Introduction
Macro- and micro-vascular complications are the
leading causes of high morbidity and mortality in patients with
diabetes (1). A causal factor
leading to the pathophysiological alterations in the diabetic
vasculature is the chronic exposure to advanced glycation end
products (AGEs), which result from non-enzymatic glycation and
glycoxidation of proteins and lipids when they interact with aldose
sugars for an extended period of time (2–4). A
study demonstrated that the presence and accumulation of AGEs in
endothelial cells affect cell structure and function, thus
contributing to the pathophysiology of vascular diseases in
diabetes (5). Soluble AGEs
activate monocytes, and AGEs in the basement membrane inhibit
monocyte migration (5). The
migration and adhesion of monocytes to the subendothelial space,
mediated by the interaction between monocytes and molecules
expressed on the endothelial cell surface (6–8), are
partly regulated by adhesion and chemotactic factors, including
vascular cell adhesion molecule-1 (VCAM-1) and monocyte
chemoattractant protein-1 (MCP-1) (9,10).
The expression of VCAM-1 and MCP-1 is mainly mediated by activation
of nuclear factor κB (NF-κB) (2,11),
which is activated by reactive oxygen species (ROS) (5,12).
Previously, we identified that high glucose levels
increase the expression of VCAM-1 and MCP-1 through activation of
the RhoA/Rho-associated protein kinase (ROCK) pathway (13). The RhoA/ROCK signaling pathway
regulates a wide range of fundamental cellular functions, including
cellular apoptosis, metabolism, migration and adhesion (13–17).
Regulation of these cellular functions is mainly dependent on the
activation of its downstream effector, ROCK (14). There is increasing evidence
supporting the hypothesis that ROCK is an important component of
signaling pathways involved in the regulation of the inflammatory
response (17–20). For example, ROCK has been
implicated in the modulation of cell adhesion (13). It has been reported that ROCK
inhibition by Y-27632, fasudil or the overexpression of
dominant-negative mutants of ROCK, reduces cell adhesion through a
loss of focal adhesion complexes and reduced expression of adhesion
molecules, including VCAM-1, MCP-1, intracellular adhesion molecule
1 (ICAM-1) and endothelial leukocyte adhesion molecule 1 (13,19,20).
In addition, activation of NF-κB is also associated with ROCK
(20,21). However, it is unknown whether ROCK
inhibition attenuates AGE-induced monocyte-endothelial adhesion
through reduction of ROS and downregulation of NF-κB.
Therefore, in the present study, we aimed to
investigate the involvement of the Rho/ROCK pathway and NF-κB
signaling in the pathogenesis of AGE-induced endothelial injury and
the usefulness of fasudil to prevent the disorder in
vitro.
Materials and methods
Preparation of AGEs
AGEs were prepared by the incubation of 50 mg/ml
human serum albumin with 1 M glucose in phosphate-buffered saline
(PBS; pH 7.4) in the presence of 1.5 mM phenylmethylsulfonyl
fluoride (PMSF), 1 mM ethylenediaminetetraacetic acid (EDTA), 100
μg/ml penicillin and 40 μg/ml gentamicin for at least
12 weeks in the dark at 37°C under sterile conditions as previously
described (22). Following
incubation, unreacted sugar was removed prior to extensive dialysis
against PBS. Then, the solution was separated into aliquots and
stored at −20°C before use.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from Clonetics Cell Discovery Systems (San Diego, CA,
USA) and routinely propagated as described previously (13). The human leukemic monocytic cell
line THP-1 was obtained from the Institute of Biochemistry and Cell
Biology (Shanghai, China). Cells were maintained in RPMI-1640
medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 2 mM
L-glutamine and 10% fetal bovine serum.
Following detachment of 90% confluent HUVECs from
flasks with 0.025% trypsin, the cells that were derived between the
third and tenth passages were plated in 6-well plates. Based on the
concentrations of AGEs and fasudil indicated previously (13,23),
HUVECs were then stimulated with AGEs or AGEs + fasudil (Tianjin
Red Sun Pharmaceutical Co., Ltd., Tianjin, China; 2 ml, 3 mg) for
24 h as follows: i) without any treatment (control); ii) with 200
μg/ml AGEs; iii) with 400 μg/ml AGEs; iv) with AGEs
(400 μg/ml) + fasudil (1 nM); and v) with AGEs (400
μg/ml) + fasudil (10 nM). In other experiments, HUVECs were
also treated with AGEs (400 μg/ml) or AGEs (400
μg/ml) + fasudil (10 nM) for various times (0, 6, 12, 24 and
48 h). Total RNA and proteins were extracted for further study.
Analysis of monocyte-endothelial
adhesion
Cell adhesion was performed as described previously
(19). HUVECs were grown in 6-well
plates and pretreated with various concentrations of AGEs or AGEs +
fasudil for 12 or 24 h. THP-1 cells passaged regularly were labeled
with 10 μg/ml 2′,7′-bis
(carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester
(BCECF/AM; Sigma, St. Louis, MO, USA) at a final concentration of
10 μM in RPMI-1640 medium for 1 h at 37°C. Then, labeled
THP-1 cells were added (1×106 cells/ml) to mono-layers
of HUVECs in 6-well plates. When the cells had been incubated for 1
h, a number of bound cells were assayed by fluorescence excitation
(488 nm) and emission (535 nm) using fluorescence microscopy (Leica
DMI6000, Leica, Wetzlar, Germany) and the number of bound monocytes
was calculated by fluorescence intensity. Three fields were
captured for each experimental condition and experiments were
performed at least three times.
RNA extraction and quantitative reverse
transciption-polymerase chain reaction (RT-PCR)
Total RNA was isolated from HUVECs with TRIzol. RNA
(1 μg) was used for first-strand cDNA synthesis with
PrimeScript® RT reagent kit (Takara Bio Inc., Otsu,
Japan). The mRNA expression was determined by real-time
quantitative RT-PCR using the ABI Prism 7900HT (Applied Biosystems,
Foster City, CA, USA). Quantitative data of relative gene
expression was calculated by the comparative Ct method
(ΔΔCt) with SYBR® Premix Ex Taq™ (Takara Bio
Inc.) as described previously (13). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was the endogenous control gene.
