Introduction
Spinal cord injury (SCI) is a global problem that
may lead to permanent disability. As such, the reversal of SCI has
become the focus of numerous studies. In recent years, tissue
engineering, as a new technology, has been utilized in a number of
areas in bioscience. There are three key elements of tissue
engineering: seed cells, scaffold materials and growth factors
(1). The use of this technology to
repair SCI is, at present, a key subject of scientific study
(2). The predominant factor that
determines the success of the repair process is the interaction
between the seed cells and the scaffold (3,4);
therefore, the selection of the appropriate cells and scaffold with
the desired biocompatibility is crucial.
Since adipose tissue-derived stem cells (ADSCs) are
readily accessible and demonstrate rapid proliferation, they are
commonly used as seed cells (5,6). In
tissue engineering technology, the scaffold acts as an artificial
extracellular matrix (ECM); it provides a template that supports
cell attachment, guides cell proliferation and differentiation and
acts as a carrier for the transportation of cells to the site of
the defect (7). A number of
natural and synthetic polymers are currently being used as scaffold
materials. These include silk-fibroin (SF), which possesses
desirable mechanical properties, biocompatibility and the ability
to support several cell types, but when dry is brittle and
challenging to handle (8,9). Another polymer that has been
investigated for use as a scaffold in tissue engineering is
chitosan (CS); its biocompatibility, biodegradability and toxicity
have been extensively investigated. However, pure CS scaffolds have
been demonstrated to degrade rapidly and display a high-swelling
property in aqueous solution (10). In order to avoid the problems
presented by these individual polymers, the blending of the
materials has been suggested (11,12).
Silk fibroin-chitosan scaffolds (SFCSs) have been demonstrated to
possess desirable mechanical properties and may therefore be used
in the repair of SCI (13,14). However, the biocompatibility of
ADSCs with SFCSs remains largely unexplored.
The aim of the current study was to extract,
identify and differentiate ADSCs from Sprague-Dawley (SD) rats and
to assess their biocompatibility with SFCSs. Furthermore, the study
aimed to provide a foundation for further experiments investigating
the use of tissue engineering technology in the repair of SCI.
Materials and methods
Experimental animals
Three-month-old SD rats, weighing 300±50 g, were
provided by the Experimental Animal Center of the Medical School of
Xi’an Jiaotong University (Xi’an, China). All the experiments were
approved and supervised by the Ethics Committee of Xi’an Jiaotong
University.
Isolation and culture of ADSCs
Pentobarbital sodium (40 mg/kg; Sigma, St. Louis,
MO, USA) was used to anesthetize the rats. The adipose tissue was
then harvested, sheared into pieces and incubated for 1 h with 0.1%
type I collagenase (Sigma) in a centrifuge tube containing
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Sigma) with 20%
fetal bovine serum (FBS; Hyclone, Logan, UT, USA) at 37°C.
Following this, the mixture was centrifuged at 3,000 × g for 12
min, the supernatant was discharged and phosphate-buffered saline
(PBS; Sigma) was added. This was subsequently centrifuged at 1,200
× g for 8 min, prior to the sediment being suspended with DMEM/F12.
The cell density was then adjusted to 1×105 / ml and the
cells were seeded in a 25 cm2 cell culture flask, which
was placed into a cell culture box at a temperature of 37°C, 5%
CO2 concentration and 95% humidity. When the original
cell generation reached 90% confluence, the entire medium was
aspirated and the cells were washed with prewarmed PBS. This was
achieved by pipetting the solution over the cell layer several
times, in order to clean the cells thoroughly of any tissue
fragments and blood cells. Following this, 0.25% trypsin/0.02% EDTA
solution (Sigma) was added to the cells. The process was observed
using an inverted phase contrast microscope, and when the cells
detached from the culture flask, DMEM/F12 was added to terminate
the digestion. Following this, centrifugation was performed at
1,700 × g for 10 min. The supernatant was then aspirated and the
cells were suspended with DMEM/F12 (containing 20% FBS), to enable
the passage of cells according to a 1:3 ratio.
Induction of the cells into adipogenic
and osteogenic directions
The third generation of cells were seeded at a
density of 1×104 /well in six-well plates, prior to
being divided into control and experimental groups. When the cells
reached 70% confluence, the osteogenic experimental group was
placed in an osteogenic induction medium consisting of 10%
FBS/L-DMEM, 0.1 μmmol/l dexamethasone, 10 mmol/l sodium
β-glycerol phosphate, 50 mg/l vitamin C and 0.01 μmol/l
vitamin D3 (Sigma). The control group was maintained in
DMEM/F12 (containing10% FBS), and, after 2 and 4 weeks, alkaline
phosphatase and Alizarin Red staining were performed, respectively.
