Introduction
Leukemia, a malignant hematopoietic tumor, is a
cancer of the blood or bone marrow that is characterized by the
abnormal proliferation of white blood cells. It is the sixth
highest ranking variety of human tumor worldwide (1). Leukemias are classified into acute
lymphocytic, originating in the lymphocytes in bone marrow, and
myelogenous leukemia, originating from granulocytes or monocytes
(2,3). However, leukemia is highly resistant
to chemotherapy, and there is no effective cure for patients in the
advanced stages of the disease. To overcome these challenges, novel
therapeutic strategies are required for the more efficacious
treatment of this disease. Moreover, effective chemopreventive
treatments for leukemia are likely to have a significant impact on
leukemia morbidity and mortality.
Apoptosis is an active process of programmed cell
death that has been characterized as a fundamental cellular
activity to maintain the physiological balance of the organism. In
general, apoptosis is mediated through two major pathways, the
extrinsic [death receptor (DR)-mediated] and intrinsic
(mitochondrial-mediated) pathways (4,5). The
products of several genes have been demonstrated to be critical in
the regulation of apoptosis, including the Bcl-2 and inhibitor of
apoptosis protein (IAP) family members and the caspase cascades. In
addition, apoptosis is involved in the immune defense machinery,
and functions as a protective mechanism against carcinogenesis by
eliminating damaged cells or abnormal excess cells that proliferate
due to the induction of various chemical agents (6–8). A
number of investigations have indicated that the induction of
apoptosis in tumor cells is the most common anti-cancer mechanism,
utilized by numerous cancer therapies. Therefore, the induction of
apoptotic cell death by certain chemotherapeutic agents is an
important mechanism in the anti-cancer properties of many
drugs.
Sarijang is a bamboo salt soy sauce that is made by
fermenting Rhynchosia nulubilis, a plant that exhibits
potent detoxifying properties, boiling it with sulfur-fed duck,
Ulmus davidiana var. japonica Nak., Allium
sativum (garlic) and sap of the lacquer tree, mixing this
combination with bamboo salt and then aging the mixture, as
previously described (Choi E-A: A method for producing healthful
soy sauce. Korean Patent. Filed: July 30, 2004; issued: June 13,
2005). It has been suggested that sarijang may exert medicinal
effects due to the fact that bamboo salt, which is a major raw
material of sarijang, is known to exhibit anti-inflammatory and
anti-cancer effects (9–13). R. nulubilis contains high
levels of genistin, daidzin, genistein and daidzein, which are
isoflavones present in general beans, in addition to high levels of
aglycone, existing in a state that is not bound to glycoside.
Therefore, R. nulubilis is expected to possess anti-cancer,
anti-inflammatory and immunity-enhancing effects, as well as being
effective in the prevention of menopausal osteoporosis (14–16).
Furthermore, the dried bark of U. davidiana has been
demonstrated to be highly effective at protecting against
cytotoxicity and preventing osteoporosis and asthma, in addition to
exerting anti-inflammatory and immunity-boosting effects (17–19).
Garlic, which is a source of sulfur-containing compounds (20–22),
and sulfur-fed duck extract have been revealed to possess excellent
anti-inflammatory and immunity-boosting properties, as well as
anti-cancer or cancer-preventing effects. Although the components
of sarijang have yet to be analyzed, the results of previous
analyses of the major raw materials of sarijang suggest that
sarijang may exhibit the effects demonstrated by each of the
individual diverse components. However, there is insufficient
scientific evidence to support this. Therefore, the aim of the
present study was to examine the anti-cancer effects of sarijang,
as part of an investigation into its medicinal efficacy. As such,
we evaluated whether sarijang was able to inhibit cell growth and
induce apoptosis in an in vivo U937 human leukemia cell
model.
Materials and methods
Reagents and antibodies
Fetal bovine serum (FBS), RPMI-1640, penicillin,
streptomycin and trypsin-EDTA were purchased from Gibco-BRL
(Gaithersburg, MD, USA). 4′,6-Diamidino-2-phenylindole (DAPI),
propidium iodide (PI), paraformaldehyde,
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), RNase A and proteinase K were obtained from Sigma-Aldrich
(St. Louis, MO, USA), and an enhanced chemiluminescence (ECL) kit
was purchased from Amersham Corp. (Arlington Heights, IL, USA).
Caspase activity assay kits were obtained from R&D Systems,
Inc. (Minneapolis, MN, USA) and the pan-caspase inhibitor,
z-VED-fmk, was obtained from Calbiochem-Novabiochem Corp. (San
Diego, CA, USA). DNA ladder size markers were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA), while the
antibodies of tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL), DR4, DR5, Fas, Fas ligand (FasL), X-linked IAP
(XIAP), cellular IAP (cIAP)-1, cIAP-2, survivin, Bcl-2, Bcl-xL,
Bax, Bad, Bid, caspases-3, -8 and -9, poly(ADP-ribose)-polymerase
(PARP), β-catenin, phospholipase C-γ1 (PLC-γ1) and actin were
purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA). Horseradish peroxidase (HRP)-conjugated anti-mouse and
anti-rabbit secondary antibodies were obtained from Amersham Corp,
while any additional chemicals not specifically cited here were
purchased from Sigma-Aldrich.
