Introduction
Metastasis and invasiveness are two of the most
significant characteristics of malignant tumor cells. Among the
proteins involved in metastasis and invasiveness, matrix
metalloproteinase-9 (MMP-9), a member of the MMP family, is
particularly important, due to its ability to degrade type IV
collagen fibers and the extracellular matrix (1). Studies have demonstrated that the
promoter region of MMP-9 contains cis-acting elements and binding
loci for the transcription factors nuclear factor-κB (NF-κB) and
activator protein AP-1. Cytokines and phorbol 12-myristate
13-acetate (PMA) are able to stimulate the production of MMP-9 by
activating NF-κB and AP-1, which indicates that the expression of
MMP-9 is inducible (2). As a
result, proteins regulating the expression and activity of MMP-9
are promising drug targets for antitumor studies (3).
Numerous extracts of herbal medicines have been
demonstrated to exhibit antitumor activities, including the
inhibition of tumor cell growth and metastasis. Andrographolide
(AD) is a type of diterpenoid extracted from the medicinal plant
Andrographis paniculata, which is usually used to treat
infectious diseases (4). It has
been observed that AD inhibits the proliferation of multiple types
of tumor cells, including leukemia, glioma, prostatic carcinoma and
breast cancer cells (5,6). However, its effect in the treatment
of lung cancer is unknown. The aim of the present study was to
investigate how AD affected PMA-induced MMP-9 expression, in
addition to studying the potential regulatory molecules and the
mechanisms involved.
Materials and methods
Reagents
AD and gelatin were purchased from Sigma-Aldrich
(St. Louis, MO, USA) and PMA was obtained from Calbiochem (La
Jolla, CA, USA). Anti-p65, β-actin and anti-IκB antibodies were
purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA), while anti-phospho-IκB antibody was purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Any additional
analytical reagents were purchased from Sangon Biotech (Shanghai)
Co., Ltd. (Shanghai, China) and Amerco (Reno, NV, USA).
Cell culture
Non-small-cell H3255 lung cancer cells were
purchased from ATCC (Manassas, VA, USA) and cultured in RPMI-1640
medium (pH 7.4) containing 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C with 5%
CO2. Cell density was adjusted to 1×105
cells/ml prior to the tests. The cells were divided into several
AD-treated groups, which were treated with 1, 5 or 10 μM AD at 37°C
for 24, 48 or 72 h. The negative control groups were treated with
equal volumes of RPMI-1640 medium, containing dimethylsulfoxide
(DMSO) at a final concentration of 0.1%.
MTT assay
Cells were inoculated onto 96-well plates at
1×104 cells per well, treated with PMA for 24 or 48 h
and then supplemented with 1.0, 5.0 and 10.0 μM of AD. Following
processing, the supernatant was disposed of and DMSO solution
containing MTT was added. The absorbance values were determined at
550 nm.
RT-PCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and 2 μg
total RNA was used to prepare the cDNA with a
SuperScript® First Strand cDNA Synthesis System kit
(Invitrogen Life Technologies). The PCR products were stained with
ethidium bromide, following electrophoresis in 2% agarose gel. The
primers used for MMP-9 were 5′-TCCCTGGAGACCTGA GAACC-3′ and
5′-CGGCAAGTCTTCCGAGTAGTT-3′, while the primers used for
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were
5′-CCATCACCATCTTCCAGGAG-3′ and 5′-CCTGCTTCACCACGTTCTTG-3′.
Gelatin zymography
The cells were cultured in serum-free medium for 24
h. Following this, the supernatant was collected, supplemented with
Laemmli sample buffer and subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 1 mg/ml
gelatin (separation gel 10%). Subsequent to the electrophoresis,
the gel was kept in renaturation buffer containing 2.5%
Triton-X-100 for 30 min, in order to remove the SDS, and then
incubated in a buffer containing 50 mM HCl (pH 7.4), 5 mM
CaCl2 and 1 μM ZnCl2 at 37°C overnight. The
gel was subsequently stained with 0.05% Coomassie Brilliant Blue
R-250 at room temperature for 30 min, prior to being decolorized
with deionized water for photography and grey-scale scanning.