The adipogenic experimental group was placed in an adipogenic
induction medium consisting of 10% FBS/L-DMEM, 0.1 μmmol/l
dexamethasone, 10 μg/ml insulin, 0.5 mmol/l
3-isobutyl-1-methylxanthine (IBMX) and 0.01 μmmol/l
indomethacin (Sigma), while the corresponding control group was
maintained in DMEM/F12 (containing 10% FBS). Oil Red O staining
(Xi’an chemical reagent factory, Xi’an, China) was conducted after
2 weeks.
Incorporation of the ADSCs into the SFCS
and the assessment of the cell adhesion rate
The SFCS (50% SF and 50% CS) was made using a freeze
drying method and was subsequently sterilized with ethylene oxide,
prior to the application of a polylysine coating (Sigma). The SFCS
was placed into a 96-well plate and was soaked with cell culture
liquid. Third generation ADSCs were obtained, and the cell density
was adjusted to 1×106 / ml. The cells were then divided
into control and experimental groups. In the experimental group,
200 μl cell suspension fluid was added to each scaffold,
prior to the scaffold being rested for 4 h. Following this, a
further 2 ml cell suspension fluid (cell density, 1×106
/ml) was added into each well. The control group consisted solely
of cells, without the scaffold. The experimental and control groups
were placed into the cell culture box (37°C, 5% CO2
concentration and 95% humidity), and the cell culture solution was
replaced every 3 days. Following 6, 12, 18 and 24 h, prewarmed PBS
was used to wash the SFCS several times, in order to remove the
cells that had not adhered, and the cells had had adhered to the
scaffold were digested with 0.25% trypsin/0.02% EDTA solution. The
cell adhesion rate was calculated according to the following
formula: Cell adhesion rate (%) = number of adhered cells / total
number of cultured cells × 100. At each time point, seven wells
were used to determine the mean and standard deviation values for
the two groups (experimental and control).
Assessment of cell proliferation
rate
The experimental group consisted of a composite of
the ADSCs with the SFCS, while the control group consisted solely
of cells. The cell culture medium was exchanged every 2 days. Cell
culture plates were obtained following 2, 4, 6, 8 and 10-day
culture periods. The entire medium was aspirated, prior to the
addition of 1 ml DMEM/F12 cell culture liquid and 200 μl MTT
(5 mg/ml; Sigma) to each well and 5 h incubation at 37°C. Following
this, the supernatant was aspirated and 450 μl DMSO
(Sigma)was added to each well. The ADSCs were then agitated for 15
min, prior to 200 μl of the liquid being removed and
transferred to a 96-well plate. An enzyme-linked immunosorbent
assay detector was used to monitor the photometric value at a
wavelength of 570 nm (A570). The A570 was
proportional to the rate of cell proliferation. At each time-point,
seven wells were used to determine the mean and standard deviation
values for the two groups (experimental and control).
Observation of cellular morphology
through scanning electron microscopy (SEM)
Following the incorporation of the ADSCs into the
SFCS for 2 and 6 days, the composite scaffold was removed.
Subsequent to this, glutaraldehyde fixation, graded alcohol series
dehydration, critical point drying and metal spraying were
performed. SEM (TESCAN, Brno, Czech Republic) was then used to
observe the adherence of the ADSCs onto the SFCS scaffold.
Observation of cellular morphology
through hematoxylin and eosin (H&E) staining
Following the incorporation of the ADSCs into the
SFCS for 2, 8 and 10 days, the composite scaffold was removed and
the cells were fixed in 4% polyformaldehyde (Xi’an chemical reagent
factory). H&E staining was then conducted.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 statistical software (SPSS, Inc., Chicago, IL, USA)
and group comparisons were conducted using a t-test. All values are
presented as the mean ± standard error (SE). P<0.05 was
considered to indicate a statistically significant difference.
