Introduction
In general, the female body undergoes marked changes
during pregnancy, with alterations in nutritional status,
metabolism, endocrinology and circulation. Gestational weight gain
occurs as a result of the growth of the fetus and the mother: The
fetal weight increases and fetal appendages develop, and there is
an increase in the size of the mother’s uterus, breasts,
circulating blood volume and extracellular fluid volume (1). Hytten and Leitch have estimated
through metabolic analysis that the physiological weight gain in
pregnant females with an average build is ∼12.5 kg (2). By contrast, the average weight gain
observed in the average Japanese female during pregnancy is only
9.8–10.5 kg (3). At present, it is
accepted that there is a positive correlation between gestational
weight gain and the birth weight of the the infant (4). However, this correlation appears to
decrease when the prepregnancy body mass index (BMI) of the female
is high (4). A study performed in
the USA has demonstrated that a higher weight gain in females
during pregnancy resulted in a large baby and an increased
frequency of cesarean section, with the birth of low-weight infants
a rarity (5). In Japan, the
correlation between gestational weight gain and pregnancy-induced
hypertension (PIH) is particularly prominent, and priority has been
allocated to helping pregnant females limit their weight gain. This
is one reason for the recent reduction in infant birth weights in
Japan. In addition, it is possible that the desire in young females
to be thin, thereby reducing their BMI, is a contributory factor in
birth weight reduction.
Maternal and fetal outcomes are at risk with raised
and lowered prepregnancy BMIs. Females with a prepregnancy BMI
<18.5 kg/m2 are at risk of premature delivery and a
low birth weight of the infant (6–9).
However, studies have revealed that females with a BMI >29
kg/m2 are at risk of PIH, gestational diabetes, cesarean
section, a large baby and neural tube defects (10–14).
Similar to prepregnancy BMI, gestational weight gain also has a
significant impact on maternal and fetal outcomes. Gluckman and
Hanson previously reiterated the concept of the developmental
origins of health and disease (DOHaD), stating that ‘environmental
factors acting during the phase of developmental plasticity
interact with genotypic variation to change the capacity of the
organism to cope with its environment later in life’ (15). The maintenance of appropriate
nutrition in prepregnant and pregnant females is important in the
prevention of future illness.
Previous studies have revealed correlations among
prepregnancy BMI, gestational weight gain and resultant infant
birth weight. However, as a variety of indices relating to the
physique have been used to assess the optimal weight of pregnant
women, no conclusions have yet been established regarding the
Japanese population. Therefore, the aim of this study was to
investigate the correlations among prepregnancy BMI, gestational
weight gain and the birth weight of the infant in primiparous and
multiparous females. The study was a retrospective analysis of the
deliveries at a single birthing center in a provincial city in
Japan over a 10-year period.
Materials and methods
Study design
This study was a retrospective analysis of pregnancy
charts from a single birthing center (the Fukushi Birth Center,
Goshogawara, Aomori, Japan) from August 1998 until the end of
September 2007. The study was conducted in compliance with the
principles of the Declaration of Helsinki (as revised in Seoul,
2008) and the ethical guidelines for epidemiological research
provided by the Ministry of Education, Culture, Sports, Science and
Technology as well as the Ministry of Health, Labour and Welfare in
Japan (2008). All data used in the present study were coded and
obtained from the pregnancy charts of subjects without disclosing
their identity.
Selection of the study population
The criteria for inclusion were a singleton,
low-risk, full-term pregnancy (duration, 37–42 weeks), resulting in
a spontaneous vaginal delivery. Mothers with chronic diseases
(including diabetes, hypertension and hyperthyroidism), gestational
diabetes and PIH were excluded from this study. In accordance with
the World Health Organization (16) and the Japan Society for the Study
of Obesity (17), prepregnancy BMI
was classified into four groups: underweight (<18.5
kg/m2), normal (18.5 to <25 kg/m2),
overweight (25 to <30 kg/m2) and obese (≥30
kg/m2).
Data collection
A chart review was conducted of the pregnancy charts
of 579 females who delivered at the Fukushi Birth Center. Records
with unknown or missing data regarding obstetric factors were not
included. A total of 560 cases were available for the final
analysis. Perinatal data was collected on maternal age, parity,
self-reported prepregnancy weight, prepregnancy BMI, gestational
weight gain, chronic diseases, delivery mode, duration of
pregnancy, duration of labor, neonatal gender, neonatal size and
the weight of the mother, one month postpartum.
