Introduction
A pulmonary nodule (PN) is defined as a spherical,
radiographic opacity <3 cm in diameter that is entirely
surrounded by lung tissue (1,2).
18F-fluorodeoxyglucose positron emission
tomography/computed tomography (18F-FDG-PET/CT) has been
widely used in the differential diagnosis of multiple PNs, since it
is able to effectively detect any PNs, as well as nodules elsewhere
in the body, in addition to monitoring the metabolic status of the
nodules (3,4). PNs with a high metabolic activity are
often considered to be metastatic (5). According to the clinical practice
guidelines of the American College of Chest Physicians (ACCP)
(6), the current therapeutic
strategies for patients with multiple highly metabolically active
PNs include radiographical follow-up, tissue sampling or surgical
resection. Clinicians are required to discuss the risks and
benefits of alternative management strategies and elicit patient
preferences; however, there is no general consensus with regard to
the optimal management of cases, which results in certain
challenges.
The current study describes the case of a patient
with multiple PNs with high metabolic activity, where an initial
diagnosis of lung cancer metastasis was proposed. However, it was
not possible to obtain sufficient clarification of the diagnosis
through conventional methods, including transbronchial lung biopsy
(TBLB), and therefore multiple nodule biopsies were performed by
video-assisted thoracic surgery (VATS). This resulted in an
