Introduction
Severe or intractable asthma is a common clinical
problem and exposure to particular allergens and sensitisation to
these allergens have an important role in its pathogenesis
(1). Fungi are closely associated
with asthma and studies suggest that the mortality, hospitalisation
rate, respiratory symptoms and peak expiratory flow (PEF) of
patients with asthma are associated with a high fungal spore
content in outdoor air (2–6). Fungi, including Alternaria
alternata, Penicillium, Aspergillus and
Cladosporium, are common allergens of patients with asthma
(7). Sensitisation to these fungal
allergens is related to the acute aggravation and severity of
asthma (8–11). Among patients with life-threatening
asthma, patients who are positive in the dermal sensitivity test to
A. alternata are extremely common (12).
Few studies concerning the correlation of fungal
sensitisation with asthma severity exist and the correlation
remains unclear. Moreover, the distribution of allergenic fungi
varies in different regions. Considering the close association of
fungal allergens with asthma, we selected cases of outpatients and
inpatients with asthma in Beijing Shijitan Hospital from 2010 to
2011 to detect the levels of specific immunoglobulin E (sIgE) to
various fungal and non-fungal allergens and investigate the
correlation of fungal sensitisation with asthma severity in the
local region. This study is likely to be significant in the
prevention and treatment of severe asthma.
Subjects and methods
Case collection
A total of 100 cases of outpatients and inpatients
diagnosed with bronchial asthma were selected from 2010 to 2011.
All patients complied with the diagnostic criteria in the
Prevention and Treatment Guideline of Bronchial Asthma prepared by
the Asthma Study Group under the Respiratory Disease Branch of the
Chinese Medical Association in 2008. According to the clinical
manifestations of the patients, lung function prior to treatment
and treatment modes for maintaining asthma control, the asthma
severity of the patients was classified into mild severity (grades
1 and 2), moderate severity (grade 3) and severe severity (grade 4)
(13). Patients with chronic
obstructive pulmonary disease, complicated active, acute or chronic
lung diseases, severe autoimmune diseases, therioma, coronary heart
disease and hypertension were excluded. Pregnant and lactating
women were also excluded. Horizontal comparisons of age, gender,
disease history, lung function, total IgE (tIgE) and sIgE were
conducted for all patients. This study was conducted in accordance
with the Declaration of Helsinki and with approval from the Ethics
Committee of Beijing Shijitan Hospital. Written informed consent
was obtained from all participants.
sIgE detection
A refrigerated sIgE kit (FOOKE GmbH, Borken,
Germany) was used and maintained at room temperature for 30 min.
The concentrated washing solution (20 times) was diluted with
distilled water for repeated use. An adequate number of
enzyme-encapsulated plates was fixed onto the frame. The standard,
test sample and blank control wells were set up and the positions
of the various wells were recorded. In the standard well, 50
μl standard solution was added. In the test sample well, 10
μl test sample solution was initially added (five times the
test sample dilution solution). No solution was added to the blank
control well. Then, the plate was incubated in an incubator at 37°C
for 30 min. The liquid in each well was removed and the plate was
dried with bibulous paper. Subsequently, each well was filled with
washing solution. After standing for 1 min, the washing solution
was removed, the plate was dried with bibulous paper and the plate
was washed repeatedly four times. Approximately 50 μl enzyme
working fluid was added to each well, with the exception of the
blank control well. The plate was incubated at 37°C for 30 min. The
liquid in each well was then removed and the plate was dried with
bibulous paper. Subsequently, each well was filled with washing
solution. After standing for 1 min, the washing solution was
removed, the plate was dried with bibulous paper and the plate was
washed repeatedly four times. Approximately 50 μl
chromogenic agent liquid A was placed into each well and then 50
μl chromogenic agent liquid B was added. After the plate had
been agitated on the flat mixing device for 30 sec, development was
conducted at 37°C for 15 min in the dark. Then, the enzyme-linked
immunosorbent assay (ELISA) plate was removed and 50 μl
termination solution was added to each well to terminate the
reaction. Zone adjustment was conducted in the blank well. The
absorbance values [optical density (OD)] of the various wells were
measured at 450 nm within 15 min of the termination of the
reaction. The linear regression equation of the standard curve was
calculated according to the standard concentrations and
corresponding OD values. The corresponding sample concentration was
calculated based on the sample OD value using the linear regression
equation. The final concentration is calculated as the product of
the measured concentration multiplied by the dilution factor.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Measurement data are expressed as mean ±
standard deviation. One-factor analysis of variance was used for
comparison of means among multiple groups, whereas the Chi-square
test was used for comparison of classification data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical features of the patients
For the clinical features of the three groups of
patients with asthma, patients in the mild group and the moderate
group were significantly younger compared with those in the severe
group (P=0.015, P=0.0001). No significant differences among the
various groups were observed in terms of gender, asthma onset age
< 40 years old, smoking, allergic rhinitis disease history and
positive family history of asthma. In the clinical examinations, no
significant difference was noted for tIgE, eosinophil percentage,
forced expiratory volume in 1 sec (FEV1) and FEV1/forced vital
capacity (FVC) among the three groups (Table I).