Data analysis
Statistical analysis was performed using SPSS
software, version 16.0 (SPSS Japan, Inc., Tokyo, Japan) for
Windows. Descriptive statistics are presented as the arithmetic
mean ± standard deviation. A two-sample t-test was performed to
determine differences across the three groups, while a
χ2 analysis was used to analyze categorical variables.
Univariate analysis was performed using the Pearson’s correlation
coefficient, and a multiple linear regression analysis was
performed to determine any correlation between the birth weight of
the infant (object functions) and maternal factors (explanatory
variables). P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographics and characteristics of the
study population
The population demographics and characteristics are
presented in Table I. The subjects
were either primiparous (n=220) or multiparous (n=340) females,
with an age range from 17 to 41 years (mean age, 24.1±3.8 and
28.2±4.3 years, respectively). The mean prepregnancy weight was
52.8±8.8 kg (range, 38–100 kg) in primiparous and 54.3±9.0 kg
(range, 40–96 kg) in multiparous females, while the mean
prepregnancy BMI was 20.8±3.1 kg/m2 (range, 15.8–35.4
kg/m2) in primiparous and 21.6±3.5 kg/m2
(range, 16.2–35.3 kg/m2) in multiparous females. The
maternal age, prepregnancy weight and prepregnancy BMI were
significantly higher in the multiparous than in the primiparous
females (P<0.05). Among the primiparous females, the mean
gestational weight gain was 12.7±4.3 kg (range, 1.8–25.5 kg), while
among the multiparous females it was 11.4±4.3 kg (range, −2.0–28.0
kg). The mean postpartum weight loss was 7.6±2.3 kg (range, 0–14.5
kg) and 7.1±2.3 kg (range, 1.0–16.6 kg) in primiparous and
multiparous females, respectively. The gestational weight gain and
postpartum weight loss were significantly higher in primiparous
than in multiparous females (P<0.05).
 | Table I.Summary and comparison of maternal
factors in parity. |
Table I.
Summary and comparison of maternal
factors in parity.
| Factors | Total population
(n=560) | Primiparous
(n=220) | Multiparous
(n=340) |
|---|
| Age (years)a | 26.6±4.6 | 24.1±3.8 | 28.2±4.3g |
| Smokersb | 108 (19.3) | 51 (23.2) | 57 (16.8) |
| Number of
cigarettes/day | 10.1±5.9 | 10.6±6.8 | 9.7±5.0 |
| Prepregnancy weight
(kg)a | 53.7±9.0 | 52.8±8.8 | 54.3±9.0e |
| Height (cm)a | 158.8±5.5 | 159.1±5.2 | 158.6±5.7 |
| Prepregnancy
BMIac (kg/m2) | 21.3±3.3 | 20.8±3.1 | 21.6±3.5f |
| BMI <18.5b | 85 (15.2) | 39 (17.7) | 46 (13.5) |
| BMI 18.5–25b | 405 (72.3) | 160 (72.7) | 245 (72.1) |
| BMI 25 to
<30b | 53 (9.5) | 17 (7.7) | 36 (10.6) |
| BMI ≥30b | 17 (3.0) | 4 (1.8) | 13 (3.8) |
| Delivery weight
(kg)a | 65.7±3.0 | 65.5±9.1 | 65.8±8.7 |
| Gestational weight
gain (kg)a | 11.9±4.3 | 12.7±4.3 | 11.4±4.3f |
| Duration of pregnancy
(weeks)a | 39.5±1.2 | 39.5±1.2 | 39.5±1.2 |
| 1 month postpartum
weight (kg)ad | 58.4±8.2 | 58.1±8.5 | 58.6±8.1 |
| Postpartum weight
loss (kg)ad | 7.3±2.3 | 7.6±2.3 | 7.1±2.3e |
The overall male infant birth rate was 50.9%
(Table II). The mean birth weights
of the infants were 3,153.0±364.1 g (range, 2,160–4,220 g) and
3,262.3±370.4 g (range, 2,190–4,540 g) for the primiparous and
multiparous females, respectively, while the overall rate of low
birth weight was 1.4%. Infant birth weight, head circumference and
chest circumference were all significantly higher in multiparous
than in primiparous females (P<0.05). In addition, the low birth
weight rate was significantly higher in primiparous than in
multiparous females (P<0.05).
 | Table II.Summary and comparison of the infants
in parity. |
Table II.