unexpected diagnosis, which entailed a better prognosis and a
change in the therapeutic strategy. The aim of this study was to
assess the role of multiple nodule biopsies by VATS in the
diagnosis of multiple highly metabolically active PNs in the
context of a case observed in The First Affiliated Hospital of
Guangzhou Medical University (Guangzhou, China). Written informed
consent was obtained from the patient for the publication of this
case report and any accompanying images.
Case report
A 70-year-old male was admitted to The First
Affiliated Hospital of Guangzhou Medical University due to a cough
with sputum that had been present for half a month. The patient had
a 40-year smoking history. A physical chest examination did not
reveal any significant signs of abnormalities and the results of
laboratory tests were not indicative of a specific diagnosis. A
computed tomography (CT) scan of the chest revealed multiple PNs,
with the largest nodule measuring 1.9 cm in its maximum dimension.
This nodule was located at the basal segment of left lower lobe.
When no improvement was observed following a one-week course of
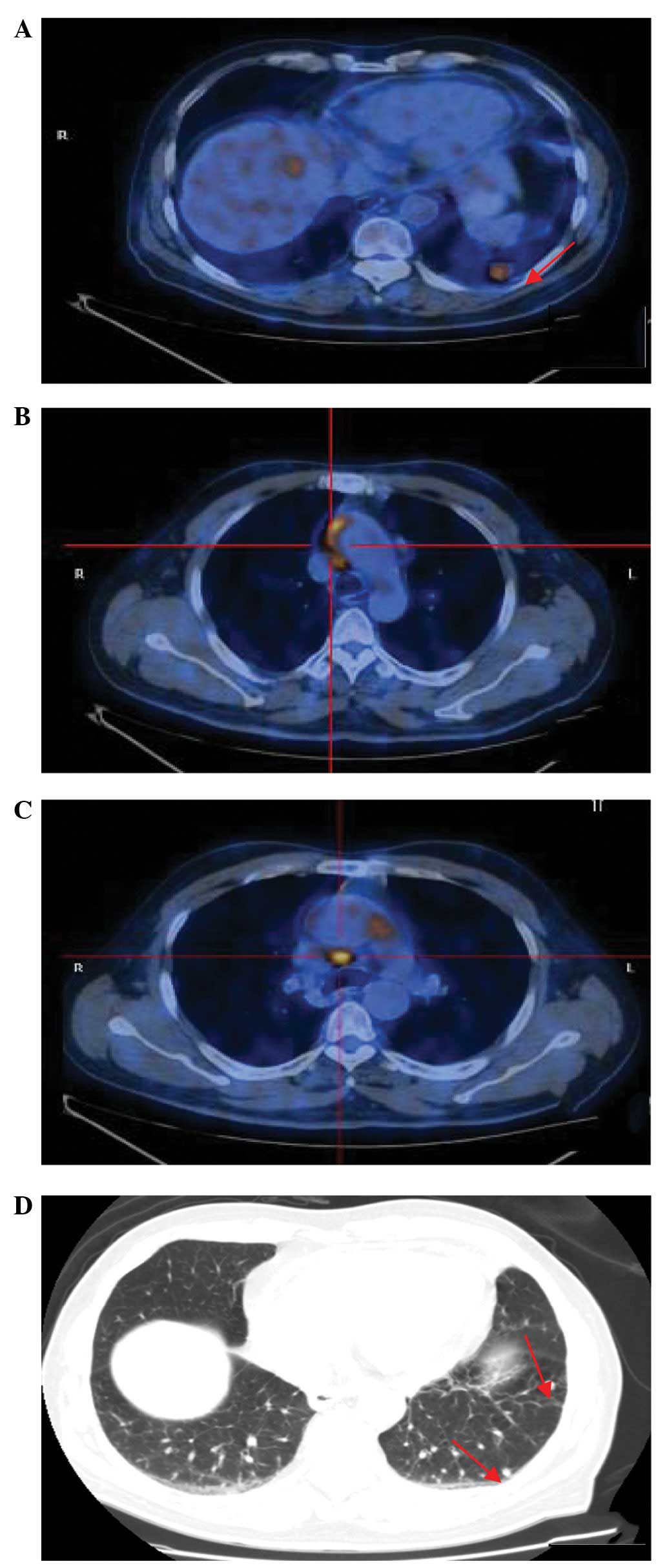
antibiotics, an 18F-FDG-PET/CT scan was performed, which
revealed an increased uptake in the largest pulmonary nodule, with
a maximum standardized uptake value (SUVmax) of 5.8
(Fig. 1A). The two foci were
located in the ascending aorta/aortic arch wall and pericardial
wall with SUVmax values of 6.4 and 8.3, respectively