Results
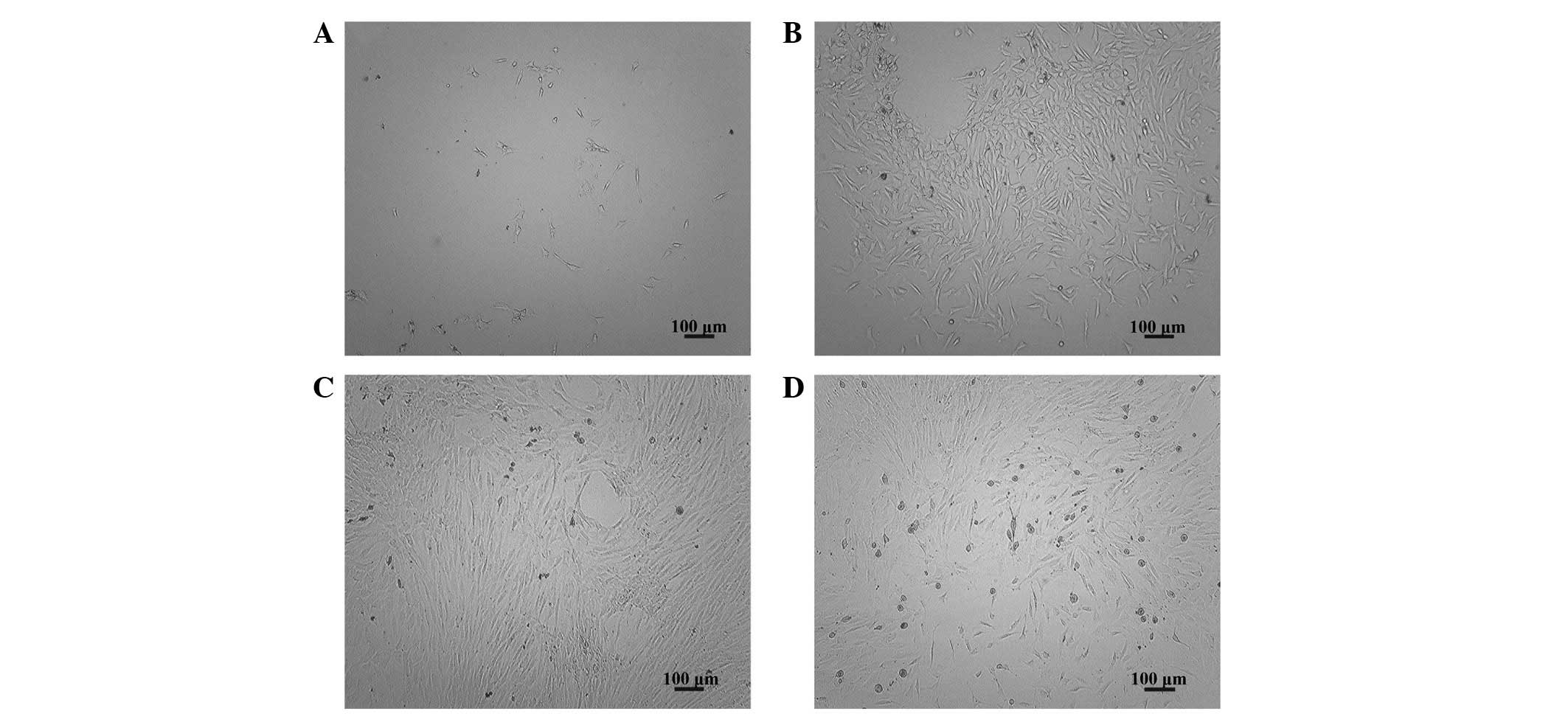
Observation of ADSC morphology
Six hours after inoculation of ADSCs, a few adherent
cells were observed (Fig. 1A). At
the 3-day time-point, an increase in the number of adherent cells
was apparent (Fig. 1B), while at
the 7-day time-point, the cells were observed to have reached 90%
confluence (Fig. 1C). There were
no marked differences in the morphology or proliferative
characteristics between the seventeenth generation of cells and the
primary cells (Fig. 1D).
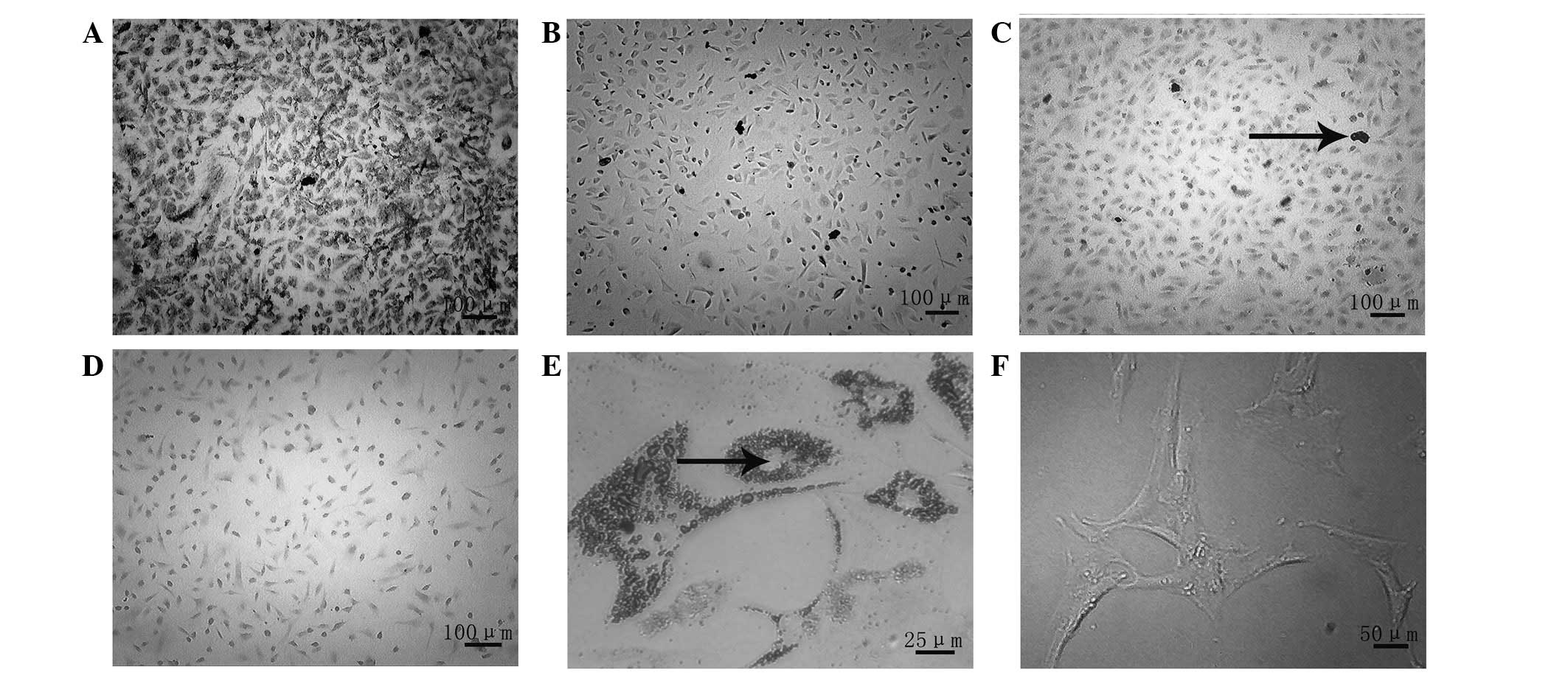
Induction of the ADSCs into osteogenic
and adipogenic directions
Osteogenic induction was observed at 2 weeks,
following alkaline phosphatase staining. In the experimental group,
black particulate material was observed in the cytoplasm of the
cells (Fig. 2A), while this was
not evident in the control group (Fig.
2B). Alizarin Red staining at the 4-week time-point revealed
the formation of calcium nodules in the experimental group
(Fig. 2C), while this was not
apparent in the control group (Fig.
2D). Oil Red O staining was performed at 2 weeks, in order to
observe the adipogenic induction: The staining revealed the
formation of fat droplets in the experimental group (Fig. 2E), whereas no fat droplets were
observed in the control group (Fig.
2F).
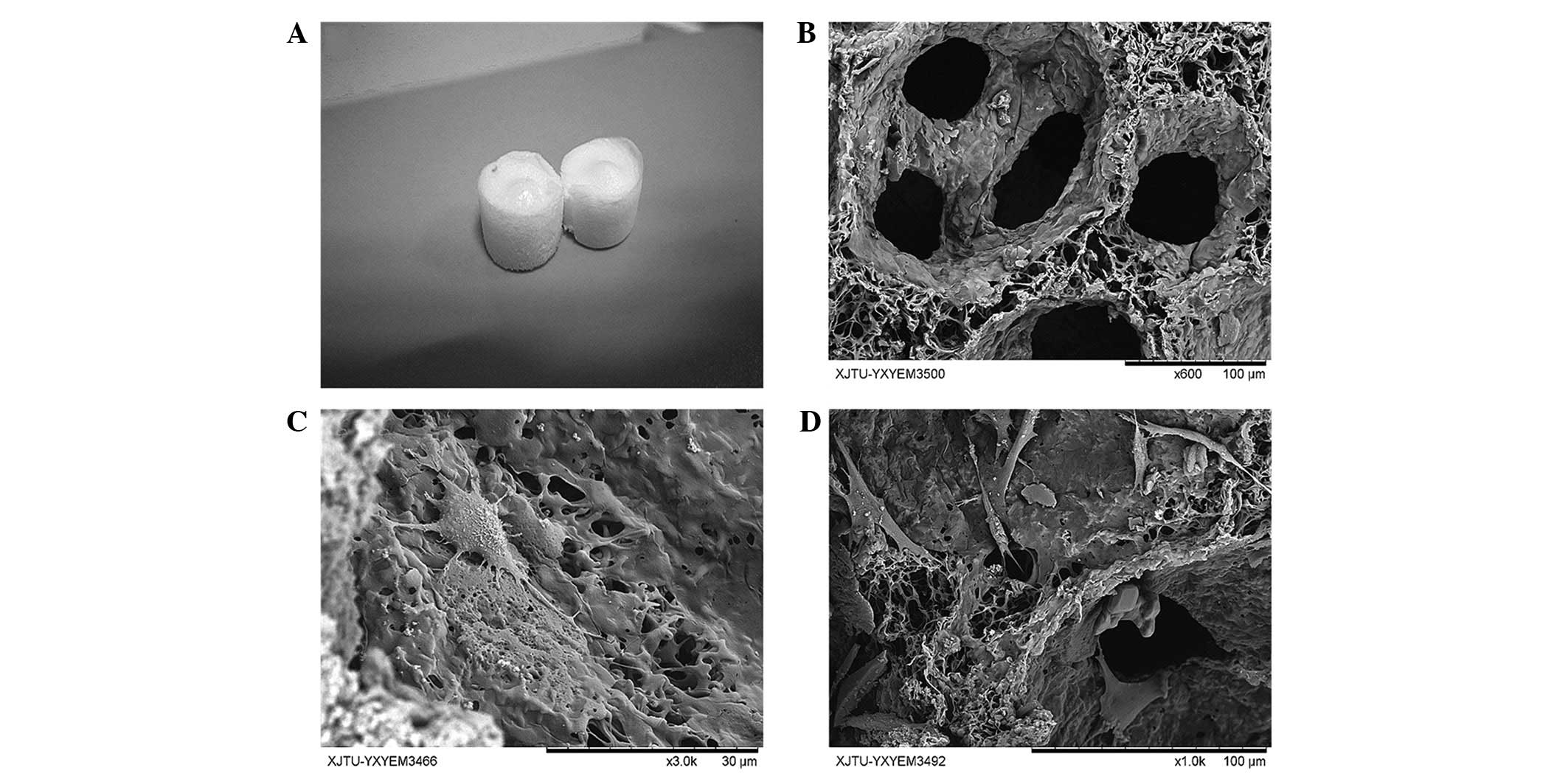