Summary and comparison of the infants
in parity.
| Factors | Total population
(n=560) | Primiparous
(n=220) | Multiparous
(n=340) |
|---|
| Malea | 285 (50.9) | 115 (52.3) | 170 (50.0) |
| Femalea | 275 (49.1) | 105 (47.7) | 170 (50.0) |
| Birth weight
(g)b | 3219.3±371.5 | 3153.0±364.1 | 3262.3±370.4d |
| Birth height
(cm)b | 49.7±1.8 | 49.5±1.9 | 49.8±1.8 |
| Head circumference
(cm)b | 33.3±1.5 | 33.1±1.6 | 33.5±1.3d |
| Chest circumference
(cm)b | 31.7±1.7 | 31.3±1.8 | 31.9±1.6e |
| Low birth
weighta | 8 (1.4) | 5 (2.3) | 3 (0.9)c |
| Placental weight
(g)b | 518.0±85.2 | 511.9±78.7 | 522.0±89.0 |
Correlation between maternal factors and
infant birth weight
The analysis of the correlation between maternal
factors and infant birth weight revealed that the birth weight was
positively correlated with prepregnancy weight, prepregnancy BMI,
delivery weight and the duration of the pregnancy. However, no
correlations were observed among birth weight, maternal age and
gestational weight gain (data not shown). In addition, birth weight
in the nonsmoking group was significantly higher than that in the
smoking group (3,234±372.0 versus 3,153.9±364.0 g, P<0.05, data
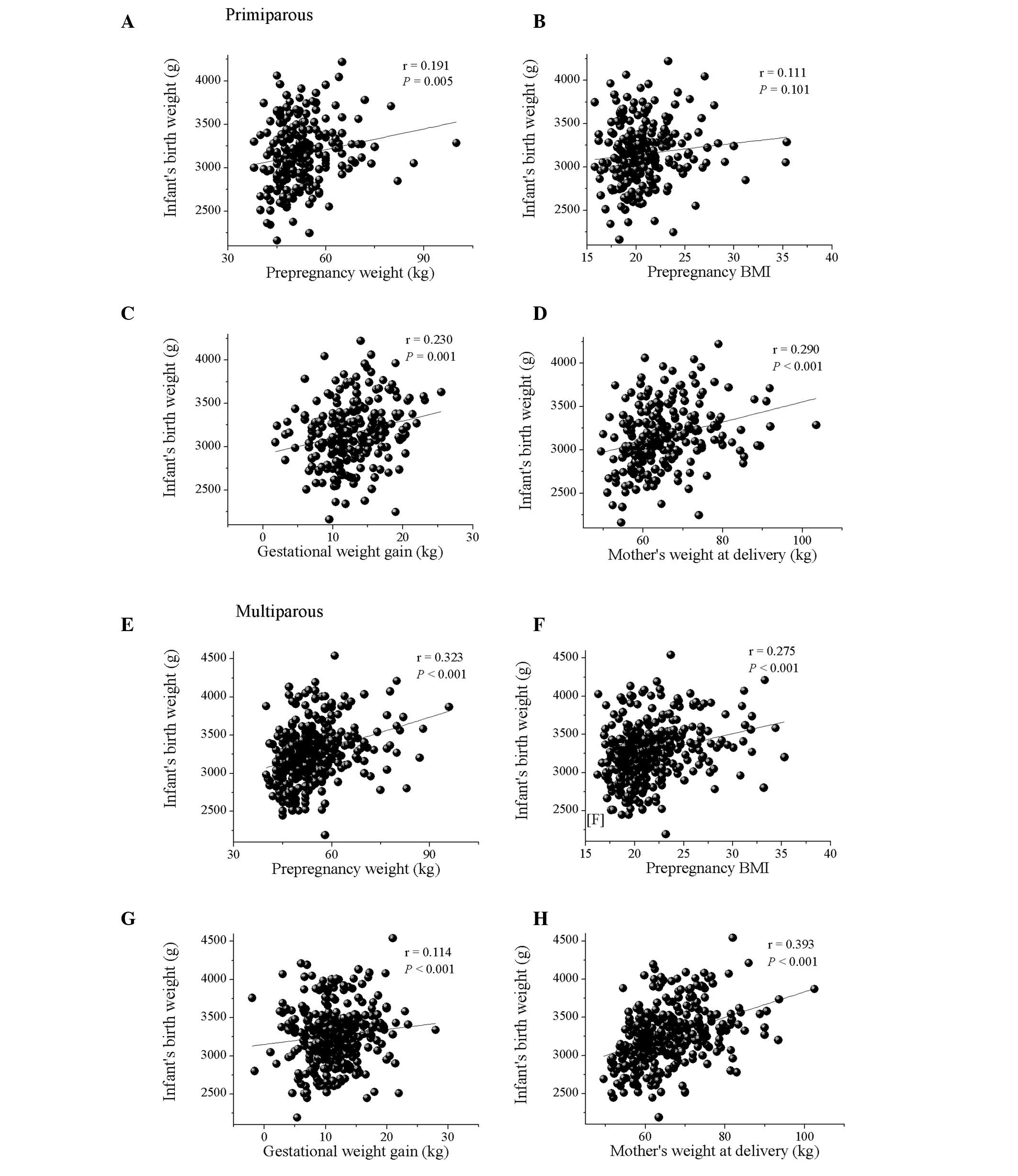
not shown). When comparing parity with the maternal factors, infant
birth weight was positively correlated with maternal delivery
weight and gestational weight gain in primiparous females (Fig. 1). However, no correlations were
observed among birth weight, prepregnancy weight and prepregnancy
BMI. In multiparous females, the birth weight of the infant was
positively correlated with prepregnancy weight, prepregnancy BMI
and maternal delivery weight, but no correlation was observed among
birth weight and gestational weight gain. Moreover, no significant
differences were revealed between the smoking and nonsmoking groups
in either primiparous or multiparous females (data not shown).
Discussion
This study analyzed the correlation between
prepregnancy BMI, gestational weight gain and infant birth weight
in primiparous and multiparous females, and was performed using a
retrospective analysis of pregnancy charts from a single birthing
center from August 1998 until the end of September 2007. The mean
age of the subjects was 26.6±4.6 years, which is lower than the
mean value for Japanese females (18). The rate of females who were
multiparous was also higher (60.7%) than the mean rate for Japan as
a whole. The average birth weight of the infants was 3,219.3±371.5
g, indicating that the rate of low birth weight was 1.4%. This
value revealed that there were fewer infants with a low birth
