Introduction
In-stent restenosis (ISR) following vascular
intervention affects the long-term curative effect markedly
(1). Although drug-eluting stents
(DESs) have favorable antiproliferative properties, ISR remains a
serious problem which should not be neglected. At present,
rapamycin-eluting stents are widely used in the clinic to reduce
restenosis. As an immunosuppressive agent, rapamycin addresses the
issue of neointimal proliferation, a pathology contributing to
restenosis. However, the inhibition of endothelial cells (ECs)
induces delayed endothelialization, which increases the risk of
in-stent thrombosis.
Asiaticoside is a white needle-like crystalline
material, which is a saponin component extracted from Centella
asiatica, a plant of the Umbelliferae family, which has been
used for the treatment of hypertrophic scars for numerous years and
is an ingredient in Chinese traditional herbal medicines. Previous
studies have demonstrated that asiaticoside has a variety of
biological effects, including anti-inflammatory (2) and anti-ulcerative properties
(3), tumor cell apoptosis-inducing
activity (4), anti-hepatofibrotic
(5) and anti-anxiety actions
(6), and wound-healing activity
(7). It has been reported that
asiaticoside may suppress scar formation by inhibiting the
proliferation of fibroblasts and extracellular matrix (ECM)
synthesis (8). However, the
precise pathological mechanism of action of asiaticoside at the
molecular and gene expression levels remains unknown.
Transforming growth factor β (TGF-β) belongs to a
family of cytokines with a variety of functions relating to
fibrosis, growth, differentiation and apoptosis (9). TGF-β is upregulated following
coronary angioplasty (10).
Several studies have demonstrated the important role of TGF-β in
intimal thickening and arterial remodeling, which contribute to ISR
(11). Shi et al observed
that TGF-β1 induces myofibroblast migration as well as arterial
remodeling by collagen deposition (12).
TGF-β1 promotes synthesis of the ECM by upregulating
the α2 (type I) collagen gene, which results in an increase in the
synthesis of type I collagen in fibroblasts; the increased ECM
contributes to artery remodeling. The Smad signaling pathway is the
primary signaling pathway for TGF-β. Among the Smad family, Smad7
is a general antagonist of the TGF-β family. Smad7 regulates TGF-β
signaling via a negative feedback loop and mediates the crosstalk
between TGF-β and other signaling pathways (13). Matrix metalloproteinase 1 (MMP1)
belongs to the family of MMPs which degrade ECM. TIMP1 is an
inhibitor of MMP1. A reduction of the TIMP1/MMP1 ratio value may
inhibit the synthesis of collagen (14). von Willebrand factor (vWF),
platelet endothelial cell adhesion molecule (PECAM-1) and
endothelial nitric oxide synthase (eNOS) are considered to be
functional markers of vascular ECs.
Materials and methods
Materials
Bare metal stents (BMSs) were purchased from
Shanghai MicroPort Medical (Group) Co., Ltd. (Shanghai, China),
asiaticoside was purchased from Guangxi Changzhou Natural
Pharmaceutical Co., Ltd., (Nanning, China), rapamycin was obtained
from Shanghai Gene Biotechnology company (Shanghai, China), Shandon
Excelsior ES™ Tissue Processor was purchased from Thermo Fisher
Scientific Inc. (Waltham, MA, USA) and the EXAKT 310 CP Basic
cutting system was purchased from Exakt Technologies, Inc.
(Oklahoma City, OK, USA). Human aortic fibroblasts (HAFs), human
aortic smooth muscle cells (HASMCs) and human coronary artery
endothelial cells (HCAECs) were purchased from ScienCell Research
Laboratories (Carlsbad, CA, USA). Trypsin 0.25% (w/v), 0.53 mM
EDTA, endothelial cell medium, fibroblast medium, smooth muscle
cell medium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), TRIzol and SuperScript® II were purchased
from Invitrogen Life Technologies (Carlsbad, CA, USA).
SYBR® Premix Ex Taq™ II (Perfect Real Time) was obtained
from Takara Bio, Inc. (Shiga, Japan). ABI PRISM® 7900HT
Sequence Detection system was purchased from Invitrogen Life
Technologies and the enzyme-linked immunosorbent assay (ELISA) kit
for collagen type I was purchased from Shanghai BlueGene Biotech
Co., Ltd. (Shanghai, China).
