Introduction
The concentration of particulate matter (PM) with a
mass median aerodynamic diameter (a density-dependent unit of
measure used to describe the diameter of a particle) ≤2.5 μm
(PM2.5) is more closely associated with respiratory
effects and subsequent mortality than larger particles of mass
median aerodynamic diameter ≤10 μm (PM10) (1). A noteworthy aspect of the
epidemiological data is that health impacts of PM2.5 are
predominantly identified in subjects with predisposing factors to
pneumonia, bronchial asthma, chronic obstructive pulmonary disease,
compromised immune disorders and an age of >65 years (2). Diesel exhaust particles (DEPs), the
main constituents of PM2.5 in urban areas, are
epidemiologically considered to be harmful for respiratory systems
and diseases (3). In accordance
with this, the respiratory toxicity of DEPs has been biologically
demonstrated, in the presence or absence of predisposing factors
(4–6).
DEP sizes have become progressively smaller due to
advancements in the automobile industry, leading to the production
and release of particles <100 nm in mass median aerodynamic
diameter (defined as nanoparticles). This trend may increase the
level of airborne nanoparticles, and consequently possess a greater
health concern (7,8). However, there have been few studies
that have examined the effects of exposure to relevant types of
nano-level DEPs on health, in individuals with or without
predisposing factors. We have focused on nanotoxicity in allergic
asthma. Since asthma is a chronic airway inflammatory disease and
patients with asthma are reportedly highly sensitive to PM
(9), we have demonstrated that
inhaled nanoparticle-rich DE (NR-DE) exacerbated allergic airway
inflammation in mice (10). The
aggravation was concomitant with the amplified expression of the
allergy-associated cytokines interleukin-5 (IL-5) and eotaxin, in
the lung. However, the mechanisms for NR-DE-mediated aggravation of
the allergic asthma model have not been fully investigated. The
formation of 8-hydroxydeoxyguanosine (8-OHdG) is the main DNA
modification induced by reactive oxygen species (ROS) and may be
responsible for DNA base mutations. It has been demonstrated that
oxidative DNA adducts accumulate and are only repaired through
enzyme pathways, resulting in further DNA damage (11). Oxidative DNA damage may be observed
in lung inflammation, such as that induced by lipopolysaccharides
(12). In addition, 8-OHdG
expression is induced or enhanced in the lung as a result of
several types of oxidative stress burdens, such as ozone (13), DEPs (14) or asbestos (15), in vitro and in vivo.
In the present study, we investigated the levels of 8-OHdG in the
lung by means of enzyme immunoassay (EIA) and immunohistochemistry,
to gain insights into the mechanistic pathway of the NR-DE-mediated
aggravation of allergic airway inflammation.
Materials and methods
Animals
Female Institute for Cancer Research (ICR) mice
(age, 6 weeks; weight, 29–33 g; Clea Japan, Inc., Tokyo, Japan)
were used in this study. The mice were housed in an animal facility
maintained at 24–26°C with 55–75% humidity and a 12 h light/dark
cycle, and fed a commercial diet (Clea Japan, Inc.) with ad
libitum access to water.
Generation of NR-DE inhalation
systems
An 8-l diesel engine (J08C; Hino Motors, Ltd., Hino,
Japan) was used for generating the nanoparticles as previously
described (10,16). Four exposure chambers were set
according to the conditions of the gases, and included a control
(control air: CA), low-concentration (36.3 μg/m3: D1)
NR-DE, high-concentration (168.8 μg/m3: D2) NR-DE and
high concentration (168.8 μg/m3: D3) NR-DE without
particulate components. In each inhalation chamber, the temperature
and relative humidity were maintained at 20°C and 50%,
respectively.
Study protocol
The mice were exposed to one of the four different
gas compositions (CA, D1, D2 and D3) in each chamber system for 5
h/day, 5 days a week for 8 weeks. During inhalation exposure, 1
μg/body of ovalbumin (OVA) or vehicle [phosphate-buffered saline
(PBS)] was intratracheally administered every 2 weeks (a total of
five times). Finally, the mice were divided into eight groups,
sacrificed and studied 24 h following the final intratracheal
instillation (80 mice in total). The animal studies were approved
by the Institutional Review Board of the National Institute for
Environmental Studies, Tsukuba, Japan.