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of the cell culture was collected
and the MMP-9 activity was tested with a SensoLyte Plus™ 520 MMP-9
assay kit (AnaSpec, Inc., Fremont, CA, USA). The unit of enzymatic
activity was indicated as 490 nm (excitation wavelength)/520 nm
(emission wavelength).
Immunoblotting assay
The nuclear and cytoplasmic proteins were extracted
in accordance the protocol provided with the ProteoJET™ Cytoplasmic
and Nuclear Protein Extraction kit (Fermentas, Vilnius, Lithuania).
Proliferating cell nuclear antigen (PCNA) and α-tubulin were used
as internal controls for nuclear and cytoplasmic proteins,
respectively. The isolated proteins were subjected to SDS-PAGE and
transferred onto nitrocellulose membranes, prior to being blocked
in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5%
non-fat dry milk. The proteins were then incubated and treated with
primary and secondary antibodies for enhanced chemiluminescence
(ECL) detection.
Statistical analyses
The data were analyzed with the statistical analysis
software SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). The values are
presented as the mean ± standard deviation. One-way analysis of
variance (ANOVA) was used for multi-group comparisons with a
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
AD inhibits the proliferation of H3255
cells
To investigate whether AD affected the proliferation
of H3255 cells, the cells were treated with 100 nM PMA in the
absence or presence of AD (1, 5 or 10 μM) for 24, 48, or 72 h. The
negative control cells were treated with equal volumes of RPMI-1640
medium containing DMSO (0.1%) only. As shown in Table I, AD significantly inhibited the
proliferation of H3255 cells in vitro. The inhibition rates
of the H3255 cells increased when the concentration of AD was
increased and when the treatment duration was longer. At the
concentrations used in the study, AD did not appear to exert any
toxic effects on the cells. These results demonstrate that AD
inhibited the proliferation of H3255 cells in a concentration- and
time-dependent manner.
 | Table IInhibitory effect of AD on the