Methods
Cell cultures
Primary HCAECs were cultured in EC growth medium
(EBM-2) containing 5% fetal bovine serum (FBS) and 1% endothelial
cell growth supplement (ECGS, Cat no. 1052) and 5 ml of
penicillin/streptomycin solution (P/S, Cat no. 0503), antibiotics
and antimycotics in an incubator with 5% carbon dioxide at 37°C.
Primary HASMCs were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) containing 10% FBS, 100 U/ml penicillin, 100 U/ml
streptomycin and 1 mmol/l L-glutamine in an incubator with 5%
carbon dioxide at 37°C. Primary HAFs were cultured in fibroblast
medium (FM) containing 20% FBS, 1% fibroblast growth supplement
(FGS) and 5 ml 1% penicillin/streptomycin solution (P/S) in an
incubator with 5% carbon dioxide at 37°C. All assays were performed
on cells at 80–100% confluence, between passages 1 and 2, and were
repeated at least 3 times.
Asiaticoside and rapamycin
treatment
The cells were seeded in 96-well plates with 8,000
cells per well and treated with asiaticoside, rapamycin or both
drugs (24 wells for each group) and incubated with 5% carbon
dioxide at 37°C for 24 h. For cell viability analysis, the blank
group was treated with 1% dimethyl sulfoxide (DMSO), the
asiaticoside group was treated with various concentrations of
asiaticoside (1×10−12, 1×10−13,
1×10−14, 1×10−15 mol/l) and the rapamycin
group was treated with various concentrations of rapamycin
(1×10−12, 1×10−13, 1×10−14,
1×10−15 mol/l). The combination group was treated with
asiaticoside and rapamycin, in which the rapamycin concentration
was 10−9 mol/l and asiaticoside was used in various
concentrations (1×10−12, 1×10−13,
1×10−14, 1×10−15 mol/l). For qPCR and ELISA
analysis, the blank group was treated with 1% dimethyl sulfoxide
(DMSO), the asiaticoside group was treated with 10−5
mol/l asiaticoside and the rapamycin group was treated with
10−9 mol/l rapamycin. The combination group was treated
with 10−5 mol/l asiaticoside and 10−9 mol/l
rapamycin. Following treatment with various drugs, the cells were
incubated for 48 h at 37°C. The supernatants were harvested and
centrifuged for 15 min at 10,656 × g, and then removed and stored
at −20°C for ELISA. The cells were harvested for qPCR.
Cell viability analysis by MTT
assay
Cell viability was detected using an MTT assay. MTT
(5 mg/ml) was added to each well. The cells were incubated for one
hour and then made soluble with cytolysis solution (10% Triton
X-100, 0.1 mmol/l HCl in isopropyl alcohol solution). Absorbance
was determined at 570 nm by spectrophotometry.
RNA isolation and qPCR
Briefly, total RNA was isolated using TRIzol
according to the manufacturer’s instructions. Reverse
transcription-generated cDNA was obtained using
Superscript® II. For HCAECs, the vWF, PECAM-1 and eNOS
mRNAs were detected. For HAFs, the TGF-β1, Smad7, type I collagen,
TIMP1 and MMP1 mRNAs were detected. The primer sequences are listed
in Table I. SYBR®
Premix Ex Taq™ II (Perfect Real Time) was used. The PCR reaction
was carried out with the ABI PRISM® 7900HT Sequence
Detection system. The samples were analyzed in duplicate. β-actin
was used as an internal control. PCR products were separated by
electrophoresis in a 2% agarose gel. Densitometry values
representing gene expression were first normalized to β-actin
expression (calculated as gene densitometry value/β-actin
densitometry value).
 | Table I.Primer sequences used in this
study. |
Table I.