Observation of cellular morphology
through SEM
The SFCS was made into a cylindrical shape (Fig. 3A) and observation of the SFCS using
SEM revealed the pore diameter to be 80–120 μm (Fig. 3B). At the 2-day time-point, it was
noted that a few cells had adhered to the scaffold, although the
cellular morphology was not entirely extended. A low level of
matrix secretion was observed (Fig.
3C). At the 6-day time-point, the cell number appeared to have
significantly increased compared with that at the 2-day time-point,
and the cells displayed the appropriate, fully extended morphology
with a growth-like spindle shape (Fig.
3D).
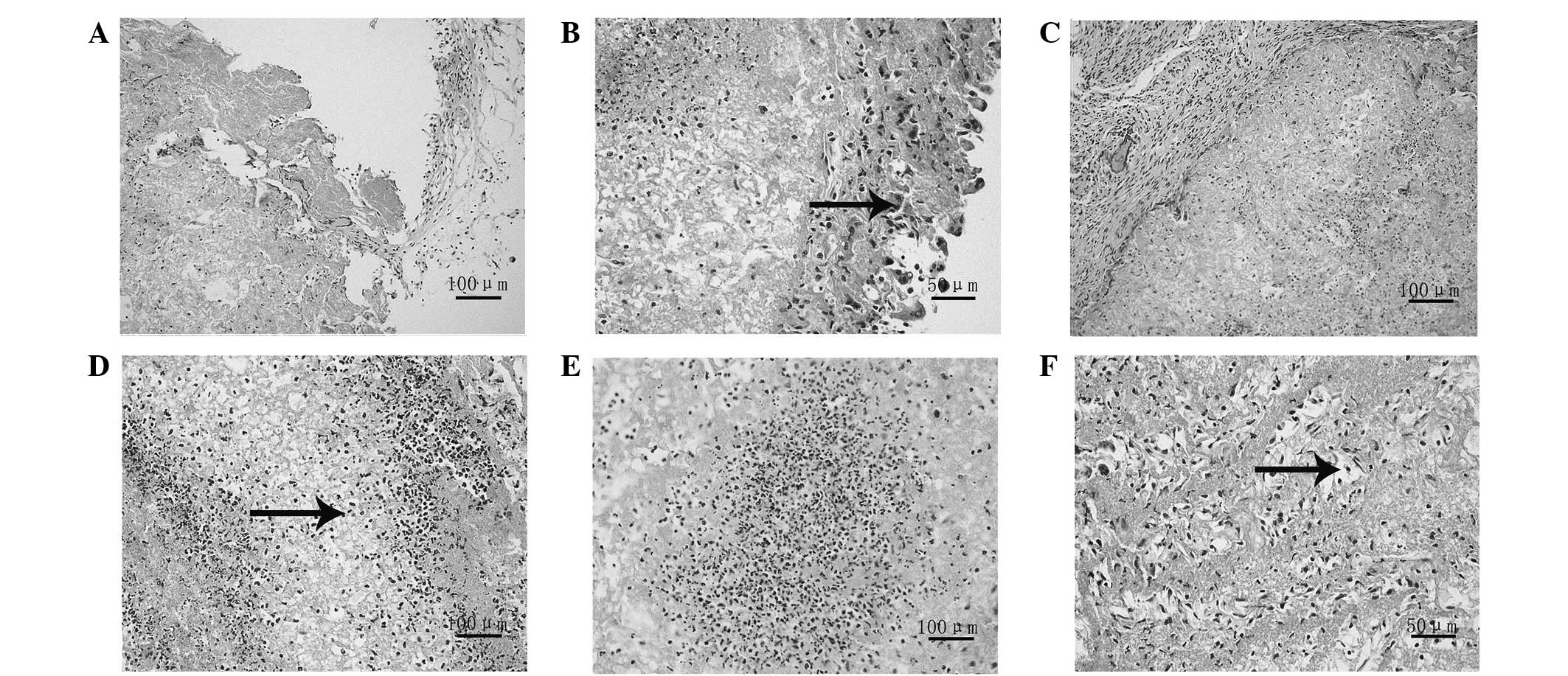
Observation of cellular morphology using
H&E staining
At the 2-day time-point, it was observed that a few
cells had adhered on to the scaffold (Fig. 4A), with the majority of the cells
located on the surface (Fig. 4B).
At the 6-day time-point, the number of cells had increased compared
with that at the 2-day time-point; however, the cells remained on
the surface (Fig. 4C). At the
8-day time-point the cells were observed to have migrated from the
surface of the scaffold into the interior (Fig. 4D), while at 10 days, it was noted
that there was a large number of cells distributed uniformly in the
inner scaffold (Fig. 4E and
F).
Cell adhesion rate
