Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common malignant tumor worldwide (1). Although therapeutic strategies have
improved in in the past two to three decades, the overall five-year
survival rate remains almost unchanged (2). The primary reasons for this are
post-treatment locoregional recurrence and distant metastasis. The
detection of tumor metabolism in the early phase is important when
devising the individual therapeutic strategy and undertaking a
prognostic evaluation. Traditionally, computed tomography (CT) and
magnetic resonance imaging (MRI) have been used to clearly display
anatomical structure. However, with regard to disease
identification, evaluation of lymph node metastasis and prognosis,
CT and MRI have certain limitations. Positron emission tomography
(PET), a functional imaging technology, is extensively applied in
clinical practice to detect tumors and evaluate cervical node
metastases in patients with HNSCC, due to its high sensitivity and
specificity and the fact that it enables the monitoring of the
disease at a molecular level (3).
Although PET has important applications in clinical
practice, the application of PET in animal experiments is
difficult, due to limitations in sensitivity and spatial
resolution. Consequently, micro-PET imaging has been designed for
this purpose. Micro-PET overcomes the shortcomings of clinical PET
and has been increasingly used in the imaging of murine models of
human diseases. However, the application of micro-PET imaging is
restricted, due to its expensive cost and single usage. The
adaptation of clinical PET for use in animal studies is
particularly challenging; resolution of this problem is likely to
provide clinical PET with another valuable function, progress the
clinical application of PET and reduce in the cost of scientific
research.
To the best of our knowledge, the current study is
the first to apply clinical PET to laryngeal squamous cell
carcinoma (LSCC) xenografts. It is likely to provide a useful tool
for exploring the mechanisms of tumor genesis and metabolism. In
this study, we established an LSCC xenograft model in nude mice and
utilized [F-18]-fluoro-2-deoxy-D-glucose (18F-FDG) as a
tracer to study the quality of PET images under various conditions.
By comparing the qualities of the images of the tumors, the most
effective handling protocol was determined. The present LSCC
xenograft study demonstrated further potential applications for
clinical PET.
Materials and methods
Cell culture and animals
The present study was conducted at the Department of
Otolaryngology Head and Neck Surgery in the Bethune International
Peace Hospital (Shijiazhuang, China), and was approved by the
Ethics Committee of the Bethune International Peace Hospital.
Hep-2 LSCC cells (Shanghai Life Science Academy,
Chinese Academy of Science, Shanghai, China) were cultured in
RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) which was
supplemented with 10% fetal bovine serum (Hangzhou sijiqing
biological engineering materials co., Ltd., Hangzhou, China), 1%
glutamine and 0.5% HEPES. Cells were cultured at 37ºC in a
humidified 5% CO2 incubator. The exponentially growing
cells were harvested with 0.25% trypsin plus
ethylenediaminetetraacetic acid, washed and suspended in
phosphate-buffered saline (PBS). The number of cells was counted
using a Coulter counter (Beckman Coulter, Inc., Brea, CA, USA).
All experiments were performed using 16–18 g male
athymic NCr-nu/nu mice, purchased from the Academy of Military
Medical Sciences Animal Center (Beijing, China). The nude mice were
maintained and used according to guidelines of Hebei province
laboratory animal management institutions and the experimental
protocols were approved by the ethics committee of Bethune
International Peace Hospital. Five animals were housed per cage and
were maintained at a constant temperature and humidity in the
Bethune International Peace Hospital Experimental Animal
Center.
Synthesis of 18F-FDG
18F-FDG was provided by the PET Center of
the Bethune International Peace Hospital and was synthesized
automatically by nucleophilic substitution on mannose triflate
(1,3,4,6-tetra-O-acetyl-2-O-trifluoro-methanesulfonyl-β-D-mannopyranose)
in a PET cyclotron unit.
Self-made warming instrument
The warming instrument comprised a cabinet that was
made of an extruding plate and sealed with adhesive tape. In this
experiment, two cabinets were used to maintain the animals at a
constant temperature. A larger cabinet was used for the animals
that had been injected with 18F-FDG and were waiting for
PET scanning, while a smaller cabinet was used for the animals that
were undergoing PET scanning. The large and small cabinets measured
60×30×18 cm and 16×12×10 cm, respectively, with sight-holes
measuring 24×16 cm and 6×4 cm, respectively. The bottom of the
cabinet was paved with aluminum foil-reflecting film, with
electrothermal film in the middle and a hole-type plastic bracket
on the surface. A power line was welded to the electrothermal film
and emerged from the side of the cabinet. At the opposite side, a
temperature probe was inserted near the bracket, to enable the
monitoring of the ambient temperature. A thermostat was situated
outside the cabinet to regulate the temperature within.
Establishment of the Hep-2 LSCC cell
xenografts in the animals
Briefly, a 1×106 cells/0.2 ml Hep-2-cell
suspension was injected subcutaneously into unanesthetized mice.
The experiments were typically performed 3–4 weeks later, when
distributions of tumors ranging from 0.8 to 1.5 cm in diameter were
observed on the backs of the mice. The average body weight of the
mice was 23.9±1.3 g. Serum glucose levels were measured prior to
the experiment in fasted mice, with the average value measured to
be 4.7±0.5 mmol/l. Following this, the mice were selected randomly
and divided into seven groups, each containing between three and
six mice. There was no statistical difference in tumor size among
these groups.
Experimental procedures
The mice were studied under the experimental
conditions summarized in Table I.