Primer sequences used in this
study.
| Primer | Sequence (5′ to
3′) |
|---|
| vWF forward |
GTGGGAAGCTGTAAGTCTGAAGTAG |
| vWF reverse |
CACATCGTTGATGTCAATGGAGTA |
| PECAM-1 forward |
TGAACTCCAACAACGAGAAAATG |
| PECAM-1 reverse |
CCGTAATGACTGTTAGCTTCCATAT |
| eNOS forward |
CGGCATCACCAGGAAGAAGA |
| eNOS reverse |
TCGGAGCCATACAGGATTGTC |
| TGF-β1 forward |
TGGACACGCAGTACAGCAAG |
| TGF-β1 reverse |
GCCCACGTAGTACACGATGG |
| Smad7 forward |
TCATGCAAACTCTTTGGTCGT |
| Smad7 reverse |
TTCTGCTTCCCCTCTTCCTAT |
| COL1A1 forward |
GAGGGCAACAGCCGCTTCAC |
| COL1A1 reverse |
GGAGGTCTTGGTGGTTTTGTATT |
| TIMP1 forward |
GGGCTTCACCAAGACCTACAC |
| TIMP1 reverse |
GGATGGATAAACAGGGAAACACT |
| MMP1 forward |
TGCTCTTTCTGAGGAAAACACT |
| MMP1 reverse |
GCTATCATTTTGGGATAACCTG |
| β-actin forward |
CTGGAACGGTGAAGGTGACA |
| β-actin reverse |
CGGCCACATTGTGAACTTTG |
ELISA
The culture supernatants were collected and stored
at −20°C. For HASMCs and HAFs, the type I collagen level was
determined using an ELISA kit for collagen type I.
Statistical analysis
The data were processed using SPSS software (version
14.0 for Windows; SPSS, Inc., Chicago, IL, USA). The results are
presented as the mean ± standard error of the mean (SEM). The
differences among experimental groups were compared by one-way
analysis of variance (ANOVA), and two sets of isolated sample data
were checked using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
Cell growth inhibitory rate by MTT
assay
Compared with the blank group, asiaticoside was able
to markedly inhibit the proliferation of HASMCs and HAFs
(P<0.01). Compared with the asiaticoside and rapamycin groups,
the combination group showed a greater inhibition of HASMCs and
HAFs. In HASMCs, the inhibitory rates were 33.12±1.35, 26.21±7.59
and 28.27±4.92%, respectively (P<0.05) and in HAFs, they were