Cell culture and treatment of
sarijang
The U937 human leukemia, Chang liver and WI-38 (an
immortalized non-tumor cell line derived from normal human liver
tissue and an embryonic lung fibroblast, respectively) cells were
purchased from the American Type Culture Collection (Rockville, MD,
USA) and maintained at 37°C in 95% humidified air and 5%
CO2 in RPMI-1640 supplemented with 10% heat-inactivated
FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml
streptomycin. Sarijang was provided by Insan Bamboo Salt Inc.
(Hamyang, Republic of Korea) and was filtration sterilized using
0.4-μl single filters, and diluted with medium to the
desired concentration prior to use.
Cell proliferation assay
Cells were seeded into 6-well plates at a density of
1×105 cells/ml and incubated for 24 h at 37°C, with the
absence and presence of variable concentrations of sarijang.
Following incubation, cells were washed with phosphate-buffered
saline (PBS), trypsinized and manually counted with a hemocytometer
through the exclusion of trypan blue. For the morphological study,
the cells were treated with sarijang for 24 h and then photographed
directly using an inverted microscope (Carl Zeiss, Oberkochen,
Germany).
Cell viability assay
The cell viability assay was performed using an MTT
assay. For the MTT assay, cells were treated with sarijang for 24
h. Following the treatments, 0.5 mg/ml MTT solution was added,
prior to incubation for 2 h at 37°C in the dark. The absorbance of
each well was measured at 540 nm with an enzyme-linked
immunosorbent assay (ELISA) reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Nuclear staining with DAPI
For DAPI staining, the cells were washed with PBS
and fixed with 3.7% paraformaldehyde (Sigma-Aldrich) in PBS for 10
min at room temperature. The fixed cells were then washed with PBS
and stained with 2.5 μg/ml DAPI solution for 10 min at room
temperature, prior to being washed twice with PBS and analyzed
using a fluorescence microscope (Carl Zeiss) (23).
DNA fragmentation assay
Following sarijang treatment, cells were lysed in a
buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA,
and 0.5% Triton X-100 for 1 h at room temperature. The lysates were
vortexed and cleared by centrifugation at 19,000 × g for 30 min at
4°C. A 25:24:1 (v/v/v) equal volume of neutral phenol : chloroform
: isoamyl alcohol was used for the extraction of the DNA in the
supernatant, followed by electrophoretic analysis on 1.5% agarose
gels containing 0.1 μg/ml ethidium bromide (EtBr).
DNA flow cytometric analysis
Following treatment with sarijang, cells were
harvested, washed twice with ice-cold PBS, and fixed with 75%
ethanol at 4°C for 30 min. The DNA content of cells was then
stained using a CycleTest™ Plus DNA staining kit (Becton Dickinson,
San Jose, CA, USA) with PI. The cellular DNA content at the sub-G1
phases was subsequently determined using a FACSCalibur™ flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA), prior to being
analyzed with Cell Quest software (Becton Dickinson).
Protein extraction and western blot
analysis
Cells were lysed with lysis buffer [20 mM sucrose, 1
mM EDTA, 20 μM Tris-Cl (pH 7.2), 1 mM dithiothreitol (DTT),
10 mM KCl, 1.5 mM MgCl2, 5 μg/ml pepstatin A, 10
μg/ml leupeptin and 2 μg/ml aprotinin] containing
protease inhibitors. A Bio-Rad protein assay (Bio-Rad, Hercules,
CA, USA) was used in accordance with the manufacturer’s
instructions, in order to determine the protein concentrations.
Following normalization, an equal quantity of protein was subjected
to electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide
gels, and then transferred to nitrocellulose membranes (Schleicher
& Schuell Bioscience, Inc., Keene, NH, USA) by electroblotting.
The membranes were blocked with 5% skimmed milk and subsequently
incubated with the primary antibodies and the HRP-conjugated
anti-mouse or anti-rabbit secondary antibodies. An ECL detection
system was used to visualize the target proteins (Amersham
Corp.).
In vitro caspase activity assay
The activity of the caspases was determined using
colorimetric assay kits, which utilized the following synthetic
tetrapeptides, labeled with p-nitroaniline (pNA):
Asp-Glu-Val-Asp (DEAD) for caspase-3, Ile-Glu-Thr-Asp (IETD) for
caspase-8 and Leu-Glu-His-Asp (LEHD) for caspase-9. In brief, the
sarijang-treated and untreated cells were lysed in the supplied
lysis buffer. The supernatants were then collected and incubated
with the supplied reaction buffer containing DTT and DEAD-, IETD-
or LEHD-pNA as substrates at 37°C. The reactions were measured by
changes in the absorbance at 405 nm, using the VERSAmax tunable
microplate reader (Molecular Devices, PaloAlto, CA, USA) (24).