Protein extraction and western blot
analysis
HUVECs were lysed with ice-cold RIPA and centrifuged
at 9,300 × g for 5 min at 4°C. Supernatants were collected and then
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). Western blot analysis was performed
using standard methods, with antibodies raised against VCAM-1
(Santa Cruz Biotechnology, Santa Cruz, CA, USA), MCP-1, RhoA,
ROCK1, phosphorylated myosin phosphatase target protein 1
(p-MYPT1), phospho-Ser32 inhibitor of NF-κB (IκB) and β-actin (Cell
Signaling Technology, Danvers, MA, USA).
Dual-luciferase reporter assay
The luciferase assay was performed as described
previously (24,25). Cells were seeded in 6-well plates
for 24 h overnight and transfected with NF-κB promoter-luciferase
constructs (Promega, Madison, WI, USA) using Lipofectamine 2000
transfection reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). After serum starvation for 24 h, transfected cells were
treated with AGEs or with AGEs + fasudil for 24 h as follows: i)
without any treatment (control); ii) with 200 μg/ml AGEs;
iii) with 400 μg/ml AGEs; iv) with AGEs (400 μg/ml) +
fasudil (1 nM); and v) with AGEs (400 μg/ml) + fasudil (10
nM). Firefly and Renilla luciferase activities in cell extracts
were measured using a Dual-Luciferase Reporter Assay system
(Promega). The relative activity was calculated by normalizing
NF-κB promoter-driven firefly luciferase activity to control
Renilla luciferase activity.
Determination of intracellular ROS
Assay for ROS was performed by measuring superoxide
anion (O2−) release into the supernatant from
HUVECs. The detection was based on its ability to cause superoxide
dismutase-inhibitable reduction of ferricytochrome c
(26). Cells were seeded at a
density of 1–3×106 cells/ml in 96-well plates overnight,
followed by treatment with serum-free media for 24 h.
Ferricytochrome c (Sigma) was added to a final concentration
of 200 μM at room temperature in the presence or absence of
150 U/ml superoxide dismutase (Sigma). HUVECs were pretreated with
various concentrations of fasudil for 2 h, followed by exposure to
AGEs for 10 min. Reduction of ferricytochrome c in the
supernatant was measured by reading the absorbance at 550 nm in a
spectrophotometer. The amount of O2− release
was calculated by dividing the difference in absorbance of the
samples, with and without superoxide dismutase. The results are
expressed in nmol/ml.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). Comparison between groups was performed using one-way
analysis of variance (ANOVA), followed by Bonferroni multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed with SPSS for Windows 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
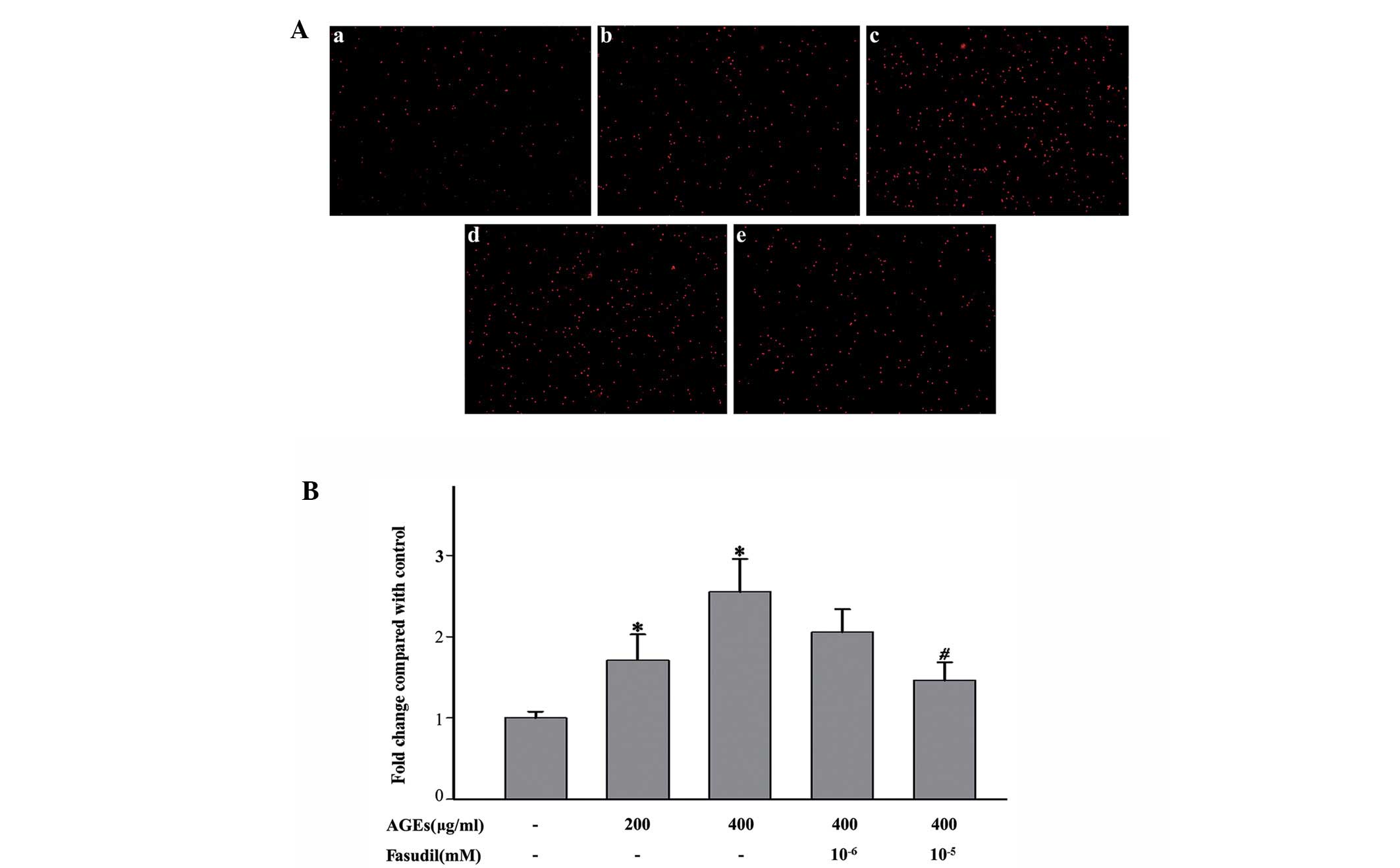
Fasudil inhibits the AGE-induced cell
adhesion in vitro
The effect of fasudil on cell adhesion was evaluated
with BCECF/AM-labeled monocytes. Incubation with AGEs for 12 h
significantly increased the adhesion of THP-1 cells to HUVECs
compared with the control group (Fig.