63.50±3.83, 53.06±8.10 and 60.34±4.93%, respectively (P<0.05).
These results showed a certain synergism between asiaticoside and
rapamycin in HASMCs and HAFs. By contrast, the combination group
showed a weaker inhibition of HCAECs compared with that observed in
the single drug groups; the inhibitory rates were 11.09±1.17,
26.22±4.24 and 34.80±2.80%, respectively (P<0.05). We suggest
that asiaticoside may antagonize the inhibitory effect of rapamycin
on vascular ECs (Fig. 1).
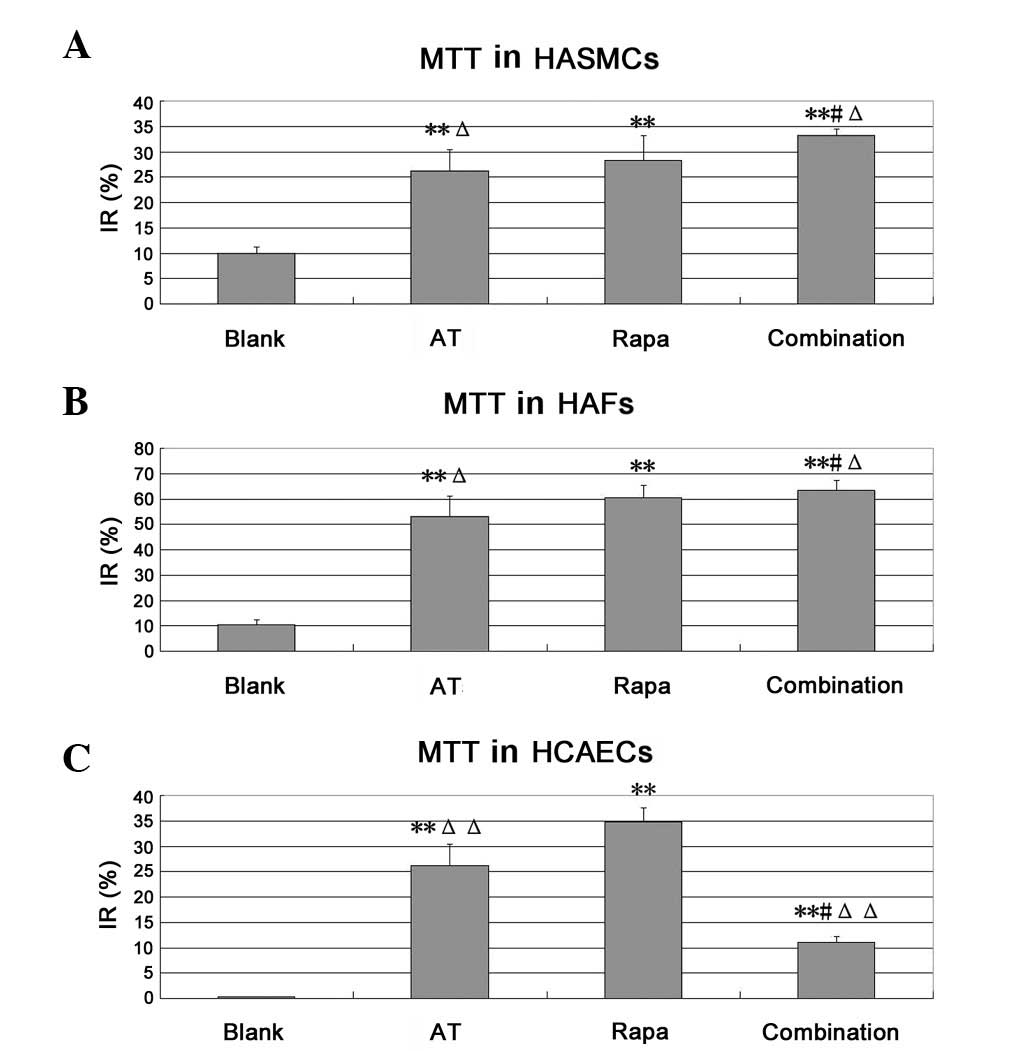 | Figure 1.Cell growth inhibitory rate of HCAECs,
HASMCs and HAFs, determined by MTT assay. The concentration of
asiaticoside (AT) is 10−5 mol/l and rapamycin (Rapa) is
10−9 mol/l. (A) HASMCs, (B) HAFs and (C) HCAECs.
**P<0.01 vs. the blank group, #P<0.05
vs. the asiaticoside group, ΔP<0.05 vs. the rapamycin
group, ΔΔP<0.01 vs. the rapamycin group. IR,
inhibitory rate=(1- medication group OD value/control group OD
value) ×100. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
HASMCs, human aortic smooth muscle cells; HAFs, human aortic
fibroblasts; HCAECs, human coronary artery endothelial cells. |
The levels of type I collagen, TGF-β1, Smad7, MMP1
and TIMP1 are shown in Fig. 2.
Asiaticoside significantly reduced the level of type I collagen
compared with that in the blank group (P<0.01). The combination
treatment was more effective than treatment with asiaticoside or
rapamycin alone (P<0.05). Compared with the blank group levels,
asiaticoside significantly upregulated Smad7 and MMP1 (P<0.01),
but downregulated TGF-β1 and TIMP1 (P<0.01 and P<0.05,
respectively). The combination group also showed more effective
results than those observed in the asiaticoside and rapamycin
groups (P<0.05), suggesting that asiaticoside had a synergism
with rapamycin.