Bronchoalveolar lavage (BAL) procedure
and 8-OHdG level in the BAL fluid (BALF)
The mice were sacrificed by etherization and
exsanguination from the abdominal aorta 24 h following the final
intratracheal administration. A cannula was inserted into the
trachea and secured with a suture. The lungs were lavaged three
times with 1.2 ml sterile saline at 37°C, which was instilled
bilaterally with a syringe. The fluid was harvested by gentle
aspiration. The collected fluid was cooled and centrifuged at 300 ×
g for 10 min, as described previously (17–19).
The collected supernatants were used for an EIA study (n=6 in each
group). In another experiment, the lungs were removed for
immunohistological examination (n=4 in each group).
The EIA for determining the 8-OHdG level in the BALF
was conducted on the basis of the competition between 8-OHdG and an
8-OHdG acetylcholine esterase conjugate (the 8-OHdG tracer) for a
limited concentration of 8-OHdG monoclonal antibody. As the
concentration of 8-OHdG varies, the concentration of the tracer
that is able to bind to the 8-OHdG monoclonal antibody is inversely
proportional to the 8-OHdG level. Following incubation with the
tracer, antibody and standard or sample in 96-well plates, the
plates were washed to remove any unbound reagents, prior to
Ellman’s Reagent being added to the well. Finally, the product of
this enzymatic reaction was read at 412 nm with conversion to
pg/ml, using values obtained from the standard with limits of
detection of 30 pg/ml.
Immunohistochemistry
The degree of expression of 8-OHdG and its
localization in the lungs were detected by immunohistochemistry
using anti-8-OHdG monoclonal antibody (N45.1, Japan Institute for
the Control of Aging, Nikken SEIL Co., Ltd., Fukuroi, Japan; n=4 in
each group). The excised mouse lungs were embedded with paraffin.
Following deparaffinization, the tissue sections were incubated
with anti-8-OHdG antibody (dilution, 1:100) overnight at 4°C, then
reacted with biotinylated secondary anti-mouse IgG antibody
(Vectastain Elite ABC kit; Vector Laboratories, Inc., Burlington,
Canada) for 30 min at room temperature. Streptavidin was added and
the color was developed with 3,3′-diaminobenzidine (DAB).
Subsequently, the tissue sections were counterstained with
hematoxylin (Merck, KGaA, Darmstadt, Germany) and examined by two
researchers independently.
Statistical analysis
Data are presented as the mean ± standard error.
Differences between groups were determined using analysis of
variance (the Student’s t-test). P<0.05 was considered to
indicate a statistically significant difference.
Results
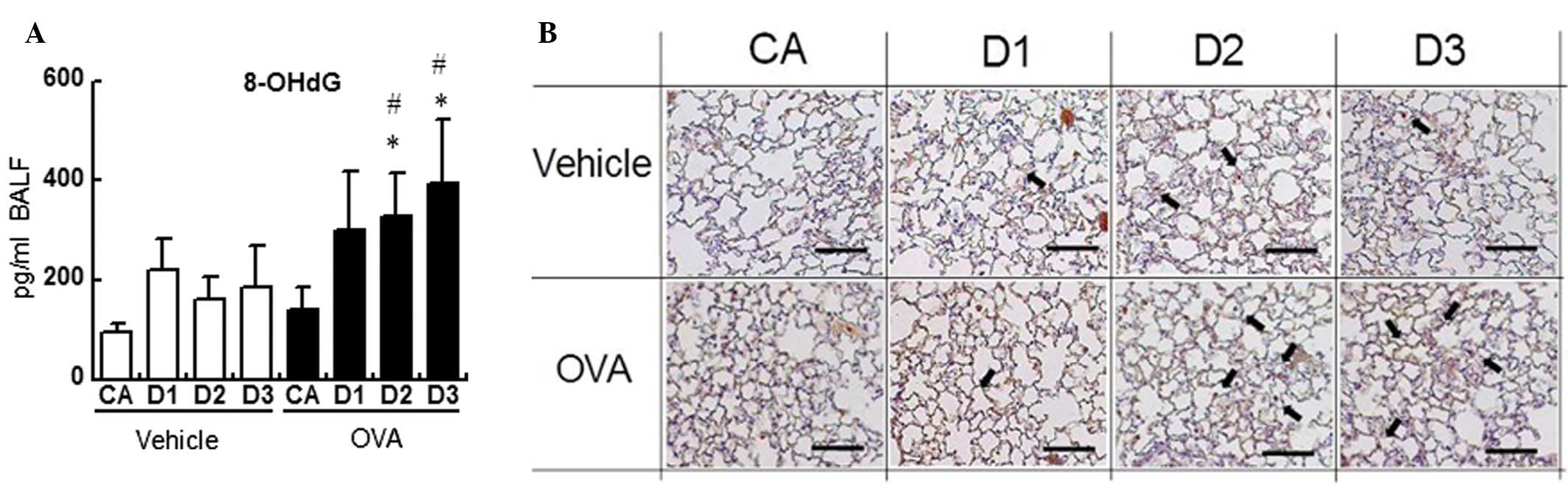
We first quantified the level of 8-OHdG in the BALF.