1; incubation with 200 μg/ml and 400 μg/ml AGEs
caused 1.7- and 2.5-fold changes, respectively; both P<0.05).
Additionally, we assessed the effect of fasudil on AGE-induced cell
adhesion. HUVECs treated with fasudil resulted in a suppression of
cell adhesion (treatment with 1 and 10 nM fasudil reduced cell
adhesion by ∼16 and 43%; P<0.10 and P<0.05, respectively;
Fig. 1). Similar results were
observed with 24 h incubation (data not shown).
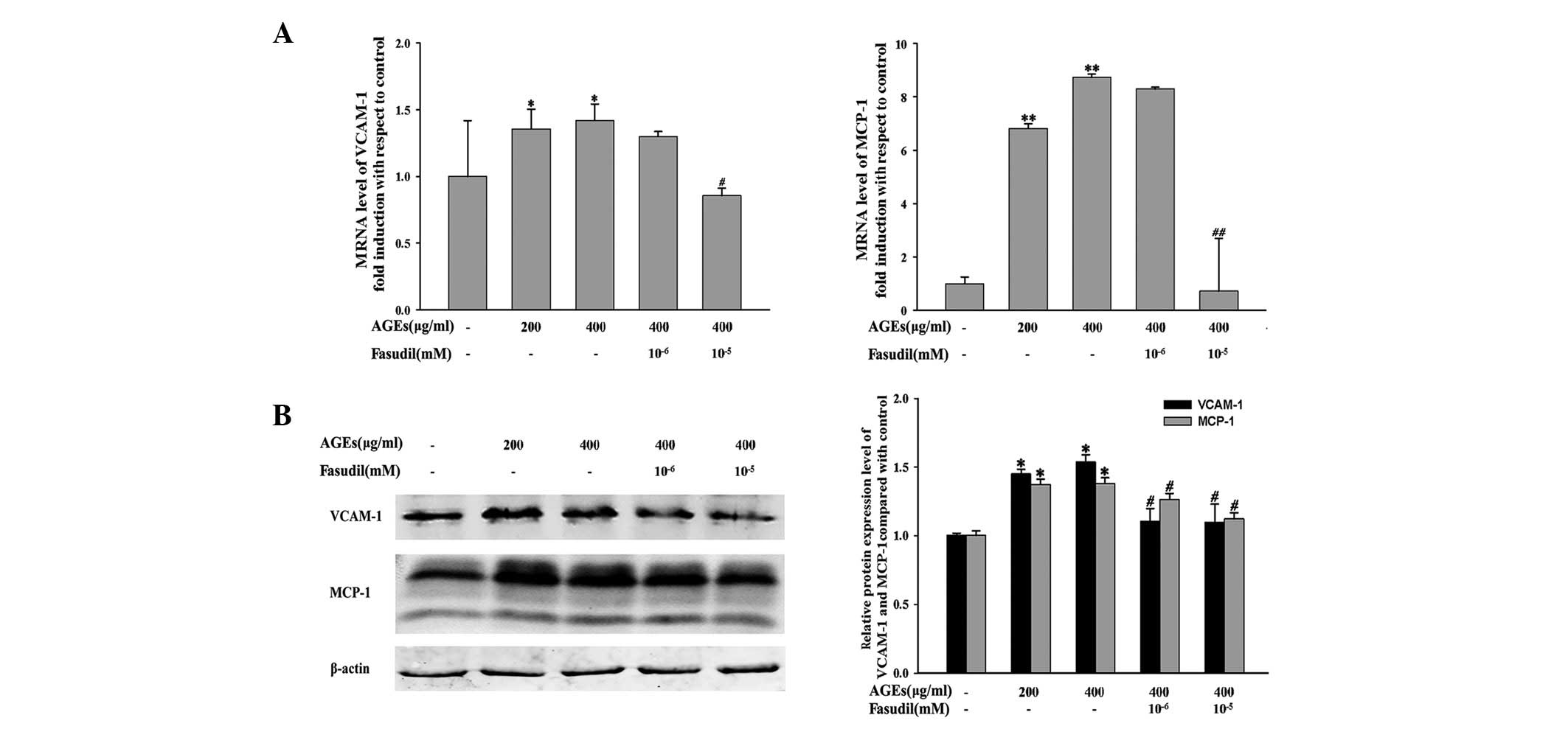
Effects of fasudil on the expression of
VCAM-1 and MCP-1
To elucidate the underlying mechanism of
monocyte-endothelial adhesion, we determined the effects of AGEs
and fasudil on the expression of VCAM-1 and MCP-1. As shown in
Fig. 2, HUVECs incubated with AGEs
(200 or 400 μg/ml) for 24 h demonstrated increased mRNA
(Fig. 2A) and protein (Fig. 2B) expression levels of VCAM-1 and
MCP-1, particularly MCP-1. Fasudil (10 or 1 nM) attenuates the
expression of adhesion and chemotactic factors. There were no