Due to the small caliber of the murine tail veins, the
administration of 18F-FDG by tail vein injection
(intravenous) is challenging. Partial paravenous injection is
common and has no significant influence on the biodistribution of
18F-FDG under different conditions. Therefore,
18F-FDG was injected intraperitoneally prior to PET
scanning.
 | Table ISummary of experimental
conditions. |
Table I
Summary of experimental
conditions.
| Group | n | Fasting | Warming | Anesthetic | Scanning start time
(h) |
|---|
| A | 3 | Yes | No | Chloral hydrate | 0 |
| B | 3 | Yes | No | Isoflurane, Chloral
hydrate | 0 |
| C | 3 | No | No | Pentobarbital | 1 |
| D | 6 | Yes | No | Pentobarbital | 1 |
| E | 4 | Yes | Yes | Pentobarbital | 1 |
| F | 4 | Yes | Yes | Pentobarbital | 1.5 |
| G | 4 | Yes | Yes | Pentobarbital | 2 |
Group A
The animals were fasted overnight, prior to
measurements of weight and serum glucose being obtained. Following
this, the animals were administered different doses of chloral
hydrate at room temperature. There was one death immediately
subsequent to the administration of 7 ml/kg 5% chloral hydrate. Two
animals were anesthetized for 30–40 min following the
administration of 5 ml/kg 5% chloral hydrate. When the dosage was
supplemented with 2 ml/kg 5% chloral hydrate, the animals ceased
breathing and died.
Group B
Animals were fasted overnight and administered an
isoflurane inhalation. Following the inhalation of isoflurane for
~10 sec, the animals rapidly lost consciousness; however, the
effect was only maintained for 1 min. The animals were then
injected with 2 ml/kg 5% chloral hydrate intraperitoneally, which
resulted in anesthesia being maintained for 30–40 min. Following
further isoflurane inhalation, the animals died.
Group C
Animals had constant access to food and drinking
water and were injected with 5 ml/kg 1% pentobarbital
intraperitoneally. Following a short (~5 min) period of anesthesia,
the animals lost consciousness and their breathing became deep and
slow. The effect was maintained for 40–50 min. When the animals
began breathing superficially, a further 2 ml/kg 1% pentobarbital
was administered, which resulted in successful anesthesia. There
were no animal deaths and therefore, in the following groups, the
intraperitoneal injection of 1% pentobarbital was adopted as the
primary anesthesia method. In group C, 18F-FDG [5–7 MBq
(200 μCi) in 0.2 ml] was injected intraperitoneally once the
animals were anesthesized with no warming and one hour later PET
scanning was performed.
Group D
Animals were fasted overnight, without any warming
treatment, and were administered an intraperitoneal injection of
18F-FDG following a short period of anesthesia with
pentobarbital. One hour later, PET scanning was performed.
Group E
Animals were fasted overnight. Following a short
period of anesthesia with pentobarbital, they were injected with
18F-FDG intraperitoneally and kept warm in the cabinet,
where the temperature was 30ºC. One hour later, the mice were
placed into the small cabinet and PET scanning was performed.
Group F
The handling conditions of the animals were
identical to those for group E. Following the intraperitoneal
injection of 18F-FDG for 1.5 h, the animals were placed
into the small cabinet and PET scanning was performed.
Group G
The handling conditions of the animals were
identical to those for group E. Following intraperitoneal injection
of 18F-FDG for 2 h, the animals were placed into the
small cabinet and PET scanning was performed.
18F-FDG PET imaging
The experiment was performed with the Philips ADAC
Allegro™ PET scanner (Koninklijke Philips NV, Amsterdam,
Netherlands). Subsequent to the animals being anesthetized and
injected with 18F-FDG, they were fixed on the bracket
with limbs stretched. According to the experimental group
requirements, the animals were either warmed in the cabinet by
thermostatic regulation or left without warming. The image data
were collected by head position 3D mode and the acquisition time
was 5 min per bed position. Through the RAMLA 3D image
reconstruction method, coronal, sagittal and cross-sectional
tomographical images were acquired. The region of interest (ROI)
technology was adopted to calculate target and non-target (T/N)
ratios, and the results were analyzed by semiquantitative
analysis.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD). Statistical comparisons were performed with SPSS
13.0 statistical software for Windows (SPSS, Inc., Chicago, IL,
USA). The differences in the T/N ratios among the experimental
groups were statistically evaluated by analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of anesthesia on the animals
In group A, different doses of 5% chloral hydrate
were administered to the mice. Observation of the mice showed that
a 7 ml/kg dosage (the highest limit for common usage) was lethal
for the nude mice, while a 5 ml/kg dosage was only able to induce
anesthesia for 30–40 min. If an additional dosage (2 ml/kg) was
administered to the nude mice, the chloral hydrate was lethal.
Isoflurane inhalation had a rapid effect on the mice (≥10 sec);
however, the duration of the anesthesia was too short (≥1 min).
When combining isoflurane inhalation with chloral hydrate
injection, a synergistic effect was observed in the course of the
anesthesia, although the combination was more dangerous. The
administration of 1% pentobarbital (5 ml/kg) was relatively safe
for the nude mice. The anesthesia had a relatively long duration
(40–50 min), and it was possible to administer an additional dosage
(2 ml/kg), according to the requirements of the experiment.
Correlation between tumor size and PET
image quality
In this study, the diameter of the tumors imaged by
PET scanning ranged from 0.8 to 1.5 cm. A comparison between the
results showed that there was no significant correlation between
tumor size and PET image quality (r=0.381, P>0.05).
Temperature change during anesthesia and
PET
When animals were maintained under anesthesia for 30
min at room temperature (24ºC), the mean body temperature of the
mice decreased from 31.72±0.46 to 24.2±0.12ºC. This marked
reduction in body temperature was avoided when the mice were kept
in the warming cabinet (body temperature subsequent to 30 min
anesthesia, 33.01±0.77ºC). There was no mouse dehydration.
Influence of different handling
conditions on the 18F-FDG uptake of the tumor