The level of 8-OHdG was higher in the D1- (299 pg/ml BALF), D2-
(375 pg/ml BALF; P<0.05) and D3- (394 pg/ml BALF; P<0.05) OVA
groups than in the corresponding vehicle groups (220, 160 and 186
pg/ml BALF, respectively), and was significantly higher in the D2-
and D3-OVA groups than in the CA-OVA group (140 pg/ml BALF;
P<0.05; Fig. 1A).
Subsequently, we investigated the expression levels
and localization of 8-OHdG in the lung specimens by means of
immunohistochemistry. NR-DE plus OVA exposure induced moderate
staining for 8-OHdG, compared with that of NR-DE alone or CA plus
OVA exposure (Fig. 1B). The 8-OHdG
expression was mainly localized to inflammatory polymorphonuclear
leukocytes, such as neutrophils and eosinophils.
Discussion
Although DEPs >200 nm in size have been
demonstrated to induce adverse effects on several respiratory
diseases, there have been few studies concerning the effects of
DEPs containing mainly nanoparticles on lung pathology. Our
previous study revealed that inhaled NR-DE exacerbated allergic
airway inflammation in mice (10).
The aggravation was concomitant with the enhanced expression of
allergy-associated cytokines, interleukin-5 (IL-5) and eotaxin, in
the lung. However, the mechanisms of the NR-DE-mediated aggravation
of allergic asthma have not been fully investigated.
Oxidative stress, such as that due to ROS, is
considered to be important in the pathogenesis of various types of
lung inflammatory diseases, including allergic asthma (20,21).
In addition, a possible association between PM2.5 and
oxidative stress has been identified. For example, environmentally
relevant concentrations of PM2.5 have been demonstrated
to exacerbate the airway inflammatory response with an increased
generation of free radicals in asthmatic patients (22,23).
On the other hand, ROS may cause oxidative DNA modification, such
as 8-OHdG formation (24). 8-OHdG
is also induced by several types of oxidative stress-producing
pollutants, such as ozone (13),
DEPs (14) or asbestos (15), in vitro and in vivo.
Concordant with these findings, it has been demonstrated that
certain types of nanoparticles (carbon black nanoparticles and
single/multi-wall nanotubes) were capable of increasing the
expression of 8-OHdG in the lung, in association with aggravated
lung inflammation or injury (19,25,26).
In the present study, we quantified the level of 8-OHdG in the
BALF. The level was greater in the D1-, D2- and D3-OVA groups than
in the corresponding vehicle groups, and was significantly greater
in the D2- and D3-OVA groups than in the CA-OVA group (P<0.05).
Furthermore, the immunohistochemical analysis revealed that NR-DE
plus OVA exposure induced moderate staining for 8-OHdG, compared
with that of NR-DE alone or CA plus OVA exposure. However, no
differences in staining were observed among the NR-DE plus OVA
groups. These results suggested that NR-DE exposure increased
8-OHdG synthesis and release in the lung, which, at least in part,
was involved in the NR-DE-mediated aggravation of the allergic
pathophysiology that was identified in our previous study (10). The significant difference between
the two parameters (EIA versus immunohistochemical staining) may be
due to the time lag during translocation from the lung parenchyma
to the bronchoalveolar spaces. Therefore, time-course studies may
be required to increase understanding of the process.