 | Table I.Clinical features of the three groups
of patients with asthma. |
Table I.
Clinical features of the three groups
of patients with asthma.
| Characteristics | Mild asthma | Moderate asthma | Severe asthma | P-value |
|---|
| Cases (n) | 52 | 24 | 24 | |
| Age (years) | 50±15.67 | 54±11.80 |
68.75±12.51a,b | <0.001a, <0.05b |
| Gender (%
female) | 65 | 58 | 75 | >0.05 |
| Asthma onset age
<40 years (%) | 58 | 42 | 75 | >0.05 |
| Smoking (%) | 19 | 8 | 33 | >0.05 |
| Allergic rhinitis
(%) | 54 | 42 | 33 | >0.05 |
| Family history
(%) | 46 | 33 | 25 | >0.05 |
| tIgE (U/ml) | 281.63±594.18 | 427.98±669.62 | 582.02±981.31 | >0.05 |
| Eosinophil (%) | 4.51±4.83 | 7.29±6.89 | 4.90±5.77 | >0.05 |
| FEV1 (litres) | 60.75±24.62 | 63.00±17.13 | 46.33±7.34 | >0.05 |
| FEV1/FVC (%) | 63.89±15.98 | 61.05±14.53 | 52.67±8.38 | >0.05 |
Allergen distributions
The percentage of patients with asthma sensitised by
fungal allergens, including Penicillium, Aspergillus
and C. albicans, was significantly higher in the severe
group than in the moderate and mild groups; the percentage in the
moderate group was significantly higher compared with that in the
mild group (P<0.05). For A. alternata-sensitised
patients, no significant difference was observed in the percentages
among the three groups. Patients with asthma caused by C.
herbarum were not observed in the three groups. The percentage
of patients sensitised by any of the five fungi was significantly
higher in the severe group than in the moderate and mild groups;
the percentage in the moderate group was significantly higher
compared with that in the mild group (P<0.001). In the severe
group, almost half of the patients with asthma were sensitised by
any of the fungi. For patients simultaneously sensitised by
multiple fungi, no significant difference was observed in the
percentages among the three groups.
For patients sensitised by non-fungal allergens,
including Dermatophagoides pteronyssinus, house dust, cat
hair, dog hair, Artemisia argyi and a tree combination
(maple/birch/beech/oak/Chinese parasol/poplar), no significant
difference was noted in the percentages among the three groups. For
patients sensitised by any of the seven non-fungal allergens, no
significant difference existed in the percentages among the three
groups. Moreover, no significant difference was observed in the
percentage of patients simultaneously sensitised by multiple
non-fungal allergens among the three groups. The percentage of
patients sensitised by combined fungal and non-fungal allergens was
significantly higher in the severe group than in the moderate and
the mild groups; the percentage in the moderate group was
significantly higher compared with that in the mild group
(P<0.001; Table II).
 | Table II.Distributions of fungal and non-fungal
allergens among the three groups of patients with asthma (%). |
Table II.
Distributions of fungal and non-fungal
allergens among the three groups of patients with asthma (%).