significance differences in the HUVECs incubated with fasudil at a
low dose (1 nM), suggesting that fasudil at a high dose had greater
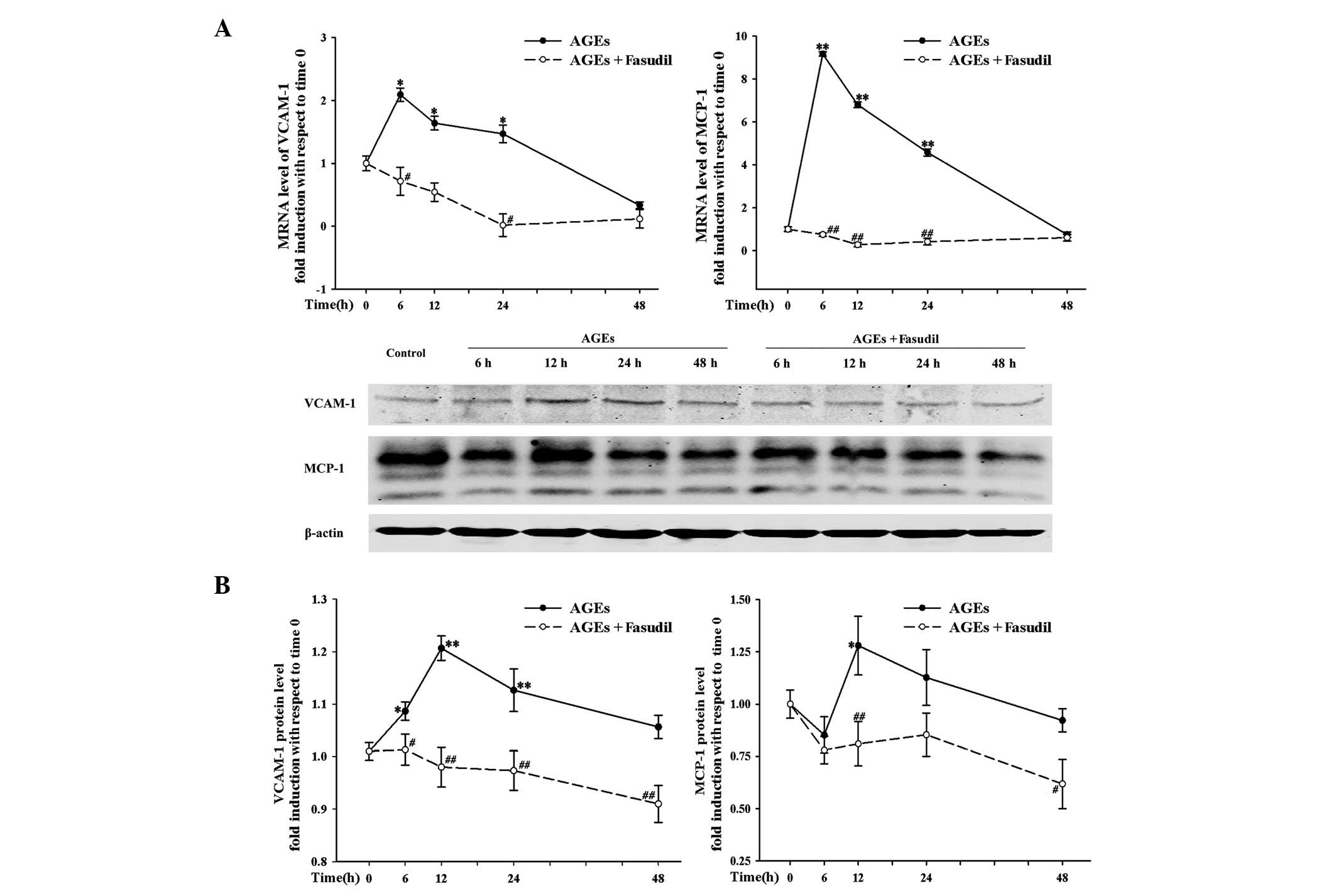
effects on HUVECs compared with at a low dose. Moreover, Fig. 3 shows the mRNA (Fig. 3A) and protein (Fig. 3B) expression of HUVECs treated with
AGEs (400 μg/ml) and AGEs (400 μg/ml) + fasudil (10
nM) for various times. At corresponding time-points, the mRNA and
protein expression levels of these factors were significantly lower
with fasudil treatment than without fasudil treatment. These
results suggest that fasudil inhibited AGE-induced cell adhesion by
reducing the expression of these adhesion and chemotactic
factors.
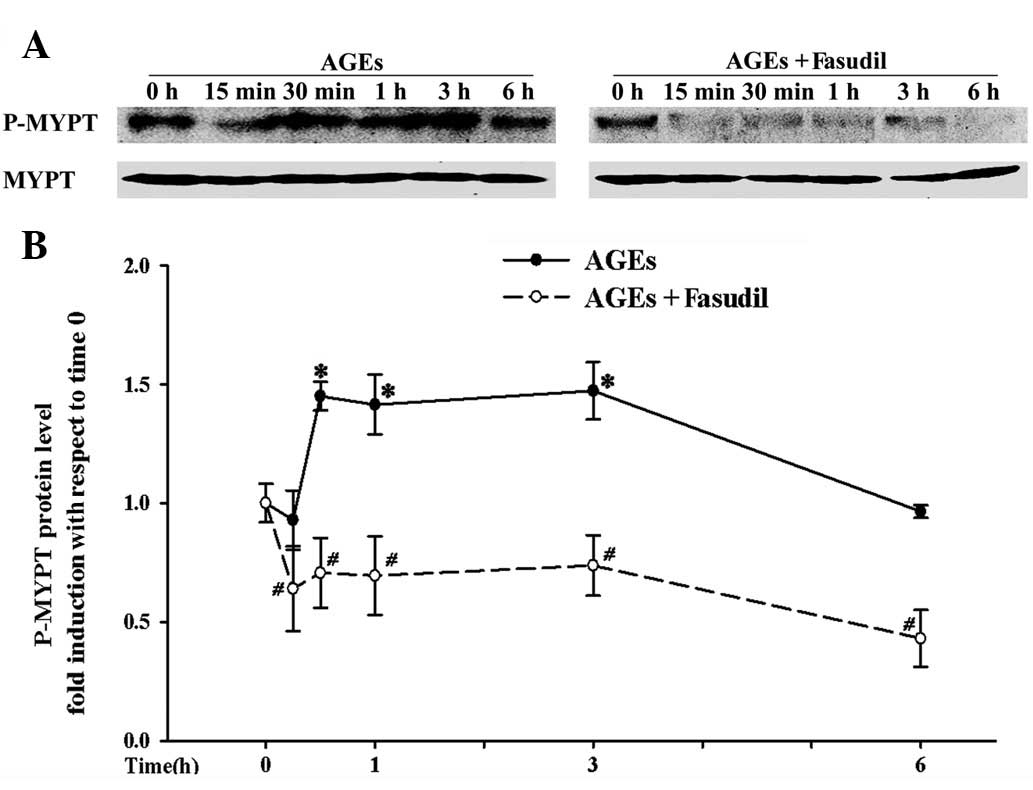
Fasudil suppresses ROCK activity and the
protein levels of Rho/ROCK
To ascertain whether AGEs increased cell adhesion
via the Rho/ROCK pathway, we assessed the levels of p-MYPT1, as
described in previous studies (13,27).
