Introduction
Clinially, pain resulting from surgical trauma
(postoperative pain) is a critical challenge for perioperative
management (1,2). Current pharmacological treatments of
postoperative pain include the use of opioids, non-steroidal
anti-inflammatory drugs (NSAIDs) and other drugs, including
tramadol and ketamine. NSAIDs exert their analgesic effects through
the inhibition of cyclooxygenase (COX), a rate-limiting enzyme that
catalyzes the conversion of arachidonic acid to prostaglandins
(PGs). COX is composed of two isoforms, COX-1 and COX-2, which are
constitutively expressed in the spinal cord. In clinial practice,
COX-2 inhibitors are widely used for postoperative pain control,
since they have a similar analgesic effect to NSAIDs without the
gastrointestinal side-effects and antiplatelet effects (3,4).
Systemic delivery of the COX-2 inhibitor parecoxib attenuates the
pain score and reduces the consumption of morphine in patients
undergoing surgery (5). It is
considered that COX-2 inhibitors produce analgesic effects by
blocking peripheral sensitization through the inhibition of the
production of COX and prostaglandin E2 (PGE2) in the local
inflammatory tissue. However, it is unknown whether central
sensitization, in particular spinal sensitization, is also involved
in the analgesic effect of COX-2 inhibitors.
In inflammatory pain, spinal sensitization plays an
important role in the analgesic effect of COX-2. In complete
Freund’s adjuvant-induced inflammatory pain, COX-2 is significantly
upregulated in the spinal cord (6). In addition, intrathecal delivery of
selective COX-2, but not COX-1 inhibitors dramatically reduces the
mechanical allodynia and thermal hyperalgesia in various types of
inflammatory pain (6,7). In contrast to inflammatory pain,
COX-2 expression in the spinal cord is only mildly upregulated in
response to surgical incision. Intrathecal delivery of a COX-2
inhibitor has only minimal effects on postoperative pain
hypersensitivity (8). These
experimental studies suggest that spinal COX-2 may not play an
important role in surgical pain. However, a clinical study
demonstrated that COX-2 inhibitor administration reduces the visual
analog scale pain score and the consumption of opioid drugs in
patients postoperatively (9). The
analgesic effect of COX-2 in postoperative pain may be associated
with the reduction of PGE2 levels in the cerebrospinal fluid (CSF)
or local tissue (10). The results
of the experimental and clinical studies strongly suggest that the
systemic delivery of COX-2 inhibitors produces an analgesic effect
through an indirect spinal mechanism.
Extracellular signal-regulated kinase (ERK) in the
spinal cord has been implicated in pain processing. In neuropathic
and inflammatory pain, activation of ERK in the spinal cord was
observed and inhibiting the activation of ERK markedly reduced the
pain behavior (11,12). Our previous study demonstrated that
phosphorylated (p)-ERK in the spinal cord is also transiently
activated following hind paw incision (13). The activation of p-ERK reached a
peak level at 5 min after incision and returned to the baseline at
10 min post-incision. Brushing the incised skin at a later time
(>10 min after incision) re-activated the expression of p-ERK.
Intrathecal delivery of an ERK inhibitor prior to incision, but not
post-incision, greatly attenuated pain hypersensitivity in response
to the incision (13). These
findings suggest that spinal ERK signaling contributes to surgical
pain.
The present study thus investigated whether spinal
ERK signaling is involved in the analgesic effect of parecoxib, a
selective COX-2 inhibitor, on surgical pain. The present study
aimed to elucidate the mechanism of the analgesic effect of COX-2
inhibitors on postoperative pain.
Materials and methods
Animals
Adult male Sprague-Dawley rats (150–250 g) obtained
from Central South University Animal Services (Changsha, China)
were used in the present study. All rats were maintained in an
air-conditioned (23–26°C, 60–70% relative humidity) vivarium with a
12 h dark/light cycle (light from 8:00 a.m. to 8:00 p.m.). The
experimental protocol complied with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals and was
approved by the Animal Care and Use Committee of Central South
University. All efforts were undertaken to minimize the suffering
of the rats.