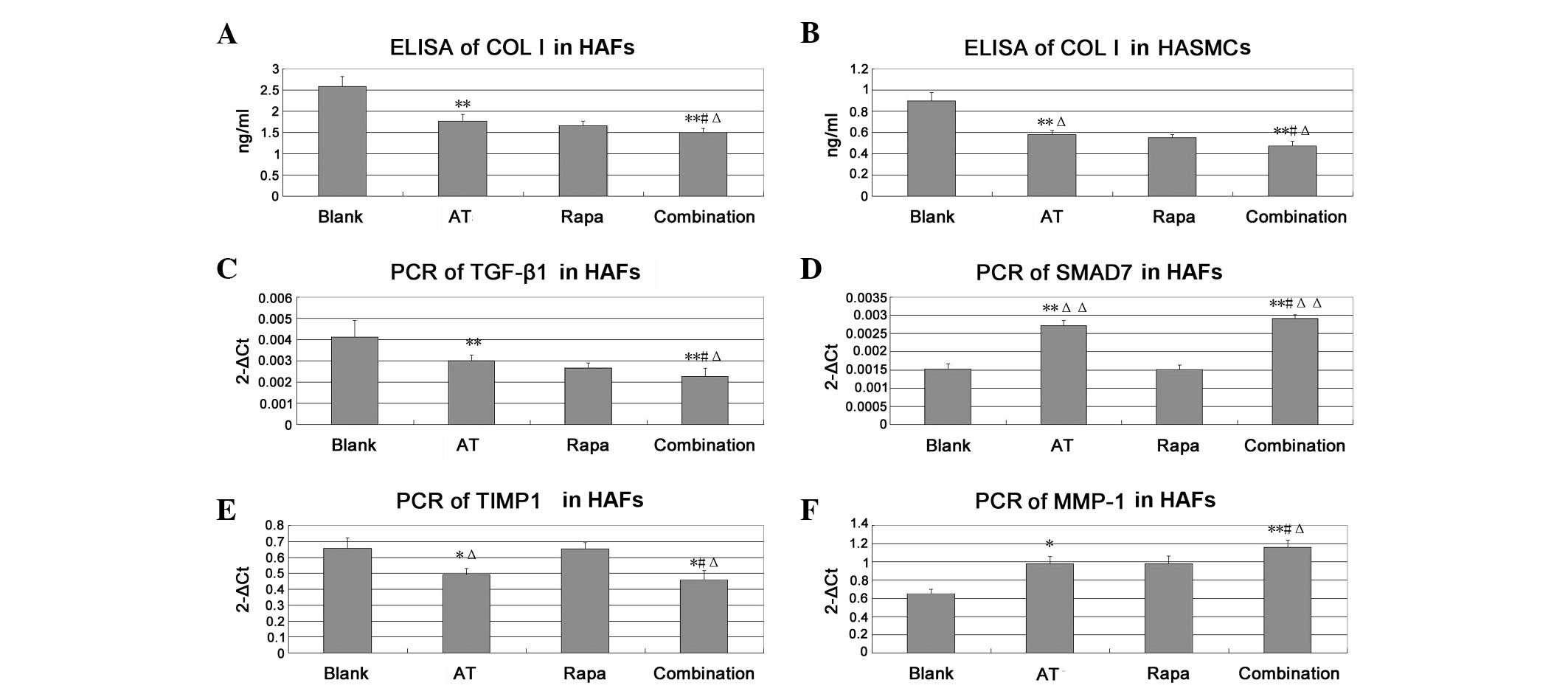 | Figure 2.Level of type I collagen in HASMCs and
HAFs as shown by ELISA and the levels of TGF-β1, Smad7, MMP1 and
TIMP1 in HAFs as shown by qPCR assay. The concentration of
asiaticoside (AT) is 10−5 mol/l and that of rapamycin
(Rapa) is 10−9 mol/l. (A and B) ELISA results. (C-F)
qPCR results. *P<0.05 vs. the blank group,
**P<0.01 vs. the blank group. #P<0.05
vs. the asiaticoside group, ΔP<0.05 vs. the rapamycin
group, ΔΔP<0.01 vs. the rapamycin group. HASMCs,
human aortic smooth muscle cells; HAFs, human aortic fibroblasts;
ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative
polymerase chain reaction; TGF, transforming growth factor; MMP,
matrix metalloproteinase. |
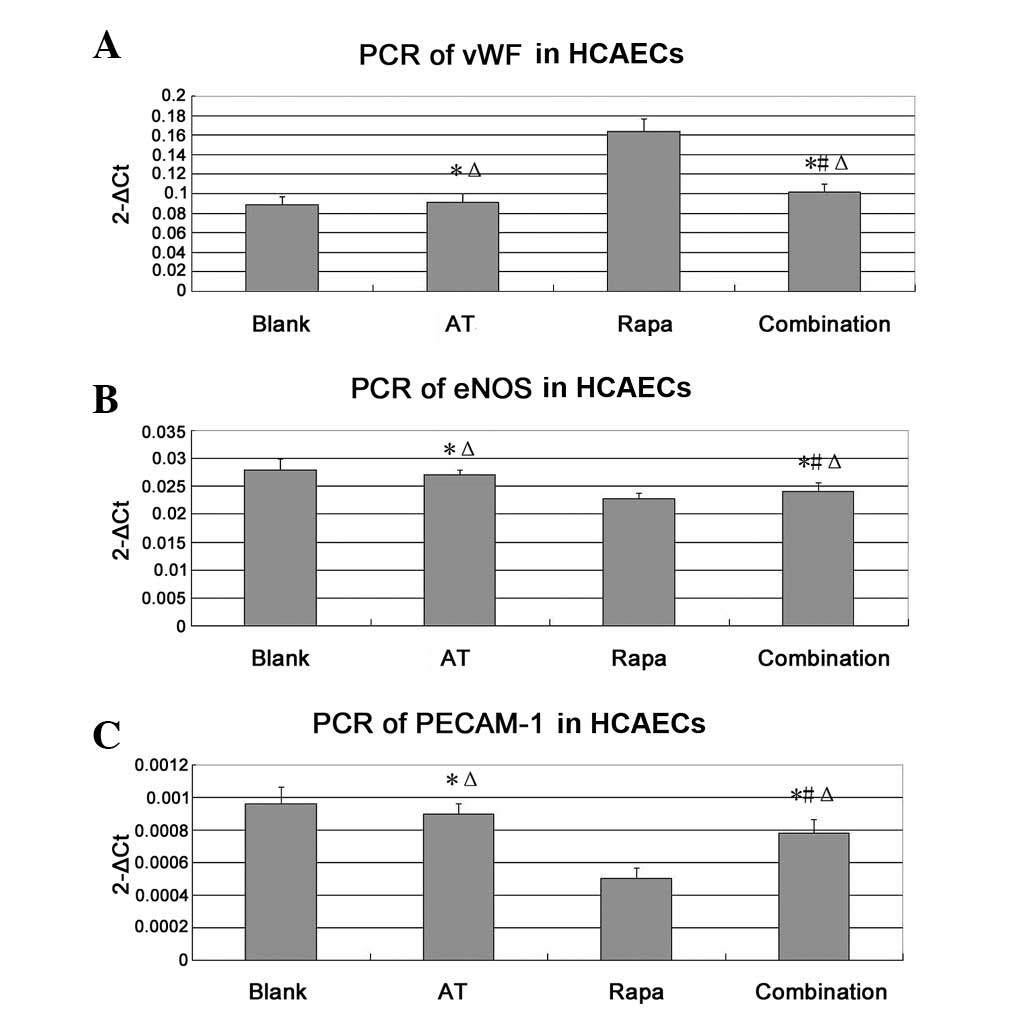
Levels of vWF, eNOS and PECAM-1 mRNAs
in HCAECs as shown by qPCR assay
As shown in Fig. 3,
compared with the level in the blank group, the vWF mRNA level of
the rapamycin group was significantly increased (P<0.05). The
mRNA expression level of the combination group was lower than that
of the rapamycin group (P<0.05), indicating that asiaticoside
may have antagonized the effect of rapamycin to downregulate the
vWF level, and thereby reduced the level of HCAEC apoptosis. The
eNOS and PECAM-1 mRNAs levels in the rapamycin group were
significantly reduced compared with those in the blank group
(P<0.05). However, in the combination group, the levels were
higher than those in the rapamycin group (P<0.05), suggesting
that asiaticoside may antagonize rapamycin and promote the
functional recovery of HCAECs by increasing the levels of eNOS and