In the present study, HUVEC exposure to AGEs resulted in an
increased p-MYPT1/MYPT1 ratio from 15 min to 3 h compared with the
control groups, indicating that AGEs activate the Rho/ROCK pathway
(Fig. 4). As expected, fasudil
demonstrated inhibitory effects on AGE-induced ROCK activation over
the experimental period.
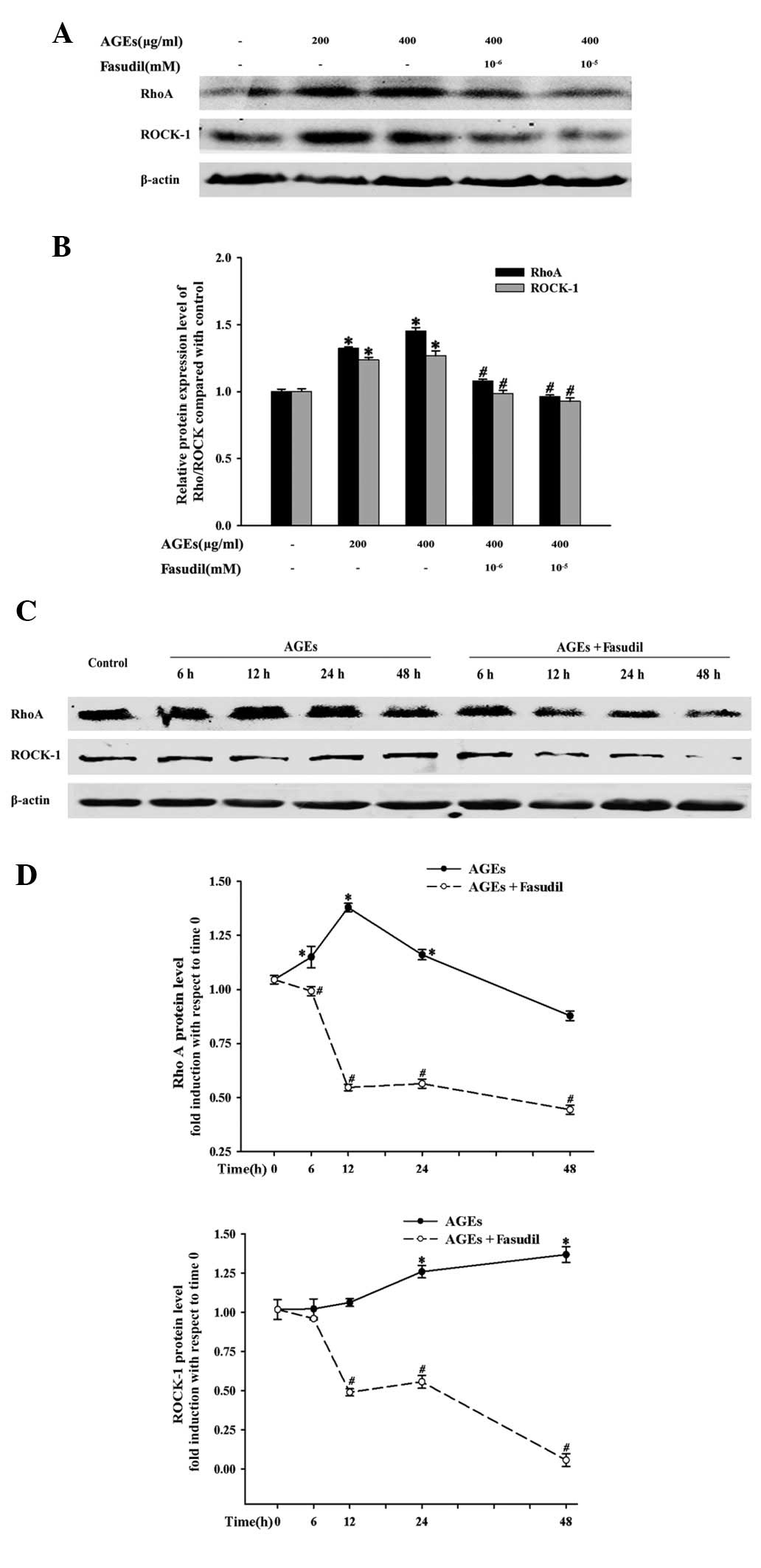
In addition, exposure to AGEs increased the protein
expression levels of RhoA and ROCK1 in a concentration- and
time-dependent manner (Fig. 5).
However, the effects were attenuated by the addition of fasudil. In
the presence of 10 nM fasudil, the AGE-induced protein expression
levels were significantly reduced from 6 to 48 h.
