönkemüller K and Malfertheiner P: Drug
treatment of functional dyspepsia. World J Gastroenterol.
12:2694–2700. 2006.
|
|
21.
|
McNally MA and Talley NJ: Current
treatments in functional dyspepsia. Curr Treat Options
Gastroenterol. 10:157–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mazzoleni LE, Sander GB, Francesconi CF,
et al: Helicobacter pylori eradication in functional
dyspepsia: HEROES trial. Arch Intern Med. 171:1929–1936. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
von Arnim U, Peitz U, Vinson B, Gundermann
KJ and Malfertheiner P: STW 5, a phytopharmacon for patients with
functional dyspepsia: results of a multicenter, placebo-controlled
double-blind study. Am J Gastroenterol. 102:1268–1275.
2007.PubMed/NCBI
|
|
24.
|
Tack J, Talley NJ, Camilleri M, Holtmann
G, Hu P, Malagelada JR and Stanghellini V: Functional
gastroduodenal disorders. Gastroenterology. 130:1466–1479. 2006.
View Article : Google Scholar
|
|
25.
|
Talley N, Vakil NB and Moayyedi P:
American gastroenterological association technical review on the
evaluation of dyspepsia. Gastroenterology. 129:1756–1780. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Talley NJ and Vakil N: Practice Parameters
Committee of the American College of Gastroenterology: Guidelines
for the management of dyspepsia. Am J Gastroenterol. 100:2324–2337.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Laheij RJ, van Rossum LG, Verbeek AL and
Jansen JB: Helicobacter pylori infection treatment of
nonulcer dyspepsia: an analysis of meta-analyses. J Clin
Gastroenterol. 36:315–320. 2003. View Article : Google Scholar
|
|
28.
|
de Artaza Varasa T, Valle Muñoz J,
Pérez-Grueso MJ, García Vela A, Martín Escobedo R, Rodríguez Merlo
R, Cuena Boy R and Carrobles Jiménez JM: Effect of Helicobacter
pylori eradication on patients with functional dyspepsia. Rev
Esp Enferm Dig. 100:532–539. 2008.(In Spanish).
|
|
29.
|
di Mario F, Stefani N, Bò ND, Rugge M,
Pilotto A, Cavestro GM, Cavallaro LG, Franzé A and Leandro G:
Natural course of functional dyspepsia after Helicobacter
pylori eradication: a seven-year survey. Dig Dis Sci.
50:2286–2295. 2000.PubMed/NCBI
|
|
30.
|
Lee KJ and Tack J: Duodenal implications
in the pathophysiology of functional dyspepsia. J
Neurogastroenterol Motil. 16:251–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Berczi L, Tamássy K, Fekete B and Kopper
L: Eosinophils and mast cells in Helicobacter pylori
infected gastric mucosa. Pathol Oncol Res. 2:229–236. 1996.
|
|
32.
|
Kikuchi T, Kato K, Ohara S, Sekine H,
Arikawa T, Suzuki T, Noguchi K, Saito M, Saito Y, Nagura H, Toyota
T and Shimosegawa T: The relationship between persistent secretion
of RANTES and residual infiltration of eosinophils and memory T
lymphocytes after Helicobacter pylori eradication. J Pathol.
192:243–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Collen MJ and Loebenberg MJ: Basal gastric
acid secretion in nonulcer dyspepsia with or without duodenitis.
Dig Dis Sci. 34:246–250. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
George AA, Tsuchiyose M and Dooley CP:
Sensitivity of the gastric mucosa to acid and duodenal contents in
patients with nonulcer dyspepsia. Gastroenterology. 101:3–6.
1991.PubMed/NCBI
|
|
35.
|
Samsom M, Verhagen MA, vanBerge Henegouwen
GP and Smout AJ: Abnormal clearance of exogenous acid and increased
acid sensitivity of the proximal duodenum in dyspeptic patients.
Gastroenterology. 116:515–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
di Stefano M, Vos R, Vanuytsel T, Janssens
J and Tack J: Prolonged duodenal acid perfusion and dyspeptic
symptom occurrence in healthy volunteers. Neurogastroenterol Motil.
21:e712–e740. 2009.PubMed/NCBI
|
|
37.
|
Lee KJ, Demarchi B, Demedts I, Sifrim D,
Raeymaekers P and Tack J: A pilot study on duodenal acid exposure
and its relationship to symptoms in functional dyspepsia with
prominent nausea. Am J Gastroenterol. 99:1765–1773. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Bratten J and Jones MP: Prolonged
recording of duodenal acid exposure in patients with functional
dyspepsia and controls using a radiotelemetry pH monitoring system.
J Clin Gastroenterol. 43:527–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Samsom M, Verhagen MA, van Berge
Henegouwen GP and Smout AJ: Abnormal clearance of exogenous acid
and increased acid sensitivity of the proximal duodenum in
dyspeptic patients. Gastroenterology. 116:515–520. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Hongo M, Harasawa S, Mine T, Sasaki I,
Matsueda K, Kusano M, Hanyu N, Nakada K and Shibata C: Large-scale
randomized clinical study on functional dyspepsia treatment with
mosapride or teprenone: Japan Mosapride Mega-Study (JMMS). J
Gastroenterol Hepatol. 27:62–68. 2012. View Article : Google Scholar : PubMed/NCBI
|