(Fig. 1B and C). In addition,
mediastinal lymph nodes with an SUVmax of 2.5 and other
PNs with normal uptakes were observed (Fig. 1D). These observations resulted in a
diagnosis of lung cancer metastasis being proposed. Following this,
a bronchoscopy and a TBLB, guided by X-ray, were performed;
however, no positive result was indicated.
To obtain a definitive diagnosis and an appropriate
treatment, a VATS lung biopsy was performed, following the
provision of signed informed consent from the patient. Three
nodules, including the largest nodule, a nodule at the lingular
segment of the left upper lobe and a nodule at the pericardial
wall, were completely enucleated during one surgical session under
the same anesthesia The histological results of the frozen sections
obtained from the nodules intraoperatively revealed that the
largest nodule was a lung carcinoma, while the remaining nodules
were indicative of tuberculosis. The standard treatment of
lobectomy with systematic mediastinal, hilar and interlobar
lymphadenectomies was completed by VATS. The pathological sections
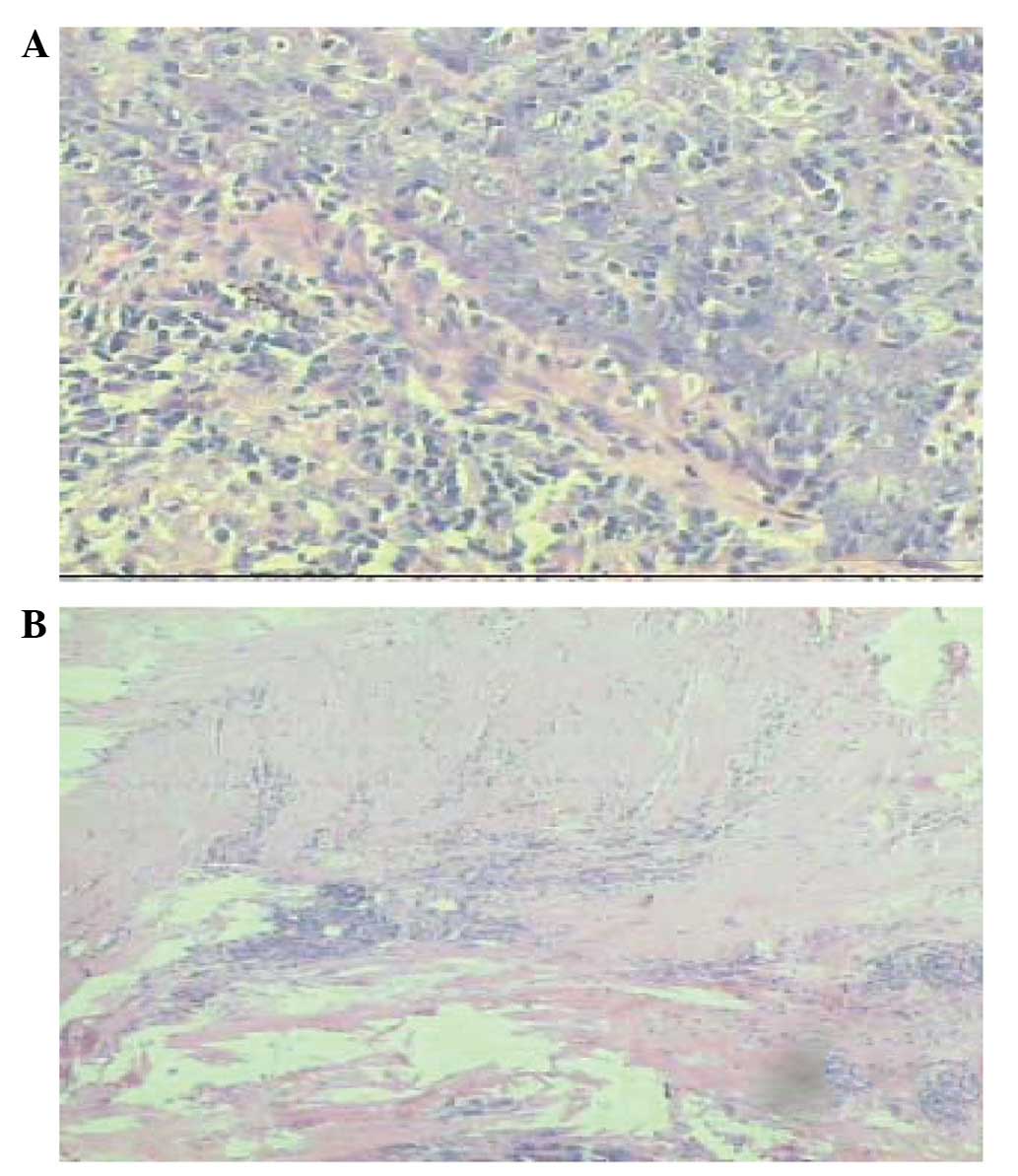
and immunohistochemistry confirmed a diagnosis of
lymphoepithelioma-like carcinoma (LELC) (stage
pT1N0M0 IA) in the largest nodule
(Fig. 2A), while the two remaining
small PNs and the pericardial nodule were confirmed as tuberculoid,
with observations of hyaline degeneration and hyperplasia of the
surrounding lymphoid tissue (Fig.
2B), indicating obsolete or active tuberculosis.