PMA-induced proliferation of H3255 cells. |
Table I
Inhibitory effect of AD on the
PMA-induced proliferation of H3255 cells.
| Duration of
treatment |
|---|
|
|
|---|
| AD concentration
(μM) | 24 h | 48 h | 72 h |
|---|
| 0.0 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| 1.0 | 15.34±2.38a | 26.84±1.41ab | 35.23±1.01ab |
| 5.0 | 29.28±2.07a | 34.25±2.51ab | 46.22±1.82ab |
| 10.0 | 41.91±1.75a | 48.51±2.31ab | 59.07±1.43ab |
AD inhibits the PMA-induced expression of
MMP-9
To detect the effect of AD on MMP-9, the total RNA
was extracted from the cells treated with or without AD, and RT-PCR
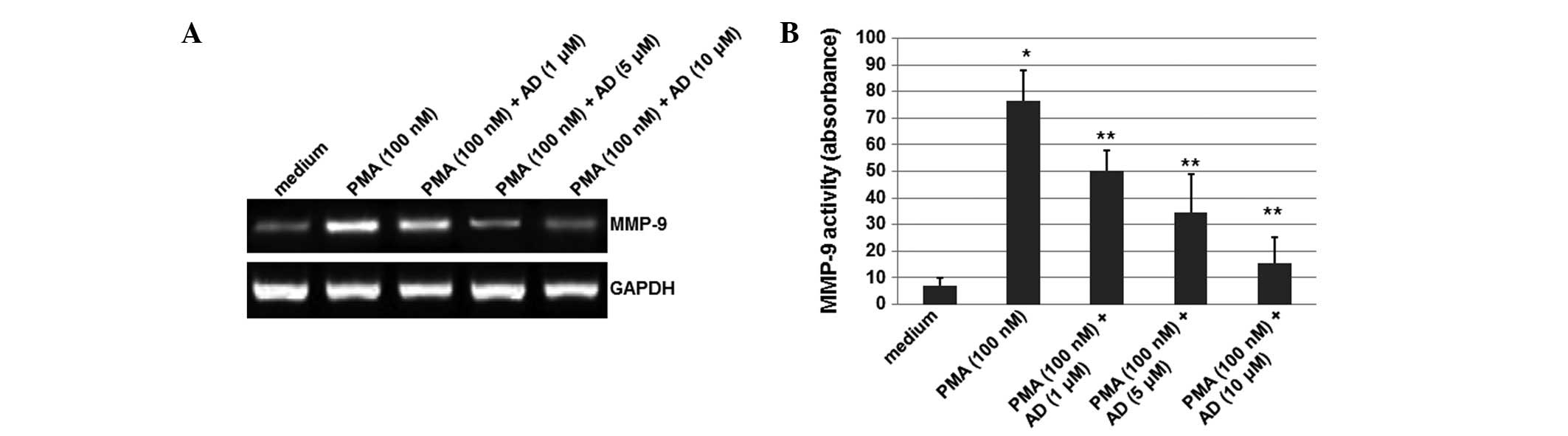
was performed. As shown in Fig.
1A, although the mRNA levels of MMP-9 were significantly
increased by PMA, this induction of MMP-9 expression was reduced by
treatment with AD (1, 5 or 10 μM) in a concentration-dependent
manner. In this experiment, GAPDH was used as an internal control.
These results suggest that AD inhibited the PMA-induced expression
of MMP-9.
AD inhibits the MMP-9 activity induced by
PMA
To detect whether the MMP-9 activity was affected by
AD, a gelatin zymography experiment was performed. As shown
Fig. 1B, although the activity of
MMP-9 increased upon treatment with PMA, AD (1, 5 or 10 μM)
decreased this activity in a concentration-dependent manner. The
MMP-9 activity was reduced by 44% when the cells were treated with
10 μM AD. These results indicate that AD inhibits the MMP-9
activity induced by PMA.
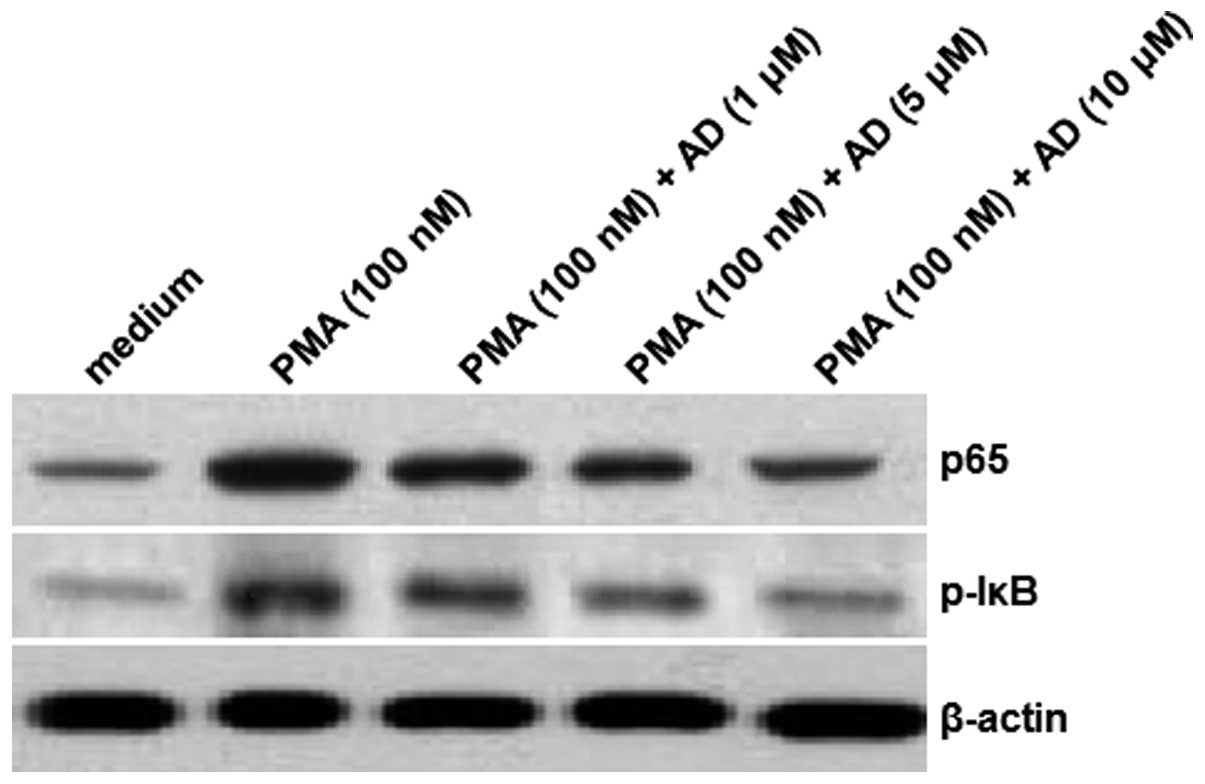
AD inhibits the PMA-induced translocation
of the NF-κB p65 subunit and the IκB phosphorylation
To further investigate the mechanisms of the effects
exerted by AD, the protein levels of the NF-κB p65 subunit were
determined using an immunoblotting assay. As shown in Fig. 2, there was an increase in the level
of the NF-κB p65 protein subunit in the nucleus following the
induction by PMA. However, the levels of p65 protein in the nucleus
were reduced following the treatments with increasing
concentrations of AD. Furthermore, although the IκB phosphorylation
was significantly increased by PMA, AD attenuated this increase in