Surgical preparation and groups
The surgical pain model was established by hind paw
incision in the rats. A detailed description of this model in rats
has been described in a previous study (14). Briefly, under anesthesia with 1.5%
sevoflurane, a 1-cm longitudinal incision was made into the planta
skin and deepened to the plantaris muscle. The muscle was then
elevated and incised longitudinally (0.5 cm). Then, the skin was
closed with 4-0 nylon sutures. A topical triple antibiotic ointment
was applied to the hind paw following surgery. Sham surgery was
performed under the same procedure with the exception of the
incision.
The rats were randomly divided into three groups:
parecoxib pretreatment group, parecoxib post-treatment group and
saline group (control group). For the parecoxib groups, parecoxib
(6 mg/kg; Pharmacia and Upjohn Co., Boston, MA, USA) was
intraperitoneally (i.p) injected 20 min before incision (parecoxib
pretreatment group) or 20 min after incision (parecoxib
post-treatment group), respectively. For the control group, 0.9%
saline was injected i.p. For behavior experiments, nocifensive
testing was performed prior to incision and 5 min, 10 min, 1 h, 6
h, 1 day and 3 days after incision in the different groups of rats
(n=10 for each group with parecoxib pretreatment or post-treatment,
n=8 for the saline control group). For immunohistochemical
experiments, rats in the control or parecoxib pretreatment groups
were sacrificed 5 min after incision. In an independent experiment,
rats in the control or parecoxib pretreatment groups 10 min after
incision were subjected to brushing of the incised skin followed by
immunohistochemical studies.
Immunohistochemistry
Rats were deeply anesthetized with chloral hydrate
(80 mg/kg) and perfused transcardially with 100 ml
phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in
0.1 M phosphate buffer. L4–L5 spinal cord segments were fixed for 4
h with 4% paraformaldehyde and then immersed in 20% sucrose in
phosphate buffer (pH 7.4) overnight. Transverse spinal cord
sections (30 μm) were cut and processed for
immunohistochemistry using the ABC method. In brief, sections were
mounted on (3-aminopropyl)triethoxysilane-coated slides and
incubated with mouse anti-p-ERK antibody (dilution 1:1000; Cell
Signaling Technology, Danvers, MA, USA) at room temperature
overnight. The secondary reagents used for localization were
biotinylated goat anti-mouse IgG and an ABC kit (Vector
Laboratories Inc., Burlingame, CA, USA). Diaminobenzidine (DAB)
tetrahydrochloride (Sigma, St. Louis, MO, USA) was used as a
peroxidase substrate.
Nociceptive testing
Mechanical allodynia was assayed by measuring the
paw withdrawal threshold (PWT) using nylon von Frey filaments
(15). In brief, rats were placed
on wire mesh platforms in clear cylindrical plastic enclosures.
Then, von Frey filaments (0.4–15.1 g) were applied to the wound
edge of the incised hind paw or the center of the plantar surface
of the unincised paw. According to the up-down method, the test was
consecutive (15). In the absence
of a paw withdrawal response, a stronger stimulus was applied;
otherwise a weaker stimulus was used. Testing proceeded in this
manner until four fibers had been applied after the first one that
caused a withdrawal response, allowing an estimation of the
PWT.