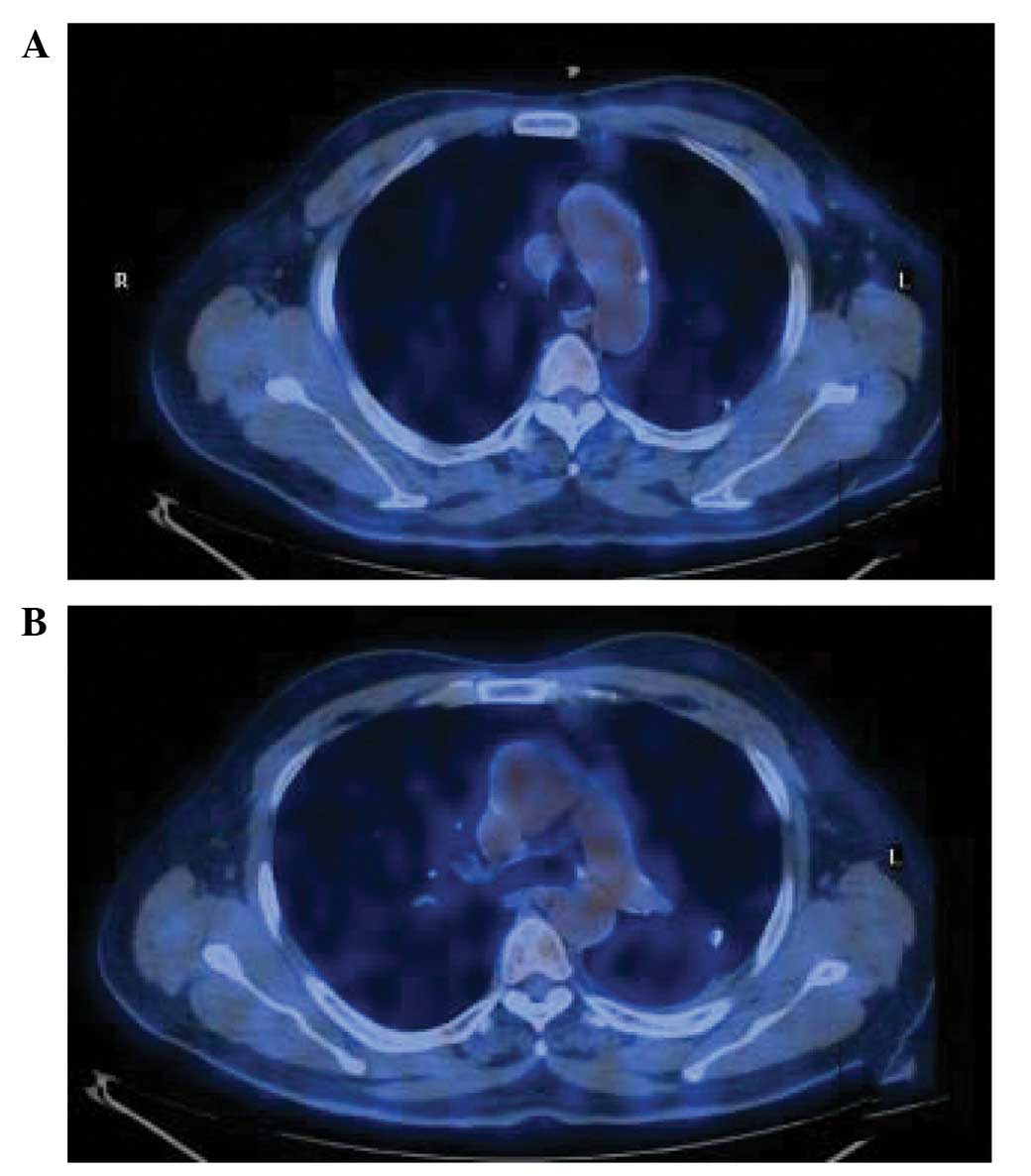
Two weeks subsequently, the patient was treated with
anti-tuberculous drugs. In the six months of follow-up, the patient
did not present with any symptoms and 18F-FDG-PET/CT
revealed that the remaining PNs were stable, with no change in
size, the nodules of the ascending aorta/aortic arch and
pericardial wall had disappeared and the 18F-FDG uptakes
were normal (Fig. 3).
Discussion
Multiple highly metabolically active PNs (in
addition to nodules elsewhere in the body) are common in clinical
practice, with the PNs frequently resulting in a differential
diagnosis of lung cancer metastasis (7–9). The
efficacy of 18F-FDG-PET/CT in the differentiation of
benign and malignant PNs >1 cm has been investigated in a number
studies (3,4); however, there is a high occurrence of
false negative results for nodules <1 cm with regard to highly
differentiated adenocarcinoma and slowly progressive malignant
tumors (10,11). Furthermore,
18F-FDG-PET/CT is not able to identify lung cancer in
combination with other metabolic diseases (12). Therefore, the presence of multiple
PNs with high metabolic activity is always associated with
diagnostic and therapeutic challenges. In the present case, taking
into consideration factors such as age, smoking history, symptoms,
treatment and the result of the PET/CT scan, lung cancer metastasis
was the primary conclusion. Therefore, there is a requirement for
the employment of precise methods to ensure the early discovery of
malignant nodules, in order to improve the prognosis.