PECAM-1.
Discussion
The results indicate that asiaticoside is likely to
be effective at reducing ISR in vivo and in vitro.
Asiaticoside combined with rapamycin exerted greater effects than
asiaticoside or rapamycin alone. Asiaticoside has a good synergism
with rapamycin to inhibit vascular smooth muscle cells (VSMSs) and
fibroblasts, while it is also antagonistic to ECs, which may
protect the vascular endothelium. The qPCR and ELISA results showed
that the combination therapy induced the downregulation of vWF,
type I collagen, TGF-β1 and TIMP1, and the upregulation of PECAM-1,
eNOS, Smad7 and MMP1. This suggests that the combination therapy
may function via the TGF-β pathway.
ISR is a process involving several pathological
pathways, in which VSMC and fibroblast proliferation, neointimal
formation, negative remodeling of the artery and epithelialization
delay play important roles. Rapamycin-eluting stents (RESs) are
widely used to treat severe stenosis of the coronary artery. As an
immunosuppressant, rapamycin binds to the cytosolic receptor
FKBP12, then inhibits mammalian target of rapamycin (mTOR), which
leads to inhibition of the down-regulation of the cyclin-dependent
kinase inhibitor p27kip1, thereby inhibiting VSMC proliferation and
migration (15). However,
rapamycin may also inhibit ECs at the same time (16) which contributes to delayed
endothelialization. The vascular endothelium is an efficient
barrier against thrombosis, lipid uptake and inflammation. In
addition, ECs produce various vasoactive substances, which maintain
vascular homeostasis (17).