Quantification and statistical
analysis
Eight non-adjacent sections from each specimen of
L4–L5 lumbar spinal cord were randomly selected and the expression
of p-ERK was determined by counting the positive cells on the L4–L5
spinal superficial dorsal horn (lamina I and II). The investigator
during data collection was blind to the treatment that the animals
had received. SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) and Prism
5.0 (Graphpad Software Inc., San Diego, CA, USA) were used for
statistical analysis. Data are presented as the mean ± standard
error of the mean (SEM). Differences between groups were compared
with one-way analysis of variance (ANOVA) followed by Dunnett’s
post hoc test or Tukey’s post hoc multiple comparison test, where
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Parecoxib pretreatment attenuates
incision-evoked pain hypersensitivity
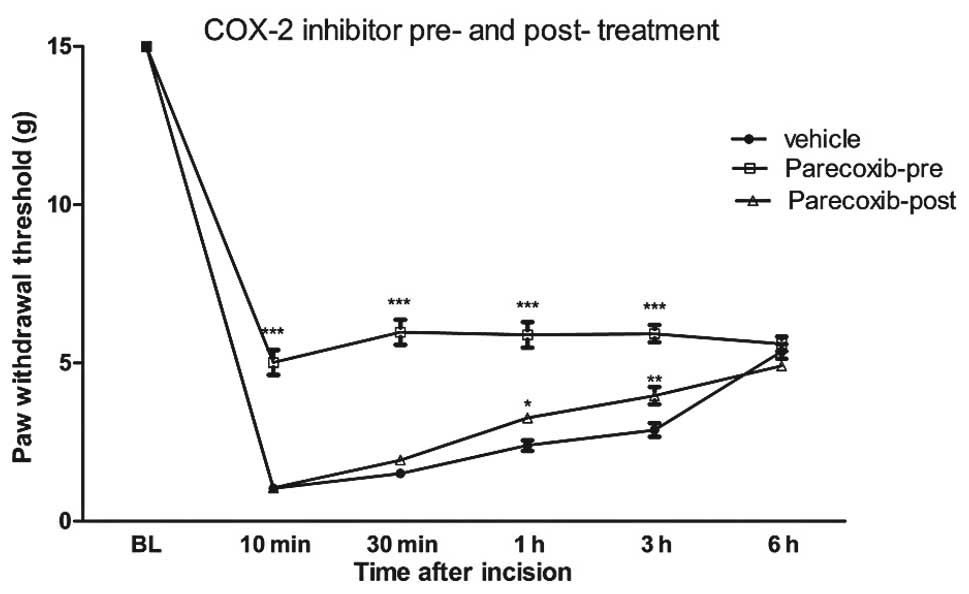
Previous studies have clearly shown that hind-paw
incision markedly reduces the PWT for >3 days (14,16).
The PWT is significantly reduced >6 h after surgical incision as
shown in Fig. 1. I.p injection of
6 mg/kg parecoxib 30 min prior to surgery significantly inhibits
the reduction in the PWT in response to incision. The analgesic
effect of parecoxib is maintained for 3 h. At 6 h after incision,