The main aims of nodule treatment include the
identification of malignant nodules at the earliest opportunity and
the avoidance of the surgical treatment of benign nodules (5,13).
TBLB is a moderately invasive technique; however, the sensitivity,
guided by radial probe endobronchial ultrasonography (14) or electromagnetic navigation
bronchoscopy (15), has potential
for improvement. For peripheral nodules, the sensitivity of
transthoracic needle aspiration (TTNA) is higher than that of TBLB,
and has been observed to vary from 70 to 100% (16,17).
However, challenges become apparent when the results are negative;
furthermore, it is easy to ignore the coexistence of other
diseases. This may lead to misdiagnosis and diagnostic errors when
one nodule has a positive pathology (15). In the present study, following the
failure of the TBLB to provide a positive result, it was relatively
difficult to perform a biopsy by TTNA and somewhat easier to
consider a diagnosis of lung cancer metastasis and then delay the
treatment if a positive pathological result was obtained from the
TBLB. Thus, TBLB and TTNA exhibit numerous limitations with regard
to the diagnosis of multiple highly metabolically active PNs.
A VATS lung biopsy possesses fundamental advantages
for the diagnosis of multiple PNs with high metabolic activity. It
reduces the surgical trauma, the duration of the hospital stay, the
postoperative pain and the time required for the complete recovery
of the patients’ normal activity. Furthermore, there have been no
intra- or postoperative mortalities or significant intraoperative
complications observed with the procedure (18). The biopsy is performed under
general anesthesia using lung ventilation via a double-lumen
endotracheal tube. Recently, it has been demonstrated that the VATS
may be performed under anesthesia with nortraceal intubation
(19). It provides excellent
visualization of the entire lung surface, chest wall and
mediastinum; moreover, it enables multiple nodule biopsies to be
performed during one surgical session. The samples obtained provide
the potential for the acquisition of a fast, certain and definitive
diagnosis by intraoperative frozen section histology. As the
procedure has been used more extensively, the range of detectable
nodule diameters has expanded, reportedly varying between 3 and 30
mm (18). When the detection of
subpleural nodules is difficult due to the nodules being too small
or too far from the pleural surface, the placement of a
preoperative CT-guided marking using a hookwire with a string may
be done promptly, to enable the VATS lung biopsy (20). However, for patients with lung
cancer metastasis, the surgery is only a means for providing a
diagnosis; therefore, a conservative treatment option is always
likely to be selected by the patients. In the present study, taking
into consideration the age, trauma, economy and the initial
diagnosis of lung cancer metastasis, the patient was not willing
for the VATS to be conducted. After the patient was persuaded to
reconsider and agree to undergo the VATS, a VATS lung biopsy was
performed for the different nodules and a diagnosis of LELC and
tuberculosis was reached. The standard method of treatment, i.e.
lobectomy with systematic mediastinal, hilar and interlober
lymphadenectomies, was then implemented, which avoided delay and
improved the prognosis of the patient.
In conclusion, in the present study the VATS lung
biopsy was demonstrated to be a safe and effective procedure that
enabled an accurate diagnosis through multiple nodule biopsies in a
minimally invasive manner. The procedure was particularly
beneficial in this case, since it avoided excessive instrumental
examinations, misdiagnoses and inappropriate treatments.
References
|
1.
|
Ost D, Fein AM and Feinsilver SH: Clinical
practice. The solitary pulmonary nodule. N Engl J Med.
348:2535–2542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Winer-Muram HT: The solitary pulmonary
nodule. Radiology. 239:34–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Herder GJ, Golding RP, Hoekstra OS, Comans
EF, Teule GJ, Postmus PE and Smit EF: The performance of
18F-fluorodeoxyglucosepositron emission tomography in
small solitary pulmonary nodules. Eur J Nucl Med Mol Imaging.