Endothelium that has regenerated following percutaneous coronary
intervention (PCI) is incompetent in terms of its integrity and
function, with poorly formed cell junctions, reduced expression of
antithrombotic molecules and reduced nitric oxide production.
Delayed endothelial healing, characterized by poor
endothelialization, is the primary cause of late and very late
stent thrombosis following PCI. One small study demonstrated
impaired endothelial function in patients presenting with ISR,
compared with matched control subjects. This supports a hypothesis
that endothelial dysfunction contributes to the development of
restenosis, following PCI (18).
Thus, protecting ECs and promoting the recovery of endothelial
function requires further study (19).
Our study shows that asiaticoside has a synergism
with rapamycin in VSMCs and fibroblasts, which results in a greater
increase of cell growth inhibition rate than using a single drug.
The combination effects are achieved via several mechanisms.
Asiaticoside may inhibit the proliferation of VSMCs and
fibroblasts, which is consistent with other studies (8,20,21).
Asiaticoside upregulates Smad7 and TGF-β1, thus reducing synthesis
of type I collagen. Pan et al have demonstrated that
asiaticoside inhibits scar fibroblast growth via the Smad signal
pathway. The Smad7 protein and mRNA levels were reported to be
increased in asiaticoside-treated fibroblasts, compared with
control fibroblasts (8,21). It is likely that asiaticoside has
different functions in different tissues, and has distinct tissue
specificity. Nowwarote et al observed that asiaticoside
enhanced the expression of type I collagen in human periodontal
ligament cells (22), which
conflicted with our findings. However, our results in vascular
cells are consistent with previous results in scar, wound and renal
fibroblasts (23). Our results
revealed that the combination reduced the ratio value of
TIMP1/MMP1; this may also be reduced by increased TGF-β1 levels,
leading to an increase in the degradation of type I collagen.
In ECs, asiaticoside shows significant activity as a
rapamycin antagonist, therefore, the inhibition of cell
proliferation in the combination group is lower than that in the
rapamycin group. There are few studies concerning the effect of
asiaticoside on ECs. Zhou et al (24) established a rabbit model and
observed that asiaticoside had an accelerating action on EC growth
and was effective in the prevention of ISR. However, the mechanism
remains unclear. vWF is a blood glycoprotein involved in
hemostasis. Increased plasma levels are presumed to arise from
adverse changes to the endothelium and may contribute to an
increased risk of thrombosis. PECAM-1 is a protein which makes up a
large portion of endothelial cell intercellular junctions. eNOS is
secreted by ECs. Thus, the reduction of vWF mRNA and the increase
of PECAM-1 and eNOS mRNAs show that asiaticoside is able to
accelerate the recovery of EC function. According to our data, the
mechanism may be associated with the enhancement of eNOS and
PECAM-1.
References
|
1.
|
Mehran R, Dangas G, Abizaid AS, et al:
Angiographic patterns of ISR: classification and implications for
long-term outcome. Circulation. 100:1872–1878. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Guo JS, Cheng CL and Koo MW: Inhibitory
effects of Centella asiatica water extract and asiaticoside
on inducible nitric oxide synthase during gastric ulcer healing in
rats. Planta Med. 70:1150–1154. 2004.
|
|
3.
|
Cheng CL, Guo JS, Luk J and Koo MW: The
healing effects of Centella extract and asiaticoside on
acetic acid induced gastric ulcers in rats. Life Sci. 74:2237–2249.
2004.PubMed/NCBI
|
|
4.
|
Al-Saeedi FJ, Bitar M and Pariyani S:
Effect of asiaticoside on 99mTc-tetrofosmin and
99mTc-sestamibi uptake in MCF-7 cells. J Nucl Med
Technol. 39:279–283. 2011.
|
|
5.
|
Dong MS, Jung SH, Kim HJ, et al:
Structure-related cytotoxicity and anti-hepatofibric effect of
asiatic acid derivatives in rat hepatic stellate cell-line, HSC-T6.