Statistical analysis
Unless otherwise indicated, the data are expressed
as the mean ± standard deviation of the results obtained from three
separate experiments. Statistical analysis was performed using a
paired Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sarijang inhibits proliferation and cell
viability in U937 cells
To investigate the effects of sarijang on U937 cell
growth, the cells were treated with various concentrations of
sarijang for 24 h, and the cell number and viability were then
measured by the trypan blue exclusion method and MTT assay,
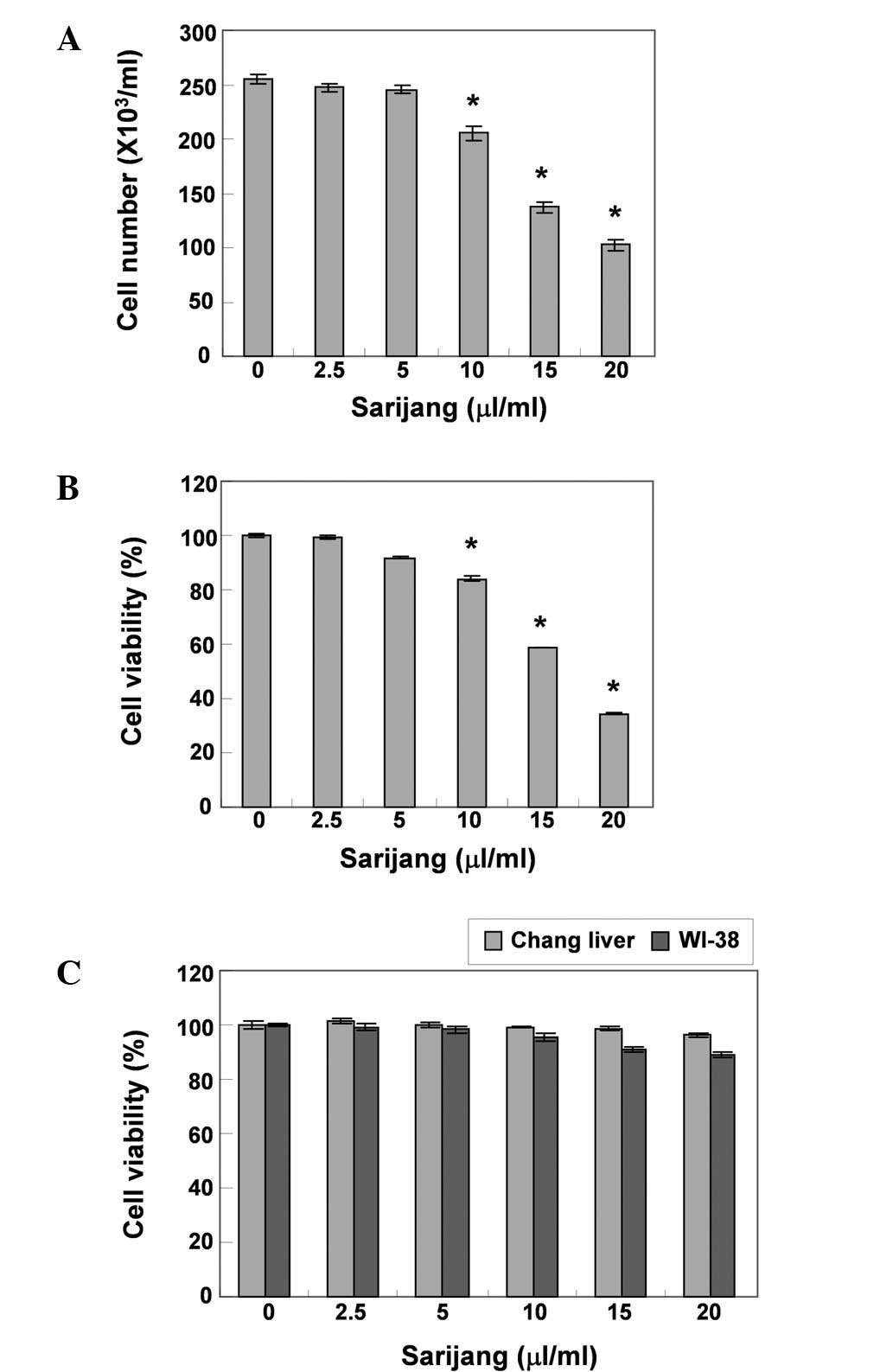
respectively. As demonstrated in Fig.