weight compared with the average for Japanese females. One
explanation may be that the birthing center only accepted females
with normal pregnancies that were expected to result in a safe
delivery and require little medical intervention. The average
prepregnancy BMI was 21.3±3.3 kg/m2, and the underweight
group (those with a BMI <18.5 kg/m2) accounted for
15.2% of the total subject population. There has been a sharp
increase in the number of underweight females aged 20–29 years in
Japan, and this study reflected that trend (19). This is therefore becoming a greater
social issue.
It is accepted that there is a linear correlation
between maternal prepregnancy BMI and the mean birth weight of the
infant (20). In addition, various
other factors have been observed to affect the birth weight of the
infant, as follows: the gender of the infant, gestational age,
smoking, obstetric history and genetic predisposition (21,22).
Neonatal anthropometric charts for gestational age are widely used
by obstetricians and pediatricians to predict neonatal risk and to
monitor infants. According to the anthropometric charts used in
Japan, certain differences have been revealed with regard to the
gestational weight gain in primiparous and multiparous females from
∼32 weeks of gestation (23). In
addition, a recent large-scale study observed that although there
were differences in the timing of the appearance of weight
differences, the birth weights of the infants of multiparous
females were higher than those of primiparous females (24). Although the mechanism for these
observed weight differences has yet to be elucidated, the birth
weight of the infant was generally higher for multiparous than for
primiparous females. The results of the present study concurred
with this, with the infants born to multiparous females being
heavier than those born to primiparous females (Table II). Furthermore, the gestational
weight gain was observed to be weakly correlated with the birth
weight of the infant in primiparous females (Fig. 1C). By contrast, prepregnancy weight
and BMI were revealed to be positively correlated with birth weight
in multiparous females (Fig. 1E and
F). The relationship between maternal physique and gestational
weight gain has been well established. However, this study
suggested that the factors influencing infant birth weight were
different for primiparous and multiparous females. In addition, the
fact that prepregnancy weight and BMI were significantly higher in
multiparous than in primiparous females may have influenced the
differences in birth weight between the two groups.