At the 6-hour time-point, 23.87% of the cells were
observed to have adhered on the scaffold in the experimental group,
compared with 32.19% adhered to the plate in the control group.
This was a significant difference between the two groups; however,
at the 12, 18 and 24-hour time-points, the cell adhesion rate in
the experimental group was consistent with that of the control
group and no significant difference was observed (Table I). These results suggested that the
ADSCs and SFCS demonstrated good biocompatibility.
 | Table I.Cell adhesion rate of the experimental
and control groups (n=7). |
Table I.
Cell adhesion rate of the experimental
and control groups (n=7).
| Time (h) | Experimental group
(%) | Control group
(%) | t value | P-value |
|---|
| 6 | 25.07±0.63a | 32.38±0.83a | −18.469 | <0.05 |
| 12 | 51.46±1.21 | 52.49±1.13 | −1.657 | >0.05 |
| 18 | 76.21±1.33 | 77.34±1.30 | −1.604 | >0.05 |
| 24 | 93.98±1.07 | 94.47±1.68 | −0.651 | >0.05 |
Cellular proliferation rate
The results of the MTT assay revealed that the ADSCs
were able to grow and proliferate effectively on the SFCS. At the 2
and 4-day time-points, a significant difference was observed
between the control and experimental groups, and the cell
proliferation of the control group was greater that of the
experimental group; however, at the 6, 8 and 10-day time-points, no