1A and B, sarijang markedly inhibited the cell proliferation
and viability of the U937 cells in a concentration-dependent
manner. The cell viability was inhibited by >41 or 63% in cells
exposed to 15 or 20 μl/ml sarijang, respectively, as
compared with untreated controls. In addition, a visual inspection
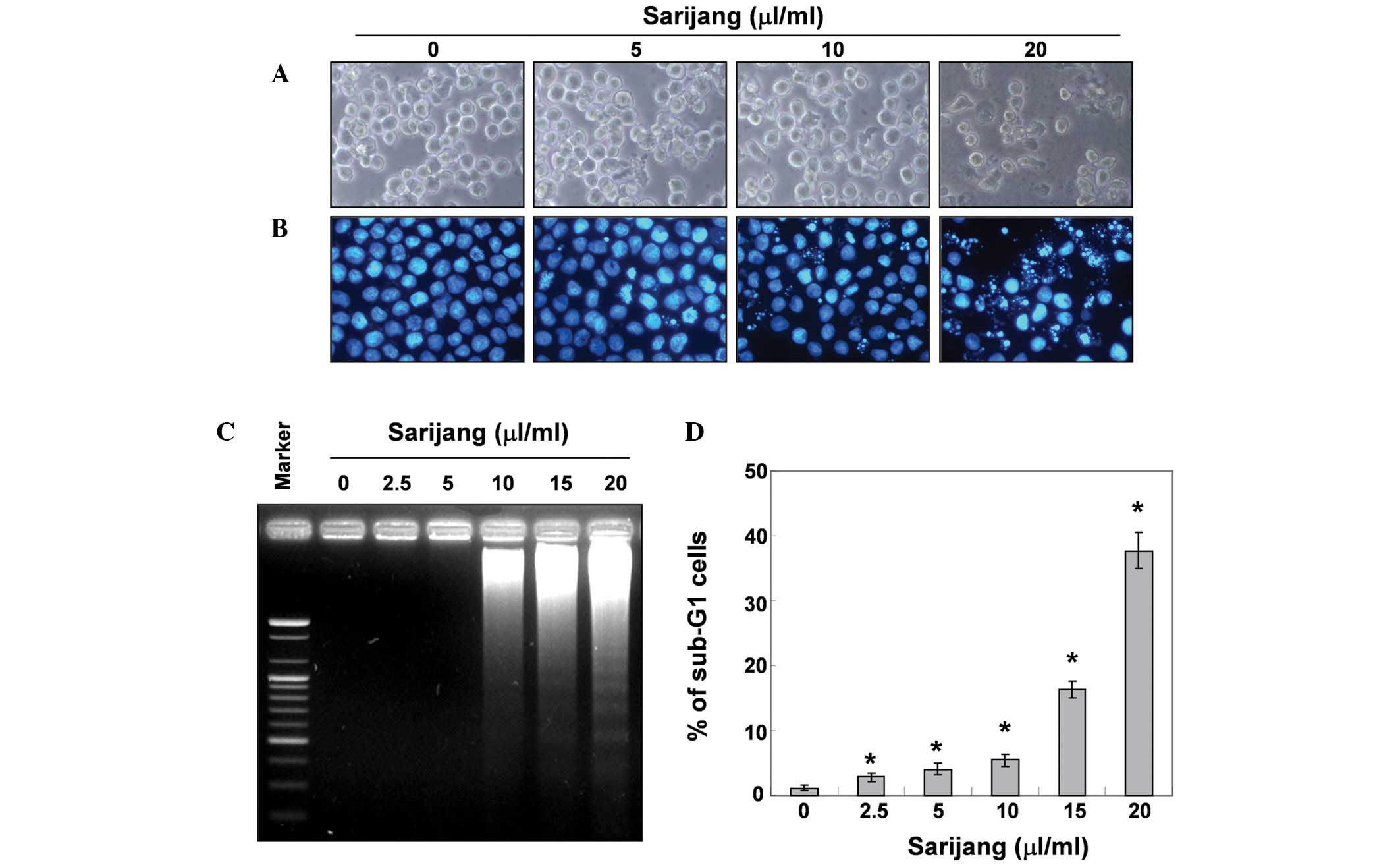
using inverted microscopy revealed that treatment with sarijang
resulted in numerous morphological changes (Fig. 2A). Furthermore, an additional
experiment was conducted using Chang liver and WI-38 cells, in
order to examine the effect of sarijang on the proliferation of
normal cells. The results of this experiment are presented in
Fig. 1C. The results demonstrated
that the survival rate of Chang liver and WI-38 cells did not
significantly change when under the same conditions as those
applied to U937 cells.
Sarijang induces apoptosis in U937
cells
In order to determine whether the growth inhibition
by sarijang was associated with the induction of apoptosis,
apoptotic features were examined by measuring the chromatin
condensation of nuclei, DNA fragmentation and the sub-G1 phase DNA
content. As demonstrated in Fig.
2B, treatment with sarijang resulted in the condensation of
chromatin in a significant number of cells, and the occurrence of
apoptotic body formation in a concentration-dependent manner. These
features were not observed in the control cells. In addition,
treatment with sarijang induced a progressive accumulation of
fragmented DNA, which appeared in a typical ladder pattern of DNA
fragmentation. This was due to the internucleosomal cleavage
associated with apoptosis, and occurred in a
concentration-dependent manner (Fig.
2C). The degree of apoptosis was determined by analyzing the
sub-G1 DNA content in the sarijang-treated U937 cells using flow
cytometry. As demonstrated in Fig.
2D, treatment with sarijang resulted in an increased
accumulation of cells with sub-G1 DNA content. These results
revealed a good correlation between the extent of apoptosis and the
inhibition of growth in U937 cells.
Effects of sarijang on the expression of
apoptosis-related genes
In order to determine which apoptosis pathway
contributed to sarijang-induced apoptosis, the DR and corresponding
pro-apoptotic ligands were examined using western blot analyses.