The primiparous and multiparous females were also
compared in terms of changes in maternal physique during pregnancy
and at one month postpartum. Prepregnancy BMI was observed to be
higher in multiparous than in primiparous females, and gestational
weight gain and weight reduction following delivery were higher in
primiparous females (Table I).
These results suggested that there may be small differences in
maternal physique between primiparous and multiparous females that
may be correlated with pregnancy and delivery. The maternal body
accumulates fat during the second trimester, and maternal lipid
metabolism then switches to a catabolic condition during the third
trimester to adapt to the increased nutritional requirements of
late pregnancy (25). Obesity
increases the risk of diseases such as hypertension, type 2
diabetes, hyperlipidemia and arteriosclerosis (26–28).
Therefore, from the perspective of disease prevention and health
maintenance, it is necessary to not only control weight during
pregnancy but also following delivery.
In conclusion, the results of the present study have
revealed significant differences between primiparous and
multiparous females, with regard to gestational weight gain and
weight reduction following delivery. In addition, the study
suggested that the factors influencing birth weight differed
between primiparous and multiparous females. The degree of
gestational weight gain and the prepregnancy physique have
significant impacts on the outcome of pregnancy for the mother and
the infant. Since these outcomes also affect the long-term health
of the mother and child, there is a requirement for health guidance
to be tailored to the individual pregnant female. To improve
maternal and fetal outcomes, further studies regarding the
correlations between prepregnancy physique and the degree of
gestational weight gain are necessary.
Acknowledgements
The authors are indebted to the
midwives of the Fukushi Birth Center (Goshogawara, Japan) for
collecting the pregnancy charts. This study was supported by a
grant for Hirosaki University Institutional Research (2011–2012).
Ikuo Kashiwakura organized the present study and took overall
responsibility, while Takako Chiba and Satoko Ebina analyzed the
data obtained in this study. Takako Chiba and Ikuo Kashiwakura
wrote the manuscript, following discussions with the other authors.
All authors contributed equally to the preparation of the
manuscript.
References
|
1.
|
Cunningham FG, Leveno KJ, Bloom SL, et al:
Maternal physiology. Williams Obstetrics. 23rd edition.
McGraw-Hill; New York, NY: pp. 107–135. 2010
|
|
2.
|
Hytten FE and Leitch I: The Physiology of
Human Pregnancy. 2nd edition. Blackwell Scientific Publications;
Oxford, United Kingdom: pp. 5991971
|
|
3.
|
Ueda Y, Maruo M and Niiya K: Study on the
usefulness of maternal weight gain at 16 weeks’ gestation for the
prediction of total weight gain during pregnancy. Acta Obstet
Gynaecol Jpn. 53:980–988. 2001.(In Japanese).
|
|
4.
|
Johnson JW, Longmate JA and Frentzen B:
Excessive maternal weight and pregnancy outcome. Am J Obstet
Gynecol. 67:353–370; discussion 370–372,. 1992. View Article : Google Scholar
|
|
5.
|
Weiss JL, Malone FD, Emig D, et al FASTER
Research Consortium: Obesity, obstetric complications and cesarean
delivery rate - a population-based screening study. Am J Obstet
Gynecol. 190:1091–1097. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Spinillo A, Capuzzo E, Piazzi G, Ferrari
A, Morales V and Di Mario M: Risk for spontaneous preterm delivery
by combined body mass index and gestational weight gain patterns.
Acta Obstet Gynecol Scand. 77:32–36. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Schieve LA, Cogswell ME, Scanlon KS, et al
The NMIHS Collaborative Study Group: Prepregnancy body mass index
and pregnancy weight gain: associations with preterm delivery.
Obstet Gynecol. 96:194–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ehrenberg HM, Dierker L, Milluzzi C and
Mercer BM: Low maternal weight, failure to thrive in pregnancy, and
adverse pregnancy outcomes. Am J Obstet Gynecol. 189:1726–1730.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hendler I, Goldenberg RL, Mercer BM, et
al: The Preterm Prediction Study: association between maternal body
mass index and spontaneous and indicated preterm birth. Am J Obstet
Gynecol. 92:882–886. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Shaw GM, Velie EM and Schaffer D: Risk of
neural tube defect-affected pregnancies among obese females. JAMA.