a concentration-dependent manner. In this experiment, β-actin
served as the internal control. These results suggest that AD
inhibits the PMA-induced increase in the levels of the NF-κB p65
subunit and IκB phosphorylation.
Discussion
A number of chemical compounds have been
demonstrated to exert antitumor effects on various tumors,
including leukemia and prostatic, breast and pancreatic cancer. AD
has been revealed to reduce the activity of
Na+/K+-ATPase and inhibit the translocation
of NF-κB through regulating proteases (7). It has been indicated that AD exhibits
certain inhibitory effects on metastasis in lung cancers (8). However, no comprehensive studies in
this field have been performed, and, therefore, further
investigations are required to study the exact molecular mechanisms
of the antitumor effect of AD.
In the present study, the effect of AD on
PMA-induced H3255 cell proliferation was investigated, and it was
demonstrated that (i) AD is able to inhibit the expression and
activity of MMP-9 and (ii) AD is capable of inhibiting
NF-κB-mediated MMP-9 expression by suppressing the activation of
NF-κB, and thus preventing the migration and invasion of tumor
cells.
Metastasis is a multi-stage complex process
involving cell proliferation and migration into the circulatory
system, and tumor growth at the primary site. The expression of
MMP-9 in numerous types of cancer is increasingly gaining focus,
and PMA is a commonly used chemical inducer of tumors in
vivo and in vitro(9).
Studies have revealed that PMA is able to enhance the migration and
invasion of tumor cells by inducing the expression of MMPs-2 and -9
in glioma, in addition to colon, liver and breast cancer (10), although the mechanisms involved in
the PMA-induced invasion in lung cancer have not yet been
elucidated. The present study demonstrated that PMA enhanced the
expression of MMP-9 in H3255 cells at the mRNA and protein levels,
and that AD was capable of inhibiting this effect. The inhibitory
effect of AD on the enzymatic activity and protein expression of
MMP-9 indicated that AD participates in the regulation of
posttranscriptional pathways.