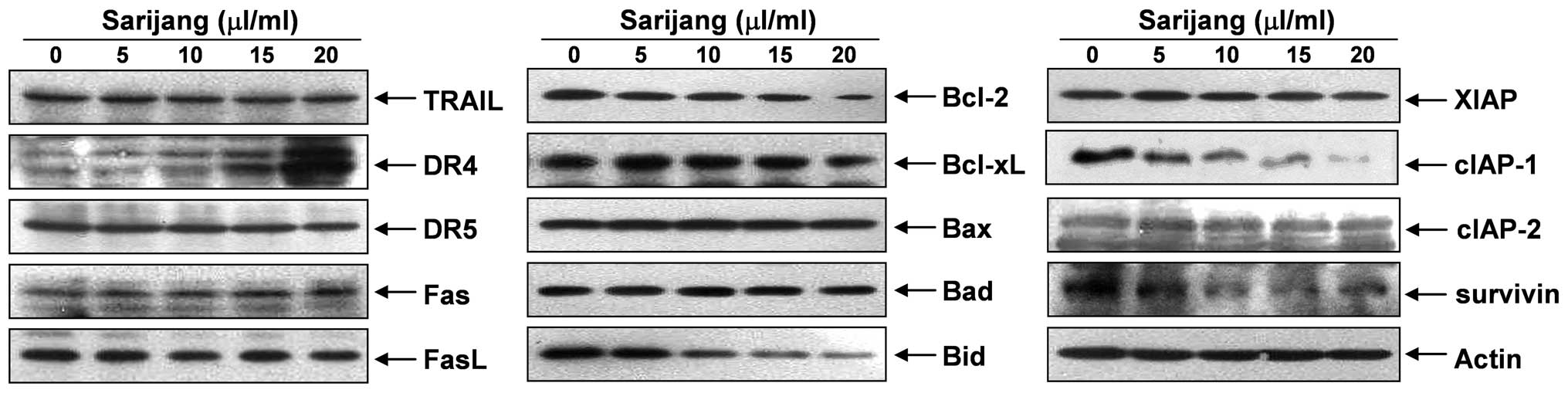
The results revealed that sarijang treatment resulted in a
concentration-dependent increase in the levels of DR4, whereas the
levels of TRAIL, DR5, Fas and FasL expression remained relatively
unchanged in response to sarijang treatment (Fig. 3). The examination of the effects of
sarijang on the levels of Bcl-2 family proteins revealed that the
levels of anti-apoptotic Bcl-2 proteins were inhibited by sarijang
treatment, while the pro-apoptotic protein, Bid, a BH3-only
pro-apoptotic member of the Bcl-2 family, was truncated in a
concentration-dependent manner (Fig.
3). By contrast, the levels of anti-apoptotic Bcl-xL and
pro-apoptotic Bax and Bad remained virtually unchanged in response
to sarijang treatment. Under identical conditions, the expression
levels of IAP family proteins were also examined. The results of
western blotting revealed that sarijang treatment resulted in a
concentration-dependent reduction in the expression levels of
survivin and cIAP-1, but not XIAP or cIAP-2.
Sarijang induces the activation of
caspases and the cleavage of PARP, β-catenin and PLC-γ1
Following the investigation into the effects of
sarijang on the expression of apoptosis-related genes, we examined
the expression levels and activities of caspases during
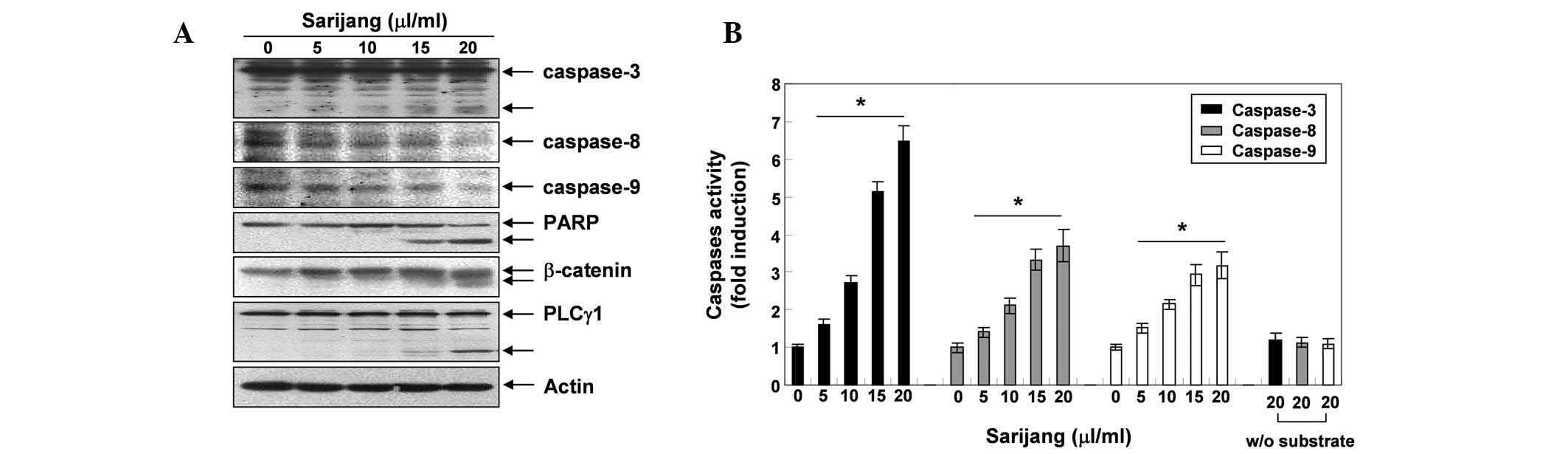
sarijang-induced apoptosis. As demonstrated in Fig. 4A, western blotting revealed that
the expression levels of the pro-caspases-3, -8 and -9 decreased
following sarijang treatment, while levels of the active forms of
caspase-3 increased, in a concentration-dependent manner. In
addition, the in vitro caspase activity in cellular extracts
of U937 cells was measured following 24 h exposure to sarijang,
using colorimetric substrates specific for each caspase. As
illustrated in Fig. 4B, 5–20
μl/ml sarijang significantly stimulated caspase-3, -8 and -9
activities in a concentration-dependent manner. In addition,
sarijang treatment led to the progressive proteolytic cleavage of
PARP, β-catenin and PLC-γ1 proteins, which are substrates of
activated caspase and indicators of apoptosis (Fig. 4A).