Arch Pharm Res. 27:512–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wijeweera P, Arnason JT, Koszycki D and
Merali Z: Evaluation of anxiolytic properties of Gotukola -
(Centella asiatica) extracts and asiaticoside in rat
behavioral models. Phytomedicine. 13:668–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kimura Y, Sumiyoshi M, Samukawa K, et al:
Facilitating action of asiaticoside at low doses on burn wound
repair and its mechanism. Eur J Pharmacol. 584:415–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Pan S, Li T and Li Y: Effects of
asiaticoside on cell proliferation and smad signal pathway of
hypertrophic scar fibroblasts. Zhongguo Xiu Fu Chong Jian Wai Ke Za
Zhi. 18:291–294. 2004.(In Chinese).
|
|
9.
|
Suwanabol PA, Kent KC and Liu B: TGF-β and
restenosis revisited: a Smad link. J Surg Res. 167:287–297.
2011.
|
|
10.
|
Chamberlain J, Gunn J, Francis SE, et al:
TGF-β is active, and correlates with activators of TGF-β, following
porcine coronary angioplasty. Cardiovasc Res. 50:125–136. 2001.
|
|
11.
|
Wolff RA, Malinowski RL, Heaton NS, et al:
Transforming growth factor-β1 antisense treatment of rat vein
grafts reduces the accumulation of collagen and increases the
accumulation of h-caldesmon. J Vasc Surg. 43:1028–1036. 2006.
|
|
12.
|
Shi Y, O’Brien JE Jr, Fard A and Zalewski
A: Transforming growth factor-β 1 expression and myofibroblast
formation during arterial repair. Arterioscler Thromb Vasc Biol.
16:1298–1305. 1996.
|
|
13.
|
Mallawaarachchi CM, Weissberg PL and Siow
RC: Smad7 gene transfer attenuates adventitial cell migration and
vascular remodeling after balloon injury. Arterioscler Thromb Vasc
Biol. 25:1383–1387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: the
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
15.
|
Marx SO and Marks AR: Bench to bedside:
the development of rapamycin and its application to stent
restenosis. Circulation. 104:852–855. 2001. View Article : Google Scholar
|
|
16.
|
Kwon YS and Kim JC: Inhibition of corneal
neovascularization by rapamycin. Exp Mol Med. 38:173–179. 2006.
View Article : Google Scholar
|
|
17.
|
Vane JR, Anggård EE and Botting RM:
Regulatory function of the vascular endothelium. N Engl J Med.
323:27–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Thanyasiri P, Kathir K, Celermajer DS and
Adams MR: Endothelial dysfunction and restenosis following
percutaneous coronary intervention. Int J Cardiol. 119:362–367.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Inoue T, Croce K, Morooka T, et al:
Vascular inflammation and repair: implications for
re-endothelialization, restenosis, and stent thrombosis. JACC
Cardiovasc Interv. 4:1057–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Xie J, Li T, Qi S, Li Z, Liang H and Wu Y:
The effects of asiaticoside on fibroblasts in vitro culture.
Academic Journal of Sun Yat-Sen University of Medical Sciences.
22:41–43. 2001.(In Chinese).
|
|
21.
|
Qi SH, Xie JL, Pan S, et al: Effects of
asiaticoside on the expression of Smad protein by normal skin
fibroblasts and hypertrophic scar fibroblasts. Clin Exp Dermatol.
33:171–175. 2008. View Article : Google Scholar
|
|
22.
|
Nowwarote N, Osathanon T, Jitjaturunt P,
et al: Asiaticoside induces type I collagen synthesis and
osteogenic differentiation in human periodontal ligament cells.
Phytother Res. 27:457–462. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wang J, Cheng XX, Yang RC, et al:
Modification of Centella asiatica compound on renal
cytokines expression profiles in mice models with focal
glomerulosclerosis. Chinese Journal of Clinical Pharmacology and
Therapeutics. 8:638–641. 2003.
|
|
24.
|
Zhou J, Jiang H, Yi L, et al: Experimental
study of the effect of asiaticoside on preventing restenosis after
percutaneous coronary intervention. Journal of Xi’an Jiaotong
University (Medical Sciences). 26:477–497. 2005.(In Chinese).
|