31:1231–1236. 2004.
|
|
4.
|
Fischer BM, Mortensen J, Langer SW, Loft
A, Berthelsen AK, Daugaard G, Lassen U and Hansen HH: PET/CT
imaging in response evaluation of patients with small cell lung
cancer. Lung Cancer. 54:41–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wahidi MM, Govert JA, Goudar RK, Gould MK
and McCrory DC; American College of Chest Physicians: Evidence for
the treatment of patients with pulmonary nodules: when is it lung
cancer?: ACCP evidence-based clinical practice guidelines (2nd
edition). Chest. 132(Suppl): 94S–107S. 2007. View Article : Google Scholar
|
|
6.
|
Rivera MP and Mehta AC; American College
of Chest Physicians: Initial diagnosis of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132(Suppl): 131S–148S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cardillo G, Regal M, Sera F, Di Martino M,
Carbone L, Facciolo F and Martelli M: Videothoracoscopic management
of the solitary pulmonary nodule: a single-institution study on 429
cases. Ann Thorac Surg. 75:1607–1612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Li Y, Su M, Li F, Kuang A and Tian R: The
value of 18F-FDG-PET/CT in the differential diagnosis of
solitary pulmonary nodules in areas with a high incidence of
tuberculosis. Ann Nucl Med. 25:804–811. 2011.
|
|
9.
|
Gould MK, Fletcher J, Iannettoni MD, Lynch
WR, Midthun DE, Naidich DP and Ost DE; American College of Chest
Physicians: Evaluation of patients with pulmonary nodules: when is
it lung cancer?: ACCP evidence-based clinical practice guidelines
(2nd edition). Chest. 132(Suppl): 108S–130S. 2007. View Article : Google Scholar
|
|
10.
|
Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi
M, Noguchi T, et al: Fluorine-18-FDG PET imaging is negative in
bronchioloalveolar lung carcinoma. J Nucl Med. 39:1016–1020.
1998.PubMed/NCBI
|
|
11.
|
Lowe VJ, Fletcher JW, Gobar L, Lawson M,
Kirchner P, Valk P, Karis J, Hubner K, Delbeke D, et al:
Prospective investigation of positron emission tomography in lung
nodules. J Clin Oncol. 16:1075–1084. 1998.PubMed/NCBI
|
|
12.
|
Shinagare AB, Cunto-Amesty G and Fennessy
FM: Multiple inflammatory nodules: a differential diagnosis of new
pulmonary nodules in oncology patients. Cancer Imaging. 10:205–208.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hodnett PA and Ko JP: Evaluation and
management of indeterminate pulmonary nodules. Radiol Clin North
Am. 50:895–914. 2012. View Article : Google Scholar
|
|
14.
|
Oki M, Saka H, Kitagawa C, Kogure Y,
Murata N, Adachi T and Ando M: Randomized study of endobronchial
ultrasound-guided transbronchial biopsy: thin bronchoscopic method
versus guide sheath method. J Thorac Oncol. 7:535–541. 2012.
View Article : Google Scholar
|
|
15.
|
Lamprecht B, Porsch P, Wegleitner B,
Strasser G, Kaiser B and Studnicka M: Electromagnetic navigation
bronchoscopy (ENB): Increasing diagnostic yield. Respir Med.
106:710–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Klein JS and Zarka MA: Transthoracic
needle biopsy. Radiol Clin North Am. 38:235–266. 2000. View Article : Google Scholar
|
|
17.
|
Klein JS, Salomon G and Stewart EA:
Transthoracic needle biopsy with a coaxially placed 20-gauge
automated cutting needle: results in 122 patients. Radiology.
198:715–720. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Prisadov GC, Wallimann H and Welcker K:
Our experience in the diagnostics and therapy of patients with
solitary peripheral lung tumours. Folia Med (Plovdiv). 53:47–52.
2011.PubMed/NCBI
|
|
19.
|
Dong Q, Liang L, Li Y, Liu J, Yin W, Chen
H, et al: Anesthesia with nontracheal intubation in thoracic
surgery. J Thorac Dis. 4:126–130. 2012.PubMed/NCBI
|
|
20.
|
Chen YR, Yeow KM, Lee JY, Su IH, Chu SY,
Lee CH, et al: CT-guided hook wire localization of subpleural lung
lesions for video-assisted thoracoscopic surgery (VATS). J Formos
Med Assoc. 106:911–918. 2007. View Article : Google Scholar : PubMed/NCBI
|