| Mild asthma
(n=52) | Moderate asthma
(n=24) | Severe asthma
(n=24) | P-value |
|---|
| Fungal allergens | | | | |
|
Aspergillus | <0.01 | 8 | 25 | <0.05 |
|
Penicillium | 4 | 8 | 25 | <0.05 |
| Alternaria
alternata | <0.01 | 8 | <0.01 | |
| Cladosporium
herbarum | 0 | 0 | 0 | |
| Candida
albicans | <0.01 | 8 | 25 | <0.05 |
| Any one fungal
sensitisation | 4 | 8 | 50 | <0.001 |
| >1 fungal
sensitisation | <0.01 | 8 | 17 | |
| Non-fungal
allergens | | | | |
| Dermatophagoides
pteronyssinus | 12 | 33 | 17 | |
| Dermatophagoides
culinae | 15 | 17 | 17 | |
| House dust | 23 | 42 | 33 | |
| Cat epithelium | 12 | 33 | 8 | |
| Dog epithelium | 15 | 42 | 8 | |
| Artemisia
argyi | 12 | 17 | 17 | |
| Tree
combination | <0.01 | 8 | <0.01 | |
| Any one non-fungal
sensitisation | 35 | 67 | 33 | |
| >1 non-fungal
sensitisation | 8 | 25 | 8 | |
| Combined fungal and
non-fungal sensitisation | <0.01 | 8 | 50 | <0.0001 |
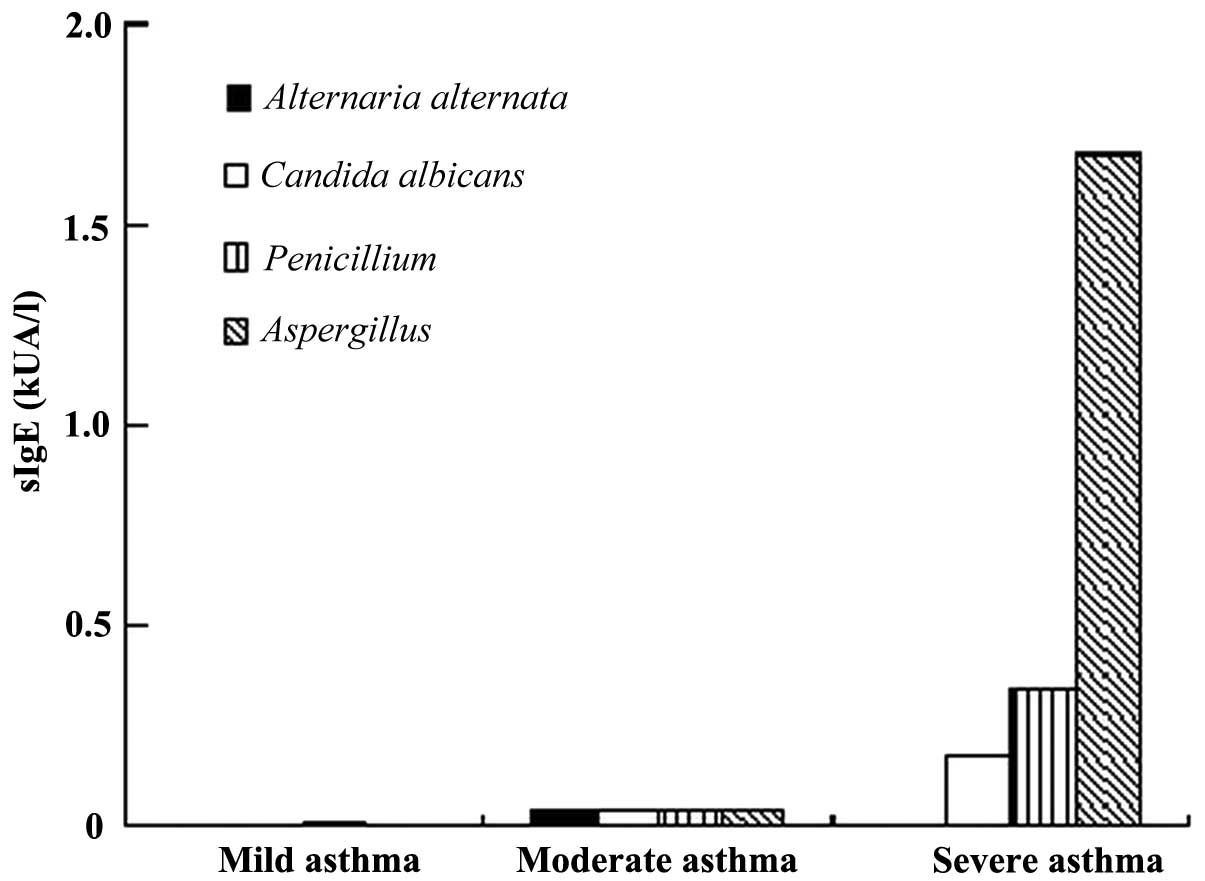
ELISA
The levels of sIgE to Aspergillus,
Penicillium and C. albicans of the patients with
asthma in the severe group were significantly higher than those in
the moderate and mild groups; those of the moderate group were
significantly higher than those of the mild group (P<0.01 or
P<0.05). Regarding the level of sIgE to A. alternata, no
significant difference was observed among the three groups of
patients with asthma. sIgE to C. herbarum among the three
groups was not present. Regarding the levels of sIgE to seven
non-fungal allergens, no significant difference was noted among the
three groups of patients with asthma (Table III, Fig. 1).
 | Table III.Levels of sIgE to fungal and
non-fungal allergens of the three groups of patients with
asthma. |
Table III.