Notably, gaseous components in the
high-concentration NR-DE without particulate components (D3)
significantly elevated the 8-OHdG levels in the BALF in the
presence of allergen compared with CA (P<0.05). This is
concordant with the allergic pathophysiology observed in the
preceding study (10). 8-OHdG
synthesis in the lung has been demonstrated to be induced or
amplified as a consequence of DNA damage following exposure to
gaseous pollutants, such as nitrogen oxides (NOx), sulphur oxides
(SOx) or ozone (23,27). Therefore, these gaseous components
of NR-DE may be responsible for the enhanced formation of 8-OHdG.
However, in the present study, particulate matter, NR-DEP,
collected in the inhalation systems, independently elevated the
8-OHdG levels in the presence of OVA (data not shown), as we have
previously identified in a study concerning nanoparticles (19). These results suggest that the
mechanism of 8-OHdG-hyperproduction may differ between
high-concentration DE with and without particulate components.
NR-DE exposure significantly elevated the 8-OHdG
level in the lung in the presence of an allergen (as compared with
CA exposure). These results suggested that amplified 8-OHdG
formation in asthmatic lungs, at least in part, is involved in the
NR-DE-mediated aggravation of the allergic pathophysiology observed
in our preceding study (10).
Acknowledgements
The authors would like to thank Dr Masako Kiyono,
Ryosuke Nakamura and Yuka Sone for their significant assistance.
This study was supported by Grants-in-Aid for Scientific Research
(B) 18390188 (to Ken-ichiro Inoue) from the Japan Society for the
Promotion of Science, and partly by The Sumitomo-Foundation (Tokyo,
Japan).
Abbreviations:
|
NR-DE
|
nanoparticle-rich diesel exhaust
|
|
8-OHdG
|
8-hydroxydeoxyguanosine
|
|
ROS
|
reactive oxygen species
|
|
PM
|
particulate matter
|
|
DEP
|
diesel exhaust particles
|
|
PM2.5
|
PM with a diameter <2.5 μm
|
|
NOx
|
nitric oxides
|
|
SOx
|
sulphur oxides
|
|
PBS
|
phosphate-buffered saline
|
|
OVA
|
ovalbumin
|
|
BAL
|
bronchoalveolar lavage
|
|
BALF
|
BAL fluid
|
|
IL
|
interleukin
|
References
|
1
|
Peters A, Wichmann HE, Tuch T, Heinrich J
and Heyder J: Respiratory effects are associated with the number of
ultrafine particles. Am J Respir Crit Care Med. 155:1376–1383.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dockery DW, Pope CA III, Xu X, et al: An
association between air pollution and mortality in six U.S. cities.
N Engl J Med. 329:1753–1759. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel MM, Chillrud SN, Correa JC, et al:
Traffic-related particulate matter and acute respiratory symptoms
among New York City area adolescents. Environ Health Perspect.
118:1338–1343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ichinose T, Furuyama A and Sagai M:
Biological effects of diesel exhaust particles (DEP). II Acute
toxicity of DEP introduced into lung by intratracheal instillation.
Toxicology. 99:153–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takano H, Yoshikawa T, Ichinose T, et al:
Diesel exhaust particles enhance antigen-induced airway
inflammation and local cytokine expression in mice. Am J Respir
Crit Care Med. 156:36–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maejima K, Tamura K, Nakajima T, et al:
Effects of the inhalation of diesel exhaust, Kanto loam dust, or
diesel exhaust without particles on immune responses in mice
exposed to Japanese cedar (Cryptomeria japonica) pollen.
Inhal Toxicol. 13:1047–1063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timonen KL, Hoek G, Heinrich J, et al:
Daily variation in fine and ultrafine particulate air pollution and
urinary concentrations of lung Clara cell protein CC161. Occup
Environ Med. 61:908–914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Y, Eiguren-Fernandez A, Hinds WC and
Miguel AH: In-cabin commuter exposure to ultrafine particles on Los
Angeles freeways. Environ Sci Technol. 41:2138–2145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kappos AD, Bruckmann P, Eikmann T, et al:
Health effects of particles in ambient air. Int J Hyg Environ
Health. 207:399–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka M, Aoki Y, Takano H, et al: Effects
of exposure to nanoparticle-rich or -depleted diesel exhaust on
allergic pathophysiology in the murine lung. J Toxicol Sci.