275:1093–1096. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cnattingius S, Bergström R, Lipworth L and
Kramer MS: Prepregnancy weight and the risk of adverse pregnancy
outcomes. N Engl J Med. 338:147–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jensen DM, Damm P, Sørensen B, et al:
Pregnancy outcome and prepregnancy body mass index in 2459
glucose-tolerant Danish females. Am J Obstet Gynecol. 189:239–244.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Bhattacharya S, Campbell DM, Liston WA and
Bhattacharya S: Effect of body mass index on pregnancy outcomes in
nulliparous females delivering singleton babies. BMC Public Health.
7:1682007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Crane JM, White J, Murphy P, Burrage L and
Hutchens D: The effect of gestational weight gain by body mass
index on maternal and neonatal outcomes. J Obstet Gynaecol Can.
31:28–35. 2009.PubMed/NCBI
|
|
15.
|
Gluckman P and Hanson M: The conceptual
basis for the developmental origins of health and disease.
Developmental Origins of Health and Disease. Cambridge University
Press; Cambridge, United Kindom: pp. 33–50. 2006, View Article : Google Scholar
|
|
16.
|
World Health Organization (WHO): Global
Database on Body Mass Index, BMI classification. http://www.who.int/bmi/index.jspuri.
Accessed November 12, 2012.
|
|
17.
|
Japan Society for the Study of Obesity
(JASSO): Obesity diagnosis by body weight and BMI criteria.
http://www.soc.nii.ac.jp/jasso/index.htmluri.
Accessed November 12, 2012 (In Japanese).
|
|
18.
|
Ministry of Health, Labour and Welfare:
Summary report of annual vital statistics report (preliminary
data). 2011, http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai11/kekka02.htmluri.
Accessed December 1, 2012 (In Japanese).
|
|
19.
|
Ministry of Health, Labour and Welfare:
National Health and Nutrition Survey. http://www.mhlw.go.jp/stf/houdou/2r98520000020qbb-att/2r98520000021c1g.pdf#search='http%3A%2F%2Fwww.mhlw.go.jp%2Fstf%2Fhoudou%2F2r98520000020qbbatt%2F2r98520000021c1g.pdfuri.
Accessed December 1, 2012 (In Japanese).
|
|
20.
|
Getahun D, Ananth CV, Peltier MR, Salihu
HM and Scorza WE: Changes in prepregnancy body mass index between
the first and second pregnancies and risk of
large-for-gestational-age birth. Am J Obstet Gynecol. 196:530.e1–8.
2007.PubMed/NCBI
|
|
21.
|
Thame M, Osmond C, Bennett F, Wilks R and
Forrester T: Fetal growth is directly related to maternal
anthropometry and placental volume. Eur J Clin Nutr. 58:894–900.
2004.PubMed/NCBI
|
|
22.
|
Frederick IO, Williams MA, Sales AE,
Martin DP and Killien M: Pre-pregnancy body mass index, gestational
weight gain, and other maternal characteristics in relation to
infant birth weight. Matern Child Health J. 12:557–567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ogawa Y, Iwamura T, Kuriya N, et al: Birth
size standards by gestational age for Japanese neonates. Acta
Neonatol Jpn. 34:624–632. 1998.(In Japanese).
|
|
24.
|
Neonatal Research Network: A Multicenter
Benchmark Research on Neonatal Outcome in Japan. http://nrn.shiga-med.ac.jp/DOC/NRNcommon/hokokusho/H21/H21HOKOKUSHO_SOKATSU_BUNTAN.pdfuri.
Accessed December 1, 2012 (In Japanese).
|
|
25.
|
Herrera E and Amusquivar E: Lipid
metabolism in the fetus and the newborn. Diabetes Metab Res Rev.
16:202–210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kaplan NM: The deadly quartet. Upper-body
obesity, glucose intolerance, hypertriglyceridemia, and
hypertension. Arch Intern Med. 149:1514–1520. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Shaper AG, Wannamethee SG and Walker M:
Body weight: implications for the prevention of coronary heart
disease, stroke, and diabetes mellitus in a cohort study of middle
aged men. BMJ. 314:1311–1317. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Schulte H, Cullen P and Assmann G:
Obesity, mortality and cardiovascular disease in the Münster Heart
Study (PROCAM). Atherosclerosis. 144:199–209. 1999.
|