Levels of sIgE to fungal and
non-fungal allergens of the three groups of patients with
asthma.
| sIgE(kUA/l)
| P-value |
|---|
| Mild asthma
(n=52) | Moderate asthma
(n=24) | Severe asthma
(n=24) |
|---|
| Fungal
allergens | | | | |
|
Aspergillus | <0.01±0.00 | 0.04±0.13 |
1.68±3.40a,b | <0.01a, <0.05b |
|
Penicillium | 0.01±0.07 | 0.04±0.15 |
0.34±0.84a,b | <0.05a, <0.05b |
| Alternaria
alternata | <0.01±0.00 | 0.04±0.13 | <0.01±0.00 | |
| Cladosporium
herbarum | 0 | 0 | 0 | |
| Candida
albicans | <0.01±0.00 | 0.04±0.12 |
0.17±0.33a,b | <0.01a, <0.05b |
| Non-fungal
allergens | | | | |
|
Dermatophagoides
pteronyssinus | 3.60±16.52 | 0.23±0.38 | 0.25±0.76 | |
|
Dermatophagoides culinae | 0.99±4.11 | 0.14±0.33 | 0.37±1.12 | |
| House dust | 3.95±12.36 | 1.03±2.13 | 1.51±2.98 | |
| Cat
epithelium | 2.33±9.93 | 0.30±0.49 | 0.10±0.34 | |
| Dog
epithelium | 1.20±3.85 | 2.53±5.73 | 1.78±6.19 | |
| Artemisia
argyi | 1.07±3.68 | 0.14±0.34 | 0.74±2.32 | |
| Tree
combination | <0.01±0.00 | 0.05±0.18 | <0.01±0.00 | |
Discussion
The results of the current study demonstrated that
fungal sensitisation is common in patients with severe asthma and
fungal allergens, including Aspergillus, Penicillium,
and C. albicans, in the local region are evidently related
to the severity of asthma in the patients. The results of the study
are in line with the results of previous studies on the correlation
of fungi with asthma severity. A 30-center survey on community
respiratory health in Europe demonstrated that asthma severity
increases with the increase of sensitisation frequency caused by
A. alternata or C. herbarum (11). The survey also indicated that the
percentage of patients sensitised by one or more allergens,
including A. alternata, C. herbarum, Epicoccum
and Helminthosporium, in a group of patients treated for
acute aggravation of asthma in an intensive-care unit (ICU) reached
54%. However, the percentage of patients sensitised by fungi in the
other group of patients with asthma who were not admitted in the
ICU was only 30% (8). An
additional study demonstrated that A. alternata
sensitisation is a risk factor for the development of severe asthma
(10).
The results of the current study indicate that
common A. alternata sensitisation is not correlated with
asthma severity, in opposition to the results of previous reports.
Asthma patients sensitised by C. herbarum were not observed
in the three groups. Given the different geographical environments,
climates and vegetations, greater differences exist in the
distribution of allergenic fungi between regions (14). A study reported that
Penicillium and Paecilomyces variotii were the
dominant colonies of fungi distributed in the air in Beijing, which
was in line with the distribution of fungi in the environment,
wherein Aspergillus yeast and A. alternata were the
dominant colonies. In the literature, A. alternata is not
reported to be the dominant fungus in the air in Beijing. In the
literature, A. alternata is not reported to be the dominant
fungus in the air in Beijing. Moreover, the data demonstrated that
the distribution of C. herbarum is not detected in Beijing
(15). These findings may be
attributed to the fact that A. alternata is not a dominant fungus
in this area. Therefore, fewer patients with asthma are allergic to
A. alternata. Given that C. herbarum content is
undetectable in the air, the possibility of C. herbarum
sensitisation is extremely minimal.
The results of studies concerning the correlation of
non-fungal allergens with disease severity in patients with asthma
are inconsistent. A number of studies suggested that certain
non-fungal allergens, including dog hair, are associated with
asthma severity, whereas other studies suggested that no
correlation existed between these components (9,16).