The promoter region of MMP-9 contains regulatory
elements for NF-κB and AP-1 (11),
making the 5′ regulatory region of MMP-9 gene highly inducible. In
order to clarify the relevant mechanisms, we focused on the nuclear
translocation of the NF-κB p65 subunit and the phosphorylation of
IκB, which have been demonstrated to be necessary for the PMA
induction of MMP-9, and which are suppressible using AD. Previous
studies have indicated that NF-κB is important in the PMA-induced
expression of MMP-9 in lung cancer (12). The results of the present study
demonstrated that the NF-κB pathway also facilitates the inhibition
of the PMA-induced expression of MMP-9 by AD.
In conclusion, the present study demonstrated that
AD is able to inhibit the migration and invasion of lung cancer
cells by suppressing the PMA-induced expression of MMP-9, the
mechanisms of which may involve the suppression of IκB
phosphorylation and the consequent NF-κB activation. It is thus
suggested that AD may a promising drug candidate for the clinical
treatment of the migration and invasion of malignant tumor
cells.
References
|
1
|
Han L, Zhang HW, Zhou WP, Chen GM and Guo
KJ: The effects of genistein on transforming growth
factor-β1-induced invasion and metastasis in human pancreatic
cancer cell line Panc-1 in vitro. Chin Med J (Engl). 125:2032–2040.
2012.
|
|
2
|
Liu N, Sun Q, Chen J, et al: MicroRNA-9
suppresses uveal melanoma cell migration and invasion through the
NF-κB1 pathway. Oncol Rep. 28:961–968. 2012.PubMed/NCBI
|
|
3
|
Rodriguez Faba O, Palou-Redorta J,
Fernández-Gómez JM, Algaba F, Eiró N, Villavicencio H and Vizoso
FJ: Matrix metalloproteinases and bladder cancer: what is new? ISRN
Urol. 2012:5815392012.PubMed/NCBI
|
|
4
|
Lee WR, Chung CL, Hsiao CJ, et al:
Suppression of matrix metalloproteinase-9 expression by
andrographolide in human monocytic THP-1 cells via inhibition of
NF-κB activation. Phytomedicine. 19:270–277. 2012.PubMed/NCBI
|
|
5
|
Zhou J, Hu SE, Tan SH, et al:
Andrographolide sensitizes cisplatin-induced apoptosis via
suppression of autophagosome-lysosome fusion in human cancer cells.
Autophagy. 8:338–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Y, Liu X and Guo SW: Therapeutic
potential of andrographolide for treating endometriosis. Hum
Reprod. 27:1300–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu WJ, Lin KH, Hsu MJ, Chou DS, Hsiao G
and Sheu JR: Suppression of NF-κB signaling by andrographolide with
a novel mechanism in human platelets: regulatory roles of the p38
MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol. 84:914–924.
2012.
|
|
8
|
Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA
and Chen JH: Inhibitory effects of andrographolide on migration and
invasion in human non-small cell lung cancer A549 cells via
down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol.
632:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang H, Lee M, Choi KC, Shin DM, Ko J and
Jang SW: N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell
invasion through suppressing NF-κB activation and inhibiting matrix
metalloproteinase-9 expression. J Cell Biochem. 113:2845–2855.
2012.PubMed/NCBI
|
|
10
|
Yu HY, Kim KS, Moon HI, Kim KM, Lee YC and
Lee JH: JNP3, a new compound, suppresses PMA-induced tumor cell
invasion via NF-κB down regulation in MCF-7 breast cancer cells.
Biochem Biophys Res Commun. 421:190–196. 2012.PubMed/NCBI
|
|
11
|
Mittelstadt ML and Patel RC: AP-1 mediated
transcriptional repression of matrix metalloproteinase-9 by
recruitment of histone deacetylase 1 in response to interferon β.
PLoS One. 7:e421522012.PubMed/NCBI
|
|
12
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|