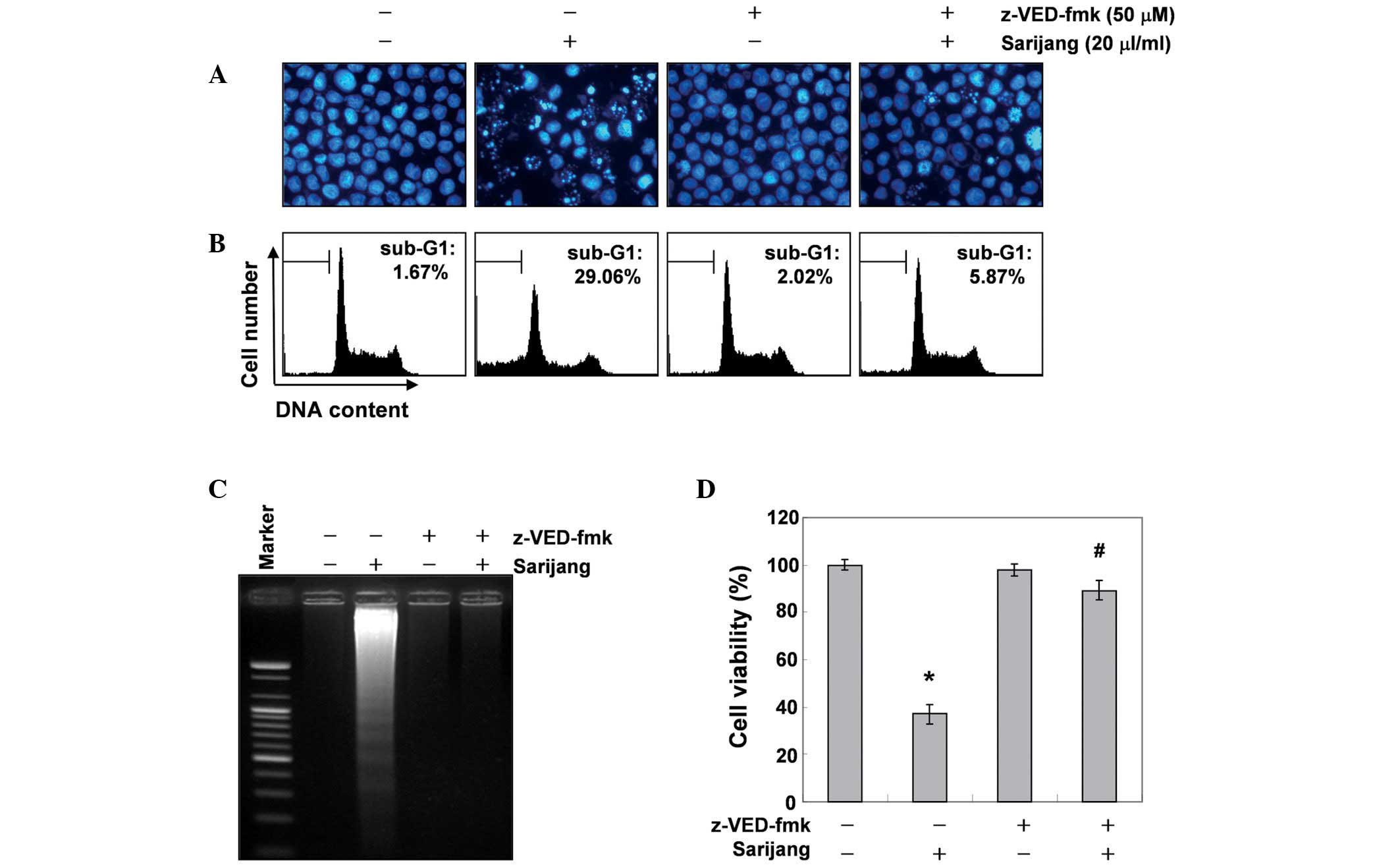
Inhibition of sarijang-induced apoptosis
by a pan-caspase inhibitor
To further confirm the involvement of caspase
activation in sarijang-induced apoptotic cell death, cells were
pre-treated with or without z-VED-fmk, a pan-caspase inhibitor, for
1 h, followed by treatment with sarijang for 24 h. The results
indicated that pre-treatment with z-VED-fmk resulted in a
significant prevention of the appearance of cells with apoptotic
features, such as chromatin condensation and apoptotic body
formation, and an attenuation of the progressive accumulation of
fragmented DNA (Fig. 5A and C).