significant differences were identified between the two groups
(Table II).
 | Table II.Photometric value (A570) of
the experimental and control groups (n=7). |
Table II.
Photometric value (A570) of
the experimental and control groups (n=7).
| Time (days) | Experimental
group | Control group | t value | P-value |
|---|
| 2 | 0.3202±0.0024a | 0.3473±0.0048a | −13.435 | <0.05 |
| 4 | 0.4135±0.0092a | 0.4224±0.0010a | −2.563 | <0.05 |
| 6 | 0.7132±0.0003 | 0.7160±0.0062 | −1.213 | >0.05 |
| 8 | 0.7403±0.0070 | 0.7448±0.0045 | −1.428 | >0.05 |
| 10 | 0.7534±0.0068 | 0.7605±0.0073 | −1.903 | >0.05 |
Discussion
The basic method utilized when using tissue
engineering to repair SCI is as follows (15): The cells are cultured at high
concentration in vitro, amplified and adhered to the
scaffold, prior to the scaffold being implanted into the spinal
cord trauma cavity. Once in place, the implanted cells continue to
proliferate in conjunction with the biological degradation and
absorption of the scaffold. Thus, a new organization with the
appropriate function and morphology is formed, ultimately resulting
in the repair of the SCI and the reconstruction of its function.
Consequently, the selection of a scaffold material and seed cells
with good biocompatibility directly affects the outcome of the
repair effort (16,17).