In the present study, the levels of sIgE to seven common non-fungal
allergens were detected and the results show that the percentage of
patients sensitised by non-fungal allergens (8–42%) is higher than
that of patients sensitised by fungal allergens (4–25%). The levels
of sIgE to non-fungal allergens are evidently higher compared with
those to fungal allergens. The sensitisation to non-fungal
allergens is stronger than that to fungal allergens and the
sensitisation to non-fungal allergens is common in all patients
with asthma. However, non-fungal allergens are not related to the
disease severity of patients with asthma.
The difference between fungal and non-fungal
allergens lies in the complex fungal allergen components. Fungal
allergens include proteases, glycosidases, protein product
components, oxidative stress-related proteins and enzymes involved
in glyconeogenesis or the pentose phosphate pathway. Proteases and
glycosidases directly affect the host, whereas the other three
components function in the metabolism process of spore germination.
Therefore, the exposure to fungal spores is equivalent to the
exposure to all fungal allergens and not just the exposure to
simple allergens, including non-fungal allergen pollen protein or
animal pelage. In addition, fungi are common in the environment;
therefore, the human respiratory tract is often exposed to fungal
spores in the air. The most common airborne fungi include C.
herbarum, A. alternata and Aspergillus. Fungal
and non-fungal allergens, including D. pteronyssinus, dog
hair and pollen, are protein allergens. However, fungal allergens
have unique features; they constantly germinate and infect the host
or implant in the respiratory tract of the host. Therefore, fungi
generate non-allergenic toxins and enzymes to pathogenic bacteria
by stimulating the defense system of the host. These substances
have an auxiliary role in sensitisation stimulation and cause a
greater impact on the host.
Fungi, including C. albicans and
Tracheophyta, are common colonisation fungi of the skin or
gastrointestinal tract. Although C. albicans is not an
airborne fungus, reports have indicated that ∼10% of patients with
mild asthma and almost 33% of patients with severe asthma are
sensitised by this fungus (9).
Trichophyton is not an airborne fungus; however, it often causes
skin and fingernail infections. One study indicated that after the
body absorbs fungal antigens, the body generates an IgE antibody to
induce air tract sensitisation and thus, cause asthma occurrence
(17). Therefore, non-airborne
fungi also induce sensitisation in the body, thereby causing asthma
occurrence. The present study also demonstrated that among patients
with asthma, certain moderate and severe asthma patients are
sensitised by C. albicans, which is significantly correlated
with asthma severity.
The effect of antifungal therapy on severe asthma
remains controversial and relevant studies are rare. In a
retrospective study, the hospitalisation rate and glucocorticoid
treatment course of Aspergillus-allergenic patients with
severe asthma who failed to comply with the diagnostic criteria of
allergenic bronchopulmonary Aspergillus was evidently
reduced following treatment with itraconazole for several months.
However, no change was observed in the tIgE and sIgE levels
(18). In another study,
fluconazole (100 mg/day) was administered for five months (once a
day) to Tracheophyta-sensitised patients with severe asthma.
Fluconazole reduced the bronchial hyper-reactivity of patients to
inspiratory Tracheophyta, reduced the hormone requirement
and increased the PEF value (19).
Moreover, another study used itraconazole to treat
fungus-sensitised patients with severe asthma and identified that
itraconazole appropriately enhanced the quality of life of the
patients, improved the rhinitis score, increased the PEF value and
reduced the tIgE level (20).
These studies suggest that antifungal therapy partially helps
fungus-sensitised patients with severe asthma; however, large-scale
clinical trials are required for confirmation.
The limitation of this study is the small number of
patients with asthma; thus, confirmation from large-scale clinical
trial data is required.
This study demonstrated that fungal sensitisation is
closely correlated with disease severity in patients with asthma by
detecting the levels to sIgE to different fungi in patients.
Non-fungal allergens are not correlated with the disease severity
of patients with asthma. Moreover, this study demonstrated that
fungi have an important role in the disease severity of patients
with asthma. Therefore, this study is significant for the future
prevention and treatment of severe asthma.
References
|
1.
|
Chung KF, Godard P, Adelroth E, et al:
Difficult/therapy-resistant asthma. Eur Respir J. 13:1198–1208.
1999.
|
|
2.
|
Targonski PV, Persky VW and Ramekrishnan
V: Effect of environmental molds on risk of death from asthma
during the pollen season. J Allergy Clin Immunol. 95:955–961. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Delfino RJ, Zeiger RS, Seltzer JM, Street
DH, Matteucci RM, Anderson PR and Koutrakis P: The effect of
outdoor fungal spore concentrations on daily asthma severity.