Similarly, pre-treatment with z-VED-fmk induced the restoration of
decreased cell viability and increased the sub-G1 cell population
(Fig. 5B and D). These results
indicate that sarijang-induced apoptotic cell death correlated with
the activation of caspases in U937 cells.
Discussion
In the present study, we demonstrated that treatment
of a U937 human leukemia cell line with sarijang resulted in the
inhibition of cell growth and viability, in addition to changes in
cellular morphology, in a concentration-dependent manner (Figs. 1 and 2A). To further confirm that the
anti-proliferative effects induced by sarijang were correlated with
apoptotic cell death, the levels of chromatin condensation, DNA
fragmentation and the induction of the sub-G1 phase were assessed
(Fig. 2B–D).
The regulation of apoptosis is critical for the
maintenance of development and tissue homeostasis (25). Dysregulated apoptosis is considered
to induce a number of pathological conditions, including cancer.
Therefore, the induction of apoptosis is an important target for
cancer therapy. In general, apoptosis is mediated through two major
pathways, the extrinsic and intrinsic pathways. The extrinsic
pathway is initiated at the plasma membrane by the binding of DRs
to their ligands, such as Fas and FasL, as well as TRAIL and DRs
and, subsequently, the activation of caspase-8. Caspase-8, an
initiator caspase, is able to directly activate downstream effector
caspases, including caspase-3 (4,26,27).
The intrinsic pathway is triggered by cell stressors and many
chemo-therapeutic agents, resulting in the induction of
mitochondrial dysfunction. Mitochondrial dysfunction induces the
activation of caspase-9 and subsequently activates effector
caspases, such as caspase-3. Following the activation of caspase-3,
several specific substrates such as PARP, β-catenin and PLC-γ1 are
cleaved, eventually leading to apoptosis (5,6). In
certain cells, caspase-8 also mediates the intrinsic pathway via
cleavage of the pro-apoptotic Bid protein (6,7). In
particular, caspases are known to be regulated by various
molecules, including members of the Bcl-2 and IAP families. Bcl-2
family proteins are involved in the control of the apoptotic
process by interactions between pro-apoptotic (such as Bax and Bad)
and anti-apoptotic (such as Bcl-2 and Bcl-xL) members, particularly
those of the intrinsic pathway, leading to mitochondrial
dysfunction. Cellular proteins of the IAP family (including XIAP,
cIAP-1, cIAP-2 and survivin) specifically inhibit the activity of
caspase-3 and -9, while they do not inhibit caspase-8 (4,5). In
the intrinsic pathway, members of the IAP family bind directly to
the principal caspases, such as pro-caspase-3 and -9, and inhibit
the apoptosis induced by Bcl-2 family proteins. Therefore, the
downregulation of IAP family proteins relieves the triggering block
of proapoptotic signaling and the execution caspases, thus
activating cell death (28,29).
In the present study, we demonstrated that sarijang markedly
upregulated the protein levels of DR4 and induced the cleavage of
Bid. In addition, the expression levels of the cIAP-1 and survivin
proteins were markedly reduced by sarijang in a
concentration-dependent manner (Fig.
3).
Caspases, a family of cysteine-containing
aspartate-specific proteases, are known to be important during
apoptosis and to lead to the initiation and execution of apoptosis.
The activation of initiator caspases, such as caspase-8 and -9, has
been demonstrated to result in the downstream activation of
effector caspases, such as caspase-3, -6 and -7 (4,5). In
particular, the activation of capase-3 is responsible for the
proteolytic degradation of numerous key proteins, including PARP
and β-catenin, leading to apoptosis (30,31).
In the present study, the data indicated that treatment with
sarijang induced the activation of capase-3, -8 and -9 and the
concomitant proteolytic degradation of PARP, β-catenin and PLC-γ1
proteins (Fig. 4). However,
pre-treatment with a pan-caspase inhibitor, z-VED-fmk, prevented
the chromatin condensation and DNA fragmentation induced by
sarijang and restored cell viability (Fig. 5). The present data demonstrated
that sarijang induced an increase in the levels of DR4 and the
enzymatic activity of extrinsic and intrinsic caspase cascades,
such as caspase-8 and -9, which was correlated with increased
levels of truncated Bid expression. In addition, caspase-3 was then
activated, and PARP, β-catenin and PLC-γ1 proteins were
progressively cleaved in sarijang-treated U937 cells.
In conclusion, the results of this study
demonstrated that sarijang triggers the apoptosis of U937 human
leukemia cells through the activation of the intrinsic caspase
pathway, along with the DR-mediated extrinsic pathway, and that the
activation of caspases is responsible for the mediation of
sarijang-induced apoptosis. Although the results of this study
provide novel information on the possible mechanisms for the
anti-cancer activity of sarijang, further studies are required to
identify the active compounds.
Acknowledgements
This study was supported by the
Blue-Bio Industry Regional Innovation Center (RIC08-06-07) at
Dongeui University (Busan, Republic of Korea) as an RIC program
under the Ministry of Knowledge Economy of Busan, Republic of
Korea.