ADSCs display no specific surface antigen (18,19),
and, therefore, the method of reductio ad absurdum was initially
adopted, in order to determine the characteristics of the ADSCs.
The assumption was that the separated cells were stem cells, and
that, as such, had the potential for multilineage differentiation.
Using third generation cells, the cells were induced into an
osteogenic direction, and the cellular morphology was observed to
change from fibroblast-like long spindles to square, polygonal or
other forms. Two weeks subsequent to the induction, the alkaline
phosphatase staining of the experimental group was positive, and
large black particulate material appeared within the cell plasma; 4
weeks following the induction, the Alizarin Red staining of the
experimental group was positive and there were evident square or
irregular calcium salt deposits. This indicated that the cells had
differentiated into osteoblasts and were exhibiting the
corresponding characteristics. Two weeks subsequent to the
adipogenic induction, Oil Red O staining revealed the formation of
lipid droplets in the cytoplasm in the experimental group, whereas
no lipid droplets were observed in the control group. This
suggested that the ADSCs had differentiated into adipocytes. These
results confirmed that the cells demonstrated the potential for
multilineage differentiation and, in combination with the fact that
the cells were extracted from the adipose tissue, determined that
the cells were ADSCs.
SF is a natural structural protein that demonstrates
no physiological activity and that has desirable biocompatibility
and degradation characteristics. When implanted in vivo, SF
elicits a mild inflammatory reaction; however, it is widely used in
the field of tissue engineering (20,21).
CS is the only alkaline polysaccharide in nature and it also
exhibits a good biocompatibility (22). A previous demonstrated that it was
possible to make a cylindrical and porous SFCS using a freezing
drying method, and that it not only improved the brittle nature of
SF in dry environments, but also reduced the cellular inhibitory
effects displayed by CS (23).
The incorporation of the third generation cells into
the scaffold and the assessment of the cell adhesion rate revealed
that there was a significant difference between the experimental
and control groups following 6 h of incorporation (P<0.05).
However, as time progressed, i.e. following 12, 18 and 24 h, no
significant difference was observed between the two groups, which
indicated that there was a favorable adhesion characteristic
between them. The examination of cellular proliferation on the
scaffold revealed that there was a statistically significant
difference between the experimental and control groups 2 and 4 days
subsequent to the commencement of the incorporation process. This
may have been due to a process of adaptation occurring following
the initial incorporation of the ADSCs into the scaffold. The fact
that the difference between the two groups at 4 days was less than
the difference at 2 days may have been due to the ADSCs
demonstrating an initial slow rate of proliferation on the
scaffold, followed by a return to a more normal proliferation rate
two days subsequently. At the 6, 8 and 10-day time-points, no
significant differences were observed between the two groups
(P>0.05). This indicated that there was good adhesion and
biocompatibility between the ADSCs and the SFCS. Using SEM to
examine the cellular morphology, it was observed that a few cells
were composite with the scaffold following 2 days of incorporation
and that the cellular morphology was not completely spindle-like.
However, at the 6-day time-point, there was a significant increase
in the cell number compared with that at the 2-day time-point and
the cellular morphology was appropriate. H&E staining results
revealed that, at the 2-day time-point, there were a few cells on
the scaffold and that the majority of them were located on the
surface of the scaffold, while at 6 days, the number of the cells
was greater than at 2 days, although the cells remained on the
surface. At the 8-day time-point, the cells had migrated from the
surface into the interior of the scaffold and at 10 days, it was
observed that there were a large number of cells distributed
uniformly across the interior of the scaffold. These results were
consistent with the results from the assessment of cellular
proliferation
In this study, ADSCs were obtained and the
multidifferentiative potential of the cells was demonstrated.
Following this, experiments were used to reveal that there were
desirable adhesion and biocompatibility properties between the
ADSCs and the SFCS. The results indicated the potential of ADSCs as
seed cells and SFCS as a scaffold material for use in the repair of
SCI. This conclusion is likely to provide a foundation for further
investigations.
Acknowledgements
The authors would like to thank
Professor Peijun Liu for the guidance provided during the
experiment.
References
|
1.
|
Madigan NN, McMahon S, O’Brien T,
Yaszemski MJ and Windebank AJ: Current tissue engineering and novel
therapeutic approaches to axonal regeneration following spinal cord
injury using polymer scaffolds. Respir Physiol Neurobiol.
169:183–199. 2009. View Article : Google Scholar
|
|
2.
|
Cadotte DW and Fehlings MG: Spinal cord
injury: a systematic review of current treatment options. Clin
Orthop Relat Res. 469:732–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Johnson PJ, Parker SR and Sakiyama-Elbert
SE: Controlled release of neurotrophin-3 from fibrin-based tissue
engineering scaffolds enhances neural fiber sprouting following
subacute spinal cord injury. Biotechnol Bioeng. 104:1207–1214.