the PWT in the parecoxib pretreatment group was similar to those in
the control and parecoxib post-treatment groups. However,
parecoxib, when delivered 20 min after incision, has only a minimal
attenuating effect on mechanical hypersensitivity following
incision. These findings suggest that pretreatment, but not
post-treatment with parecoxib attenuates pain hypersensitivity
induced by incision.
Effect of parecoxib pretreatment on p-ERK
expression following surgical incision
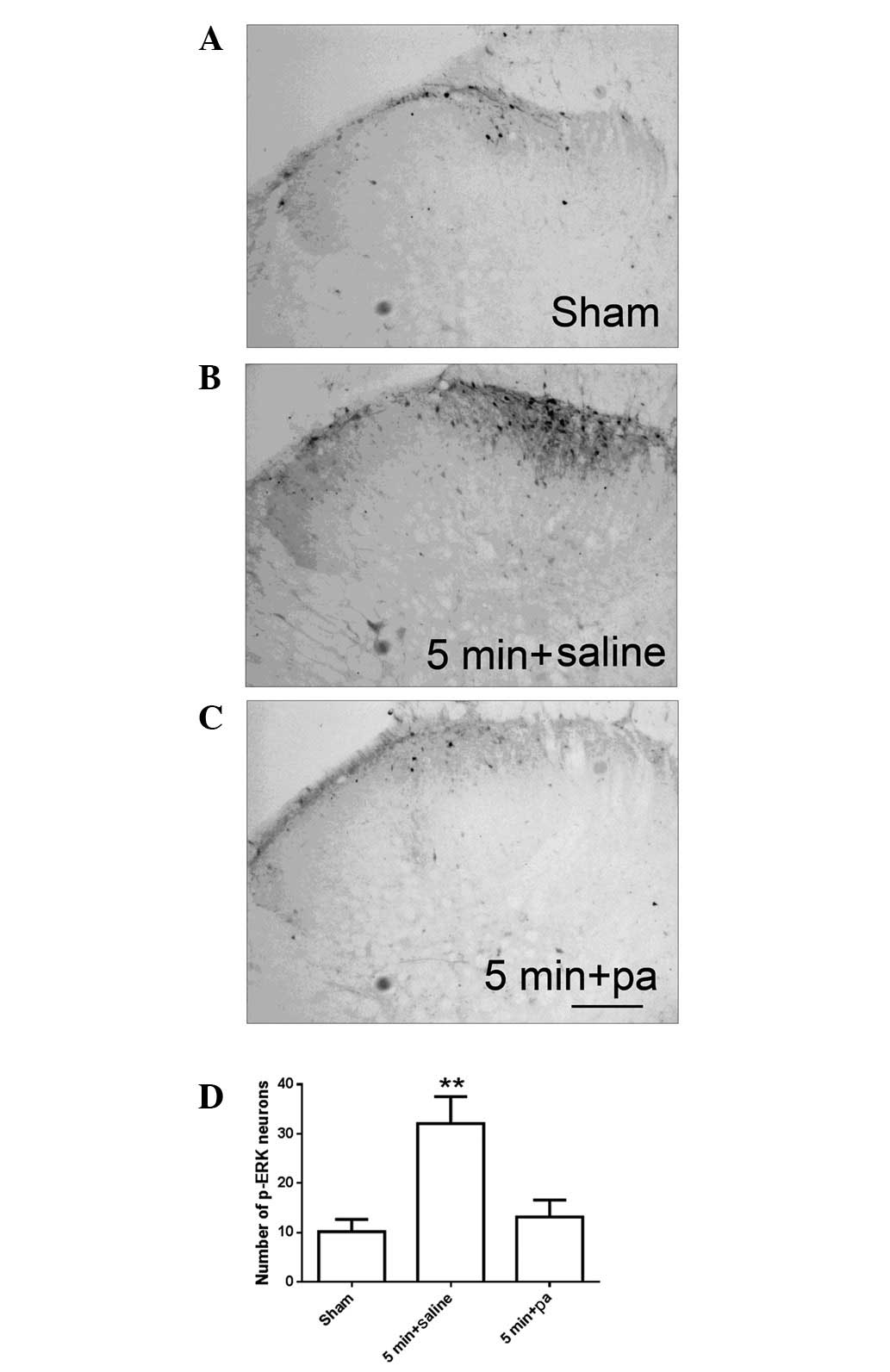
Our previous study demonstrated that p-ERK
expression is increased following hind paw incision. The increased
p-ERK expression was observed 1 min after incision, reaching a peak
level 5 min after incision and returning to the baseline 10 min
after incision and thereafter (13). As shown in Fig. 2, at 5 min after incision, increased
p-ERK immunoreactivity (Fig. 2B)
was detected as compared with the sham surgery group (Fig. 2A). Pretreatment with parecoxib
significantly inhibited the increase in the number of p-ERK neurons
exhibited at 5 min after incision (Fig. 2C and D).
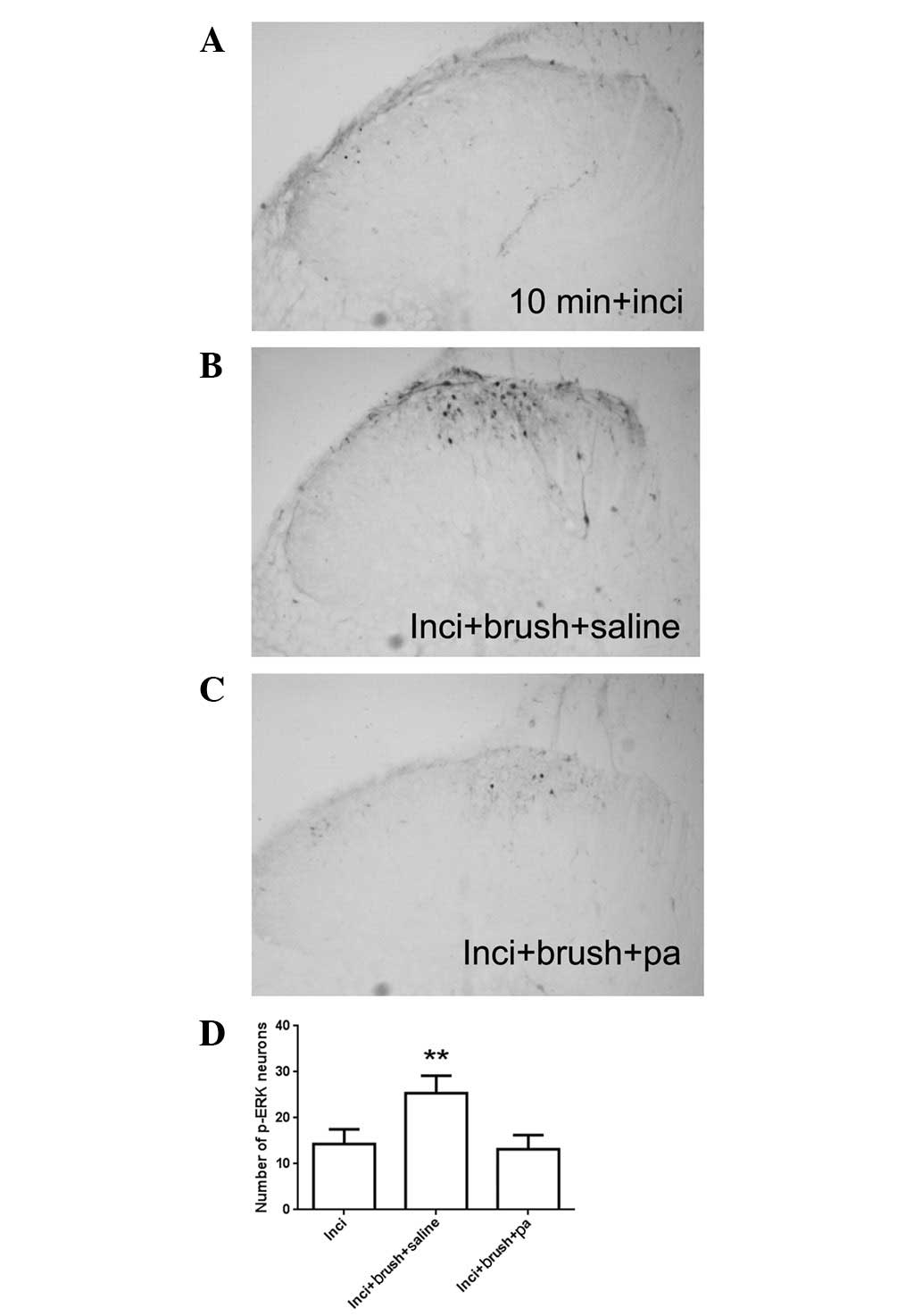
Our previous study also identified that at 10 min
after incision, when the increased p-ERK expression had returned to
baseline, brushing the incised skin re-activated p-ERK expression
(13). Confirming our previous
results, at 10 min after incision, the p-ERK expression was minimal
(Fig. 3A). However, intense
staining of p-ERK immunoreactivity was observed in the saline group
subjected to brushing at 10 min after incision (Fig. 3B). The re-activated p-ERK
expression in the spinal cord is response to brushing was also
significantly inhibited by parecoxib pretreatment (Fig. 3C and D). These findings suggest
that COX-2 regulates incisional pain through ERK signaling in the
spinal cord, at least partially.
Discussion
A number of studies have shown that systemic
administration of a COX-2 inhibitor attenuates postoperative pain
and is widely used for pain control following surgery. Experimental
and clinical studies have shown that COX-2 inhibitors inhibit the
production of PG in the local tissue. The latter in turn elicits
sensitization of peripheral nociceptor terminals and pain
hypersensitivity (10,17,18).
Despite the fact that peripheral sensitization plays important
roles in the analgesic effect of COX-2 inhibitors, whether spinal
sensitization is involved in the analgesic mechanism of COX-2
inhibitors remains to be determined. Although clinical studies have
shown that COX-2 inhibitors also reduce the level of PGE2 in the
cerebrospinal fluid (CSF) of patients undergoing vascular surgery,
the reduced PGE2 level in the CSF may be the global effect of the
reduced production of local PGE2 (10,17).