References
|
1.
|
Chang H, Lin H, Yi L, Zhu J, Zhou Y, Mi M
and Zhang Q: 3,6-Dihydroxyflavone induces apoptosis in leukemia
HL-60 cell via reactive oxygen species-mediated p38 MAPK/JNK
pathway. Eur J Pharmacol. 648:31–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Abramson N and Melton B: Leukocytosis:
basics of clinical assessment. Am Fam Physician. 62:2053–2060.
2000.PubMed/NCBI
|
|
3.
|
Gilliland DG, Jordan CT and Felix CA: The
molecular basis of leukemia. Hematology Am Soc Hematol Educ
Program. 80–97. 2004. View Article : Google Scholar
|
|
4.
|
Scaffidi C, Fulda S, Srinivasan A, Friesen
C, Li F, Tomaselli KJ, Debatin KM, Krammer PH and Peter ME: Two
CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675–1687. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lawen A: Apoptosis - an introduction.
Bioessays. 25:888–896. 2003. View Article : Google Scholar
|
|
6.
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
7.
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar
|
|
8.
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shin HY, Lee EH, Kim CY, Shin TY, Kim SD,
Song YS, Lee KN, Hong SH and Kim HM: Anti-inflammatory activity of
Korean folk medicine purple bamboo salt. Immunopharmacol
Immunotoxicol. 25:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zhao X, Deng X, Park KY, Qiu L and Pang L:
Purple bamboo salt has anticancer activity in TCA8113 cells in
vitro and preventive effects on buccal mucosa cancer in mice
in vivo. Exp Ther Med. 5:549–554. 2013.PubMed/NCBI
|
|
11.
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anti-cancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hu C, Zhang Y and Kitts DD: Evaluation of
antioxidant and prooxidant activities of bamboo Phyllostachys
nigra var. Henonis leaf extract in vitro. J Agric Food
Chem. 48:3170–3176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shin HY, Na HJ, Moon PD, Shin TK, Shin TY,
Kim SH, Hong SH and Kim HM: Inhibition of mast cell-dependent
immediate-type hypersensitivity reactions by purple bamboo salt. J
Ethnopharmacol. 91:153–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nielsen IL and Williamson G: Review of the
factors affecting bioavailability of soy isoflavones in humans.
Nutr Cancer. 57:1–10. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Chirumbolo S: The role of quercetin,
flavonols and flavones in modulating inflammatory cell function.
Inflamm Allergy Drug Targets. 9:263–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
La Ferla B, Airoldi C, Zona C, Orsato A,
Cardona F, Merlo S, Sironi E, D’Orazio G and Nicotra F: Natural
glycoconjugates with antitumor activity. Nat Prod Rep. 28:630–648.
2011.PubMed/NCBI
|
|
17.
|
Lee MY, Seo CS, Ha H, et al: Protective
effects of Ulmus davidiana var. japonica against
OVA-induced murine asthma model via upregulation of heme
oxygenase-1. J Ethnopharmacol. 130:61–69. 2010.
|
|
18.
|
Lee Y, Park H, Ryu HS, Chun M, Kang S and
Kim HS: Effects of elm bark (Ulmus davidiana var.
japonica) extracts on the modulation of immunocompetence in
mice. J Med Food. 10:118–125. 2007.PubMed/NCBI
|
|
19.
|
Choi Y, Lee MK, Lim SY, Sung SH and Kim
YC: Inhibition of inducible NO synthase, cyclooxygenase-2 and
interleukin-1beta by torilin is mediated by mitogen-activated
protein kinases in microglial BV2 cells. Br J Pharmacol.
156:933–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Resch KL and Ernst E: Garlic (Allium
sativum) - a potent medicinal plant. Fortschr Med. 113:311–315.
1995.(In German).
|
|
21.
|
Gorinstein S, Jastrzebski Z, Namiesnik J,
Leontowicz H, Leontowicz M and Trakhtenberg S: The atherosclerotic
heart disease and protecting properties of garlic: contemporary
data. Mol Nutr Food Res. 51:1365–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gullett NP, Ruhul Amin AR, Bayraktar S,
Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ and Kucuk O:
Cancer prevention with natural compounds. Semin Oncol. 37:258–281.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lee K, Lee MH, Kang YW, Rhee KJ, Kim TU
and Kim YS: Parkin induces apoptotic cell death in TNF-α-treated
cervical cancer cells. BMB Rep. 45:526–531. 2012.PubMed/NCBI
|
|
24.
|
Kim IH, Kim SW, Kim SH, Lee SO, Lee ST,
Kim DG, Lee MJ and Park WH: Parthenolide-induced apoptosis of
hepatic stellate cells and anti-fibrotic effects in an in vivo rat
model. Exp Mol Med. 2012.44:448–456
|
|
25.
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Peter ME and Krammer PH: The CD95
(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Roy N, Deveraux QL, Takahashi R, Salvesen
GS and Reed JC: The c-IAP-1 and c-IAP-2 proteins are direct
inhibitors of specific caspases. EMBO J. 16:6914–6925. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Fukuda K: Apoptosis-associated cleavage of
beta-catenin in human colon cancer and rat hepatoma cells. Int J
Biochem Cell Biol. 31:519–529. 1999. View Article : Google Scholar : PubMed/NCBI
|