2009. View Article : Google Scholar
|
|
4.
|
Silva NA, Salgado AJ, Sousa RA, et al:
Development and characterization of a novel hybrid tissue
engineering-based scaffold for spinal cord injury repair. Tissue
Eng Part A. 16:45–54. 2010.PubMed/NCBI
|
|
5.
|
Anghileri E, Marconi S, Pignatelli A, et
al: Neuronal differentiation potential of human adipose-derived
mesenchymal stem cells. Stem Cells Dev. 17:909–916. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yang LY, Liu XM, Sun B, Hui GZ, Fei J and
Guo LH: Adipose tissue-derived stromal cells express neuronal
phenotypes. Chin Med J (Engl). 117:425–429. 2004.PubMed/NCBI
|
|
7.
|
Luangbudnark W, Viyoch J, Laupattarakasem
W, Surakunprapha P and Laupattarakasem P: Properties and
biocompatibility of chitosan and silk fibroin blend films for
application in skin tissue engineering. Scientific World Journal.
2012:6972012012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Cao Y and Wang B: Biodegradation of silk
biomaterials. Int J Mol Sci. 10:1514–1524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Liu TL, Miao JC, Sheng WH, et al:
Cytocompatibility of regenerated silk fibroin film: a medical
biomaterial applicable to wound healing. J Zhejiang Univ Sci B.
11:10–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jayakumar R, Prabaharan M, Sudheesh Kumar
PT, Nair SV and Tamura H: Biomaterials based on chitin and chitosan
in wound dressing applications. Biotechnol Adv. 29:322–337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kim IY, Seo SJ, Moon HS, et al: Chitosan
and its derivatives for tissue engineering applications. Biotechnol
Adv. 26:1–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang X, Yang D and Nie J:
Chitosan/polyethylene glycol diacrylate films as potential wound
dressing material. Int J Biol Macromol. 43:456–462. 2008.PubMed/NCBI
|
|
13.
|
Chen L, Zhu Y, Li Y, Liu Y and Yu J:
Progress and prospect of electrospun silk fibroin in construction
of tissue-engineering scaffold. Sheng Wu Gong Cheng Xue Bao.
27:831–837. 2011.(In Chinese).
|
|
14.
|
She Z, Jin C, Huang Z, Zhang B, Feng Q and
Xu Y: Silk fibroin/chitosan scaffold: preparation,
characterization, and culture with HepG2 cell. J Mater Sci Mater
Med. 19:3545–3553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Johnson PJ, Parker SR and Sakiyama-Elbert
SE: Fibrin-based tissue engineering scaffolds enhance neural fiber
sprouting and delay the accumulation of reactive astrocytes at the
lesion in a subacute model of spinal cord injury. J Biomed Mater
Res A. 92:152–163. 2010. View Article : Google Scholar
|
|
16.
|
Dittmar R, Potier E, van Zandvoort M and
Ito K: Assessment of cell viability in three-dimensional scaffolds
using cellular auto-fluorescence. Tissue Eng Part C Methods.
18:198–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Valarmathi MT, Yost MJ, Goodwin RL and
Potts JD: The influence of proepicardial cells on the osteogenic
potential of marrow stromal cells in a three-dimensional tubular
scaffold. Biomaterials. 29:2203–2216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Choi YS, Dusting GJ, Stubbs S, et al:
Differentiation of human adipose-derived stem cells into beating
cardiomyocytes. J Cell Mol Med. 14:878–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zuk PA: The adipose-derived stem cell:
looking back and looking ahead. Mol Biol Cell. 21:1783–1787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kasoju N, Bhonde RR and Bora U:
Preparation and characterization of Antheraea assama silk
fibroin based novel non-woven scaffold for tissue engineering
applications. J Tissue Eng Regen Med. 3:539–552. 2009.
|
|
21.
|
Yin GB, Zhang YZ, Wang SD, Shi DB, Dong ZH
and Fu WG: Study of the electrospun PLA/silk fibroin-gelatin
composite nanofibrous scaffold for tissue engineering. J Biomed
Mater Res A. 93:158–163. 2010.PubMed/NCBI
|
|
22.
|
Dhandayuthapani B, Krishnan UM and
Sethuraman S: Fabrication and characterization of chitosan-gelatin
blend nano-fibers for skin tissue engineering. J Biomed Mater Res B
Appl Biomater. 94:264–272. 2010.PubMed/NCBI
|
|
23.
|
Gupta V, Davis G, Gordon A, et al:
Endothelial and stem cell interactions on dielectrophoretically
aligned fibrous silk fibroin-chitosan scaffolds. J Biomed Mater Res
A. 94:515–523. 2010.PubMed/NCBI
|