In the present study, pretreatment with the COX-2
inhibitor parecoxib was demonstrated to significantly inhibit the
activation of spinal ERK and attenuate mechanical hypersensitivity
following hind paw incision. These findings suggest that the COX-2
inhibitor may exert its analgesic effect through the inhibition of
spinal ERK1/2 signaling. Supporting this hypothesis, parecoxib also
suppresses the re-activation of spinal ERK in response to brushing
following incision. It is well known that ERK in the spinal cord
dorsal horn neurons are involved in the induction and maintenance
of neural plasticity, including peripheral sensitization and
central sensitization (19,20).
Only Aδ- or C-fiber stimulation or noxious peripheral stimuli
(thermal or mechanical) activate ERK in the dorsal horn, which
encodes stimulus intensity (21).
The inhibition of spinal ERK by parecoxib suggests that spinal
sensitization may also play an important role in the analgesic
effects of COX-2 inhibitors (22).
Intrathecal delivery of a COX-2 inhibitor has only marginal
analgesic effects (8). The present
study indicates that the inhibition of spinal ERK by systemic
administration of a COX-2 inhibitor may be due to the local effect
of the COX-2 inhibitor. In this scenario, the COX-2 inhibitor
reduces the production of PGE2 at local inflammatory sites, which
in turn reduces the sensitization of the nociceptive nerve fibers.
Therefore, the noxious stimuli projecting to the spinal cord
superficial dorsal horn neurons is also reduced. These findings
together indicate that COX-2 inhibitors exert their analgesic
effects through indirect inhibition of spinal sensitization.
In the present study, pretreatment, but not
post-treatment with parecoxib produced potent analgesic effects.
This finding suggests that pretreatment with a COX-2 inhibitor may
provide improved postoperative pain relief, supporting the idea of
preventive analgesia clinically. Extensive studies have shown that
delivery of analgesia prior to surgical trauma provides improved
postoperative pain control (23–25).
It is considered that analgesia delivered prior to injury prevents
the immediate and long-term effects of noxious operative afferent
input, which may induce peripheral and central sensitization and
then promote the development of postoperative pain (23–25).
However, once peripheral or central sensitization occurs as a
response to injury, it may not be totally reversed by analgesia. In
the present study, post-treatment of parecoxib had no effect on the
transient activation of p-ERK in the spinal cord dorsal horns. The
transient activation of p-ERK may subsequently activate multiple
downstream pain mediators, which the COX-2 inhibitor may not be
able to inhibit. Thus, the findings in the present study strongly
support the theory that preventive analgesia is an ideal approach
for postoperative pain control.
In conclusion, the present study demonstrated that
pretreatment, but not post-treatment with a COX-2 inhibitor
significantly attenuates incision-evoked pain hypersensitivity. The
COX-2 inhibitor parecoxib suppresses the transient activation of
spinal ERK and the reactivation of ERK in response to brushing
post-incision, suggesting that COX-2 may regulate incisional pain
through spinal ERK signaling.
Acknowledgements
The present study was supported by the
National Natural Science Foundation of China (NSFC, Grant No.
81070897) with a grant to R-P Dai.
References
|
1.
|
Costantini R, Affaitati G, Fabrizio A and
Giamberardino MA: Controlling pain in the post-operative setting.
Int J Clin Pharmacol Ther. 49:116–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wu CL and Raja SN: Treatment of acute
postoperative pain. Lancet. 377:2215–2225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lloyd R, Derry S, Moore RA and McQuay HJ:
Intravenous or intramuscular parecoxib for acute postoperative pain
in adults. Cochrane Database Syst Rev. 2:CD0047712009.PubMed/NCBI
|
|
4.
|
Myles PS and Power I: Clinical update:
postoperative analgesia. Lancet. 369:810–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Langford RM, Joshi GP, Gan TJ, Mattera MS,
Chen WH, Revicki DA, Chen C and Zlateva G: Reduction in
opioid-related adverse events and improvement in function with
parecoxib followed by valdecoxib treatment after non-cardiac
surgery: a randomized, double-blind, placebo-controlled,
parallel-group trial. Clin Drug Investig. 29:577–590. 2009.