Fasudil attenuates AGE-induced NF-κB
activation and ROS
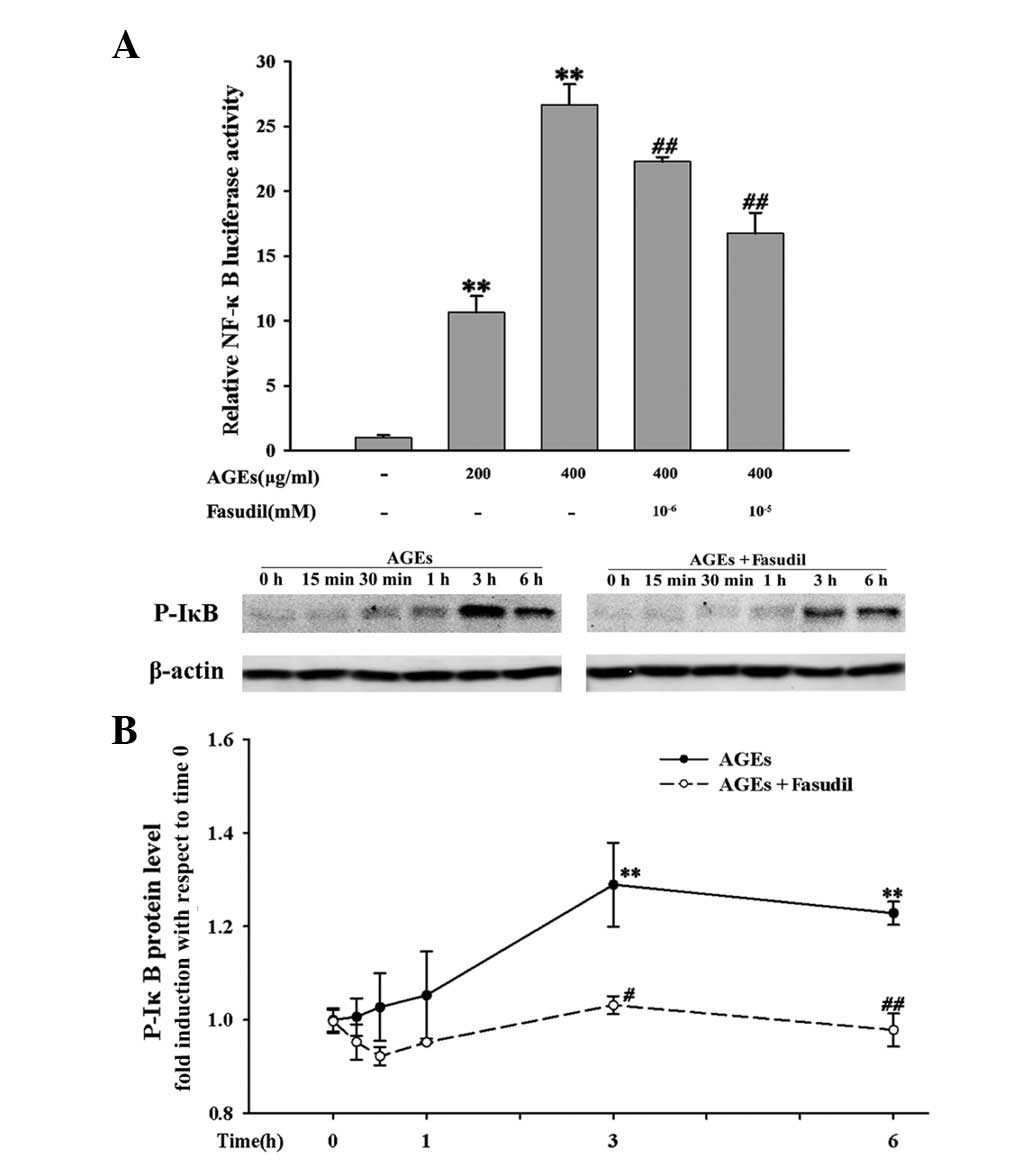
In order to investigate whether ROCK is involved in
AGE-induced NF-κB activation, we evaluated the effects of fasudil
on NF-κB-dependent transcriptional activity with a NF-κB-luciferase
reporter plasmid transiently transfected in HUVECs. As shown in
Fig. 6A, AGEs significantly
increase NF-κB-dependent transcriptional activity in a
concentration-dependent manner at 24 h (P<0.01) and fasudil
significantly inhibited the increase. Since IκB phosphorylation
plays an important role in the activation of NF-κB, we next
evaluated the effects of fasudil on IκB phosphorylation to further
investigate the molecular target of fasudil in NF-κB signaling.
Fasudil attenuated AGE-induced IκB phosphorylation from 3 to 6 h
(Fig. 6B). These results
demonstrated that ROCK is involved in the pathway that activates
NF-κB signaling.
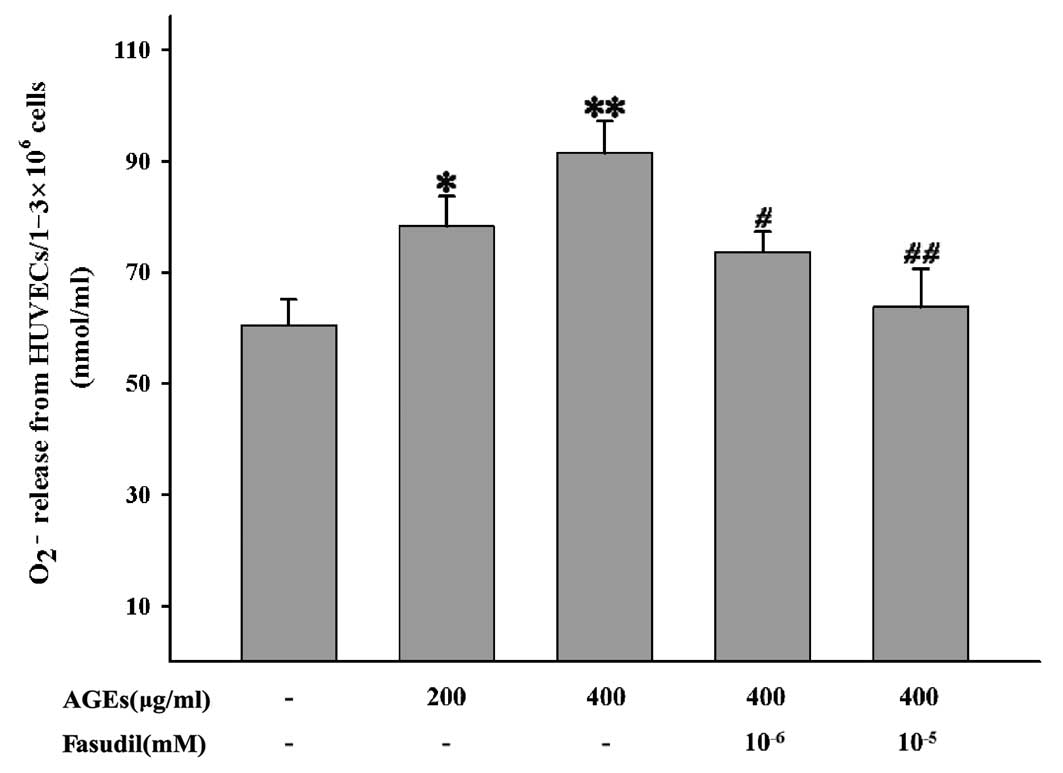
Additionally, to assess the effects of fasudil on
ROS production, we further studied O2−
release into the super-natant from HUVECs. As shown in Fig. 7, AGEs promote
O2− release; a significant increase in the
reduction of ferricytochrome c was observed in comparison
with the control groups, particularly at a high concentration.