Environ Health Perspect. 105:622–635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Neas LM, Dockery DW, Burge H, Koutrakis P
and Speizer FE: Fungus spores, air pollutants and other
determinants of peak expiratory flow rate in children. Am J
Epidemiol. 143:797–807. 1996. View Article : Google Scholar
|
|
5.
|
Salvaggio J, Seabury J and Schoenhardt E:
New Orleans Asthma. V. Relationship between Charity Hospital
admission rates, semi-quantitative pollen and fungal spore counts,
and total particulate aerometric sampling data. J Allergy Clin
Immunol. 48:96–114. 1971. View Article : Google Scholar
|
|
6.
|
Newson R, Strachan D, Corden J and
Millington W: Fungal and other spore counts as predictors of
admissions for asthma in the Trent region. Occup Environ Med.
57:786–792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Denning DW, O’Driscoll BR, Hogaboam CM,
Bowyer P and Niven RM: The link between fungi and severe asthma: a
summary of the evidence. Eur Respir J. 27:615–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Black PN, Udy AA and Brodie SM:
Sensitivity to fungal allergens is a risk factor for
life-threatening asthma. Allergy. 55:501–504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
O’Driscoll RB, Hopkinson L and Denning DW:
Mold sensitization is common amongst patients with severe asthma
requiring multiple hospital admissions. BMC Pulm Med.
5:42005.PubMed/NCBI
|
|
10.
|
Neukirch C, Henry C, Leynaert B, Liard R,
Bousquet J and Neukirch F: Is sensitization to Alternaria
alternata a risk factor for severe asthma? A population-based
study. J Allergy Clin Immunol. 103:709–711. 1999.
|
|
11.
|
Zureik M, Neukirch C, Leynaert B, Liard R,
Bousquet J and Neukirch F; European Community Respiratory Health
Survey: Sensitisation to airborne moulds and severity of asthma:
cross sectional study from European Community respiratory health
survey. BMJ. 325:411–417. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
O’Hollaren MT, Yunginger JW, Offord KP,
Somers MJ, O’Connell EJ, Ballard DJ and Sachs MI: Exposure to an
aeroallergen as a possible precipitating factor in respiratory
arrest in young patients with asthma. N Engl J Med. 324:359–363.
1991.PubMed/NCBI
|
|
13.
|
Asthma Group of Respiratory Disease
Division of Chinese Medical Association: Guidelines for the
prevention and treatment of bronchial asthma. Chinese Journal of
Tuberculosis and Respiratory Diseases. 31:177–185. 2008.(In
Chinese).
|
|
14.
|
Kleyn JG, Johnson WM and Wetzler TF:
Microbial aerosols and actinomycetes in etiological considerations
of mushroom workers’ lungs. Appl Environ Microbiol. 41:1454–1460.
1981.PubMed/NCBI
|
|
15.
|
Zhai JH, Cui H, Chen ML, Xu XZ, Shun ZH,
Hu QX and Sun RQ: Analysis and identification of airborne fungus in
Beijing and Nanjing. China Public Health. 16:10262000.(In
Chinese).
|
|
16.
|
Matsuoka H, Niimi A, Matsumoto H, et al:
Specific IgE response to trichophyton and asthma severity. Chest.
135:898–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ward GW Jr, Karlsson G, Rose G and
Platts-Mills TA: Trichophyton asthma: sensitization of bronchi and
upper airways to dermatophyte antigen. Lancet. 1:859–862. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Riding DM, Denning DW, Francis HC and
Niven RM: The effect of itraconazole therapy in severe asthma
patients allergic to aspergillus: a retrospective audit. In:
Presented at the Advances against aspergillosis conference
(abstract 75); 2004, http://www.advancesagainstaspergillosis.org/pdfs/2004syllabus.pdfuri.
|
|
19.
|
Ward GW Jr, Woodfolk JA, Hayden ML,
Jackson S and Platts-Mills TA: Treatment of late-onset asthma with
fluconazole. J Allergy Clin Immunol. 104:541–546. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Denning DW, O’Driscoll BR, Powell G, et
al: Randomized controlled trial of oral antifungal treatment for
severe asthma with fungal sensitization: The Fungal Asthma
Sensitization Trial (FAST) study. Am J Respir Crit Care Med.
179:11–18. 2009. View Article : Google Scholar : PubMed/NCBI
|