View Article : Google Scholar
|
|
6.
|
Samad TA, Moore KA, Sapirstein A, Billet
S, Allchorne A, Poole S and Woolf CJ: Interleukin-1β-mediated
induction of Cox-2 in the CNS contributes to inflammatory pain
hypersensitivity. Nature. 410:471–475. 2001.
|
|
7.
|
Yaksh TL, Dirig DM, Conway CM, Svensson C,
Luo ZD and Isakson PC: The acute antihyperalgesic action of
nonsteroidal, anti-inflammatory drugs and release of spinal
prostaglandin E2 is mediated by the inhibition of constitutive
spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci.
21:5847–5853. 2001.PubMed/NCBI
|
|
8.
|
Zhu X, Conklin DR and Eisenach JC:
Preoperative inhibition of cyclooxygenase-1 in the spinal cord
reduces postoperative pain. Anesth Analg. 100:1390–1393. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
McCrory C and Fitzgerald D: Spinal
prostaglandin formation and pain perception following thoracotomy:
a role for cyclooxygenase-2. Chest. 125:1321–1327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Reuben SS, Buvanendran A, Kroin JS and
Steinberg RB: Postoperative modulation of central nervous system
prostaglandin E2 by cyclooxygenase inhibitors after vascular
surgery. Anesthesiology. 104:411–416. 2006. View Article : Google Scholar
|
|
11.
|
Ji RR, Befort K, Brenner GJ and Woolf CJ:
ERK MAP kinase activation in superficial spinal cord neurons
induces prodynorphin and NK-1 upregulation and contributes to
persistent inflammatory pain hypersensitivity. J Neurosci.
22:478–485. 2002.PubMed/NCBI
|
|
12.
|
Zhuang ZY, Gerner P, Woolf CJ and Ji RR:
ERK is sequentially activated in neurons, microglia, and astrocytes
by spinal nerve ligation and contributes to mechanical allodynia in
this neuropathic pain model. Pain. 114:149–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shi XD, Fu D, Xu JM, Zhang YL and Dai RP:
Activation of spinal ERK1/2 contributes to mechanical allodynia in
rat model of postoperative pain. Mol Med Rep. 7:1661–1665.
2013.PubMed/NCBI
|
|
14.
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Li CQ, Xu JM, Liu D, Zhang JY and Dai RP:
Brain derived neurotrophic factor (BDNF) contributes to the pain
hypersensitivity following surgical incision in the rats. Mol Pain.
4:272008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kroin JS, Buvanendran A, Watts DE, Saha C
and Tuman KJ: Upregulation of cerebrospinal fluid and peripheral
prostaglandin E2 in a rat postoperative pain model. Anesth Analg.
103:334–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pham-Marcou TA, Beloeil H, Sun X, Gentili
M, Yaici D, Benoit G, Benhamou D and Mazoit JX: Antinociceptive
effect of resveratrol in carrageenan-evoked hyperalgesia in rats:
prolonged effect related to COX-2 expression impairment. Pain.
140:274–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Obata K and Noguchi K: MAPK activation in
nociceptive neurons and pain hypersensitivity. Life Sci.
74:2643–2653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bhave G and Gereau RW IV:
Posttranslational mechanisms of peripheral sensitization. J
Neurobiol. 61:88–106. 2004. View Article : Google Scholar
|
|
21.
|
Obata K, Yamanaka H, Kobayashi K, Dai Y,
Mizushima T, Katsura H, Fukuoka T, Tokunaga A and Noguchi K: Role
of mitogen-activated protein kinase activation in injured and
intact primary afferent neurons for mechanical and heat
hypersensitivity after spinal nerve ligation. J Neurosci.
24:10211–10222. 2004. View Article : Google Scholar
|
|
22.
|
Ji RR, Baba H, Brenner GJ and Woolf CJ:
Nociceptive-specific activation of ERK in spinal neurons
contributes to pain hypersensitivity. Nat Neurosci. 2:1114–1119.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Pogatzki-Zahn EM and Zahn PK: From
preemptive to preventive analgesia. Curr Opin Anaesthesiol.
19:551–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Katz J, Clarke H and Seltzer Z: Review
article: Preventive analgesia: quo vadimus? Anesth Analg.
113:1242–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dahl JB and Kehlet H: Preventive
analgesia. Curr Opin Anaesthesiol. 24:331–338. 2011. View Article : Google Scholar : PubMed/NCBI
|