However, the effects of AGEs on O2− release
were successfully inhibited by the addition of fasudil. These
results suggest that fasudil significantly inhibited ROS production
and that a high dose of this agent had more potent inhibitory
effects. These data together demonstrated that fasudil inhibited
ROS generation from HUVECs in response to AGEs and then inhibited
the activation of NF-κB.
Discussion
Major findings from this study demonstrated that i)
fasudil protected the vascular endothelial cells against
AGEs-induced adhesion of monocytes to the endothelium, and ii) the
effects of fasudil with regard to the inhibition of cell adhesion
were partly due to the reduction of ROS production and inhibition
of the Rho/ROCK and NF-κB signaling pathways. Our study suggests
that fasudil plays a role in the protection of the vascular
endothelium through inhibition of the Rho/ROCK pathway, reduction
of ROS generation and downregulation of NF-κB signaling. Such a
phenomenon may provide insights into molecular mechanisms of
vascular protection in diabetes.
As indicated previously, a notable feature of the
complicated inflammation process in the vasculature of diabetics is
monocyte-endothelial adhesion (6),
which is induced partly by AGEs through adhesion molecules,
including VCAM-1 and ICAM-1 (5).
Thus, it is necessary to identify effective therapies that inhibit
AGE-induced cell adhesion in diabetes; however, related treatment
for this aspect is limited. Our previous study suggested that ROCK
inhibition may have therapeutic effects in preventing high
glucose-associated vascular inflammation and atherogenesis
(13). In line with our previous
study (13), fasudil markedly
reduced AGE-induced cell adhesion by reducing the mRNA and protein
expression levels of VCAM-1 and MCP-1 in HUVECs, and fasudil at a
high dose (10 nM) provided superior efficacy. The exposure of
HUVECs to AGEs increased the protein expression of Rho/ROCK and
activated MYPT phosphorylation. Simultaneously, the effects were
significantly suppressed by fasudil. These results suggest that the
Rho/ROCK pathway was involved in the progression of AGE-induced
cell adhesion.
Since VCAM-1 and MCP-1 expression in response to
AGEs has been reported to be regulated by NF-κB signaling, we
investigated the association between ROCK inhibition and NF-κB
signaling. In the present study, we identified that treatment of
HUVECs with fasudil successfully inhibited AGE-induced NF-κB
activity and simultaneously decreased IκB phosphorylation. There
are also several lines of evidence indicating that ROCK is involved
in the pathway that activates NF-κB; however, the role of the
Rho/ROCK pathway in NF-κB signaling remains inconsistent and may
vary depending on activation stimulus. Bolick et al reported
that NF-κB is activated in the endothelial cells of
12/15-lipoxygenase transgenic mice and that ROCK inhibition blocked
NF-κB activation and monocyte adhesion (28). Moreover, thrombin and interleukin
1β (IL-1β) were shown to increase ROCK activity, the
transcriptional activation of NF-κB and then adhesion molecule
expression (20,29,30).
However, in parallel studies, researchers failed to observe the
inhibitory effects on NF-κB activation in response to tumor
necrosis factor α (TNF-α) and lipopolysaccharide (LPS) (29,30).
By contrast, our findings revealed that ROCK was involved in
AGE-induced NF-κB activation and the expression of adhesion
molecules.
Furthermore, increasing evidence indicates that ROS
also plays an important role in the pathophysiology of diabetic
vascular endothelial injury through NF-κB activation (5,31)
and ROCK enhances the production of ROS via activation of
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase
(32,33). However, ROCK involvement in NADPH
oxidase activation following the stimulation of cultured HUVECs by
AGEs remains to be identified. Our results confirmed that ROCK
activation was involved in superoxide formation. AGE administration
to HUVECs in vitro caused increased ROS production and NF-κB
activation. Treatment with fasudil significantly attenuated ROS
formation. Therefore, we may infer that ROCK inhibition plays a
role in suppressing NF-κB activity via ROS production.
In summary, our findings indicate that fasudil
attenuates AGE-induced monocyte-endothelial cell adhesion and the
expression of VCAM-1 and MCP-1 through the Rho/ROCK pathway. In
addition, the inhibitory effects of fasudil on AGE-induced
inflammatory responses were partly due to the reduction of ROS
production and inhibition of NF-κB.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (No.
30900520; 30900699; 81070107).
References
|
1.
|
Potenza MA, Gagliardi S, Nacci C, Carratu’
MR and Montagnani M: Endothelial dysfunction in diabetes: from
mechanisms to therapeutic targets. Curr Med Chem. 16:94–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Basta G, Schmidt AM and De Caterina R:
Advanced glycation end products and vascular inflammation:
implications for accelerated atherosclerosis in diabetes.
Cardiovasc Res. 63:582–592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Garay-Sevilla ME, Regalado JC, Malacara
JM, et al: Advanced glycosylation end products in skin, serum,
saliva and urine and its association with complications of patients
with type 2 diabetes mellitus. J Endocrinol Invest. 28:223–230.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Rojas A and Morales MA: Advanced glycation
and endothelial functions: a link towards vascular complications in
diabetes. Life Sci. 76:715–730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar
|
|
7.
|
Littlewood TD and Bennett MR: Apoptotic
cell death in atherosclerosis. Curr Opin Lipidol. 14:469–475. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Piconi L, Quagliaro L, Da Ros R, et al:
Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and
interleukin-6 expression in human umbilical endothelial cells in
culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost.