38:35–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuchino Y, Mori F, Kasai H, et al:
Misreading of DNA templates containing 8-hydroxydeoxyguanosine at
the modified base and at adjacent residues. Nature. 327:77–79.
1987. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawai Y, Morinaga H, Kondo H, et al:
Endogenous formation of novel halogenated 2′-deoxycytidine.
Hypohalous acid-mediated DNA modification at the site of
inflammation. J Biol Chem. 279:51241–51249. 2004.
|
|
13
|
Cheng ML, Ho HY, Huang YW, Lu FJ and Chiu
DT: Humic acid induces oxidative DNA damage, growth retardation,
and apoptosis in human primary fibroblasts. Exp Biol Med (Maywood).
228:413–423. 2003.PubMed/NCBI
|
|
14
|
Sanbongi C, Takano H, Osakabe N, et al:
Rosmarinic acid inhibits lung injury induced by diesel exhaust
particles. Free Radic Biol Med. 34:1060–1069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Upadhyay D and Kamp DW: Asbestos-induced
pulmonary toxicity: role of DNA damage and apoptosis. Exp Biol Med
(Maywood). 228:650–659. 2003.PubMed/NCBI
|
|
16
|
Fujitani Y, Hirano S, Kobayashi S, et al:
Characterization of dilution conditions for diesel nanoparticle
inhalation studies. Inhal Toxicol. 21:200–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue K, Takano H, Yanagisawa R, et al:
Pulmonary exposure to diesel exhaust particles induces airway
inflammation and cytokine expression in NC/Nga mice. Arch Toxicol.
79:595–599. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue K, Yanagisawa R, Koike K, et al:
Effects of carbon black nanoparticles on elastase-induced
emphysematous lung injury in mice. Basic Clin Pharmacol Toxicol.
108:234–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue K, Yanagisawa R, Koike E, Nishikawa
M and Takano H: Repeated pulmonary exposure to single-walled carbon
nanotubes exacerbates allergic inflammation of the airway: Possible
role of oxidative stress. Free Radic Biol Med. 48:924–934. 2010.
View Article : Google Scholar
|
|
20
|
Riedl MA and Nel AE: Importance of
oxidative stress in the pathogenesis and treatment of asthma. Curr
Opin Allergy Clin Immunol. 8:49–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YJ, Takizawa H and Kawada T: Role of
oxidative stresses induced by diesel exhaust particles in airway
inflammation, allergy and asthma: their potential as a target of
chemoprevention. Inflamm Allergy Drug Targets. 9:300–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sierra-Vargas MP, Guzman-Grenfell AM,
Blanco-Jimenez S, et al: Airborne particulate matter
PM2.5 from Mexico City affects the generation of
reactive oxygen species by blood neutrophils from asthmatics: an in
vitro approach. J Occup Med Toxicol. 4:172009.
|
|
23
|
Ren C, Fang S, Wright RO, Suh H and
Schwartz J: Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of
oxidative DNA damage induced by ambient pollution in the Normative
Aging Study. Occup Environ Med. 68:562–569. 2011.
|
|
24
|
Kuwano K, Nakashima N, Inoshima I, et al:
Oxidative stress in lung epithelial cells from patients with
idiopathic interstitial pneumonias. Eur Respir J. 21:232–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue K, Takano H, Yanagisawa R, et al:
Effects of nano particles on antigen-related airway inflammation in
mice. Respir Res. 6:1062005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai CH, Liou SH, Lin HC, et al: Exposure
to traffic exhausts and oxidative DNA damage. Occup Environ Med.
62:216–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren C, Fang S, Wright RO, Suh H and
Schwartz J: Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of
oxidative DNA damage induced by ambient pollution in the Normative
Aging Study. Occup Environ Med. 68:562–569. 2011.
|