2:1453–1459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Albelda SM, Smith CW and Ward PA: Adhesion
molecules and inflammatory injury. FASEB J. 8:504–512.
1994.PubMed/NCBI
|
|
10.
|
Gerszten RE, Garcia-Zepeda EA, Lim YC, et
al: MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular
endothelium under flow conditions. Nature. 398:718–723. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu Y, Zhu X, Liang GX, et al: Apelin-APJ
induces ICAM-1, VCAM-1 and MCP-1 expression via NF-kappaB/JNK
signal pathway in human umbilical vein endothelial cells. Amino
Acids. 43:2125–2136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang FL, Gao HQ, Wu JM, et al: Selective
inhibition by grape seed proanthocyanidin extracts of cell adhesion
molecule expression induced by advanced glycation end products in
endothelial cells. J Cardiovasc Pharmacol. 48:47–53. 2006.
View Article : Google Scholar
|
|
13.
|
Li H, Peng W, Jian W, et al: ROCK
inhibitor fasudil attenuated high glucose-induced MCP-1 and VCAM-1
expression and monocyte-endothelial cell adhesion. Cardiovasc
Diabetol. 11:652012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Noma K, Kihara Y and Higashi Y: Striking
crosstalk of ROCK signaling with endothelial function. J Cardiol.
60:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Arita R, Hata Y, Nakao S, et al: Rho
kinase inhibition by fasudil ameliorates diabetes-induced
microvascular damage. Diabetes. 58:215–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ocaranza MP, Rivera P, Novoa U, et al: Rho
kinase inhibition activates the homologous angiotensin-converting
enzyme-angiotensin-(1–9) axis in experimental hypertension. J
Hypertens. 29:706–715. 2011.PubMed/NCBI
|
|
17.
|
Shimokawa H and Rashid M: Devlopment of
Rhokinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Suzuki Y, Shibuya M, Satoh S, Sugimoto Y
and Takakura K: A postmarketing surveillance study of fasudil
treatment after aneurysmal subarachnoid hemorrhage. Surg Neurol.
68:126–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lee YJ, Kang DG, Kim JS and Lee HS: Effect
of Buddleja officinalis on high-glucose-induced vascular
inflammation in human umbilical vein endothelial cells. Exp Biol
Med (Maywood). 233:694–700. 2008.
|
|
20.
|
Okamoto H, Yoshio T, Kaneko H and Yamanaka
H: Inhibition of NF-kappaB signaling by fasudil as a potential
therapeutic strategy for rheumatoid arthritis. Arthritis Rheum.
62:82–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Segain JP, Raingeard de la Blétière D,
Sauzeau V, et al: Rho kinase blockade prevents inflammation via
nuclear factor kappa B inhibition: evidence in Crohn’s disease and
experimental colitis. Gastroenterology. 124:1180–1187.
2003.PubMed/NCBI
|
|
22.
|
Wang Y, Beck W, Deppisch R, Marshall SM,
Hoenich NA and Thompson MG: Advanced glycation end products elicit
externalization of phosphatidylserine in a subpopulation of
platelets via 5-HT2A/2C receptors. Am J Physiol Cell Physiol.
293:C328–C336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wang SH, Guo YJ, Yuan Y, et al:
PPARgamma-mediated advanced glycation end products regulate neural
stem cell proliferation but not neural differentiation through the
BDNF-CREB pathway. Toxicol Lett. 206:339–346. 2011.PubMed/NCBI
|
|
24.
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jeon KI, Xu X, Aizawa T, et al:
Vinpocetine inhibits NF-kappaB-dependent inflammation via an
IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci
USA. 107:9795–9800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Arai M, Sasaki Y and Nozawa R: Inhibition
by the protein kinase inhibitor HA1077 of the activation of NADPH
oxidase in human neutrophils. Biochem Pharmacol. 46:1487–1490.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Song J, Kost CK Jr and Martin DS:
Androgens potentiate renal vascular responses to angiotensin II via
amplification of the Rho kinase signaling pathway. Cardiovasc Res.
72:456–463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bolick DT, Orr AW, Whetzel A, et al:
12/15-Lipoxygenase regulates intercellular adhesion molecule-1
expression and monocyte adhesion to endothelium through activation
of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol.
25:2301–2307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Anwar KN, Fazal F, Malik AB and Rahman A:
RhoA/Rho-associated kinase pathway selectively regulates
thrombin-induced intercellular adhesion molecule-1 expression in
endothelial cells via activation of I kappa B kinase beta and
phosphorylation of RelA/p65. J Immunol. 173:6965–6972. 2004.
View Article : Google Scholar
|
|
30.
|
Wei CY, Huang KC, Chou YH, Hsieh PF, Lin
KH and Lin WW: The role of Rho-associated kinase in differential
regulation by statins of interleukin-1beta- and
lipopolysaccharide-mediated nuclear factor kappaB activation and
inducible nitric oxide synthase gene expression in vascular smooth
muscle cells. Mol Pharmacol. 69:960–967. 2006.
|
|
31.
|
Morita M, Yano S, Yamaguchi T and Sugimoto
T: Advanced glycation end products-induced reactive oxygen species
generation is partly through NF-kappa B activation in human aortic
endothelial cells. J Diabetes Complications. 27:11–15. 2013.
View Article : Google Scholar
|
|
32.
|
Montezano AC, Callera GE, Yogi A, et al:
Aldosterone and angiotensin II synergistically stimulate migration
in vascular smooth muscle cells through c-Src-regulated
redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol.
28:1511–1518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Jin L, Ying Z and Webb RC: Activation of
Rho/Rho kinase signaling pathway by reactive oxygen species in rat
aorta. Am J Physiol Heart Circ Physiol. 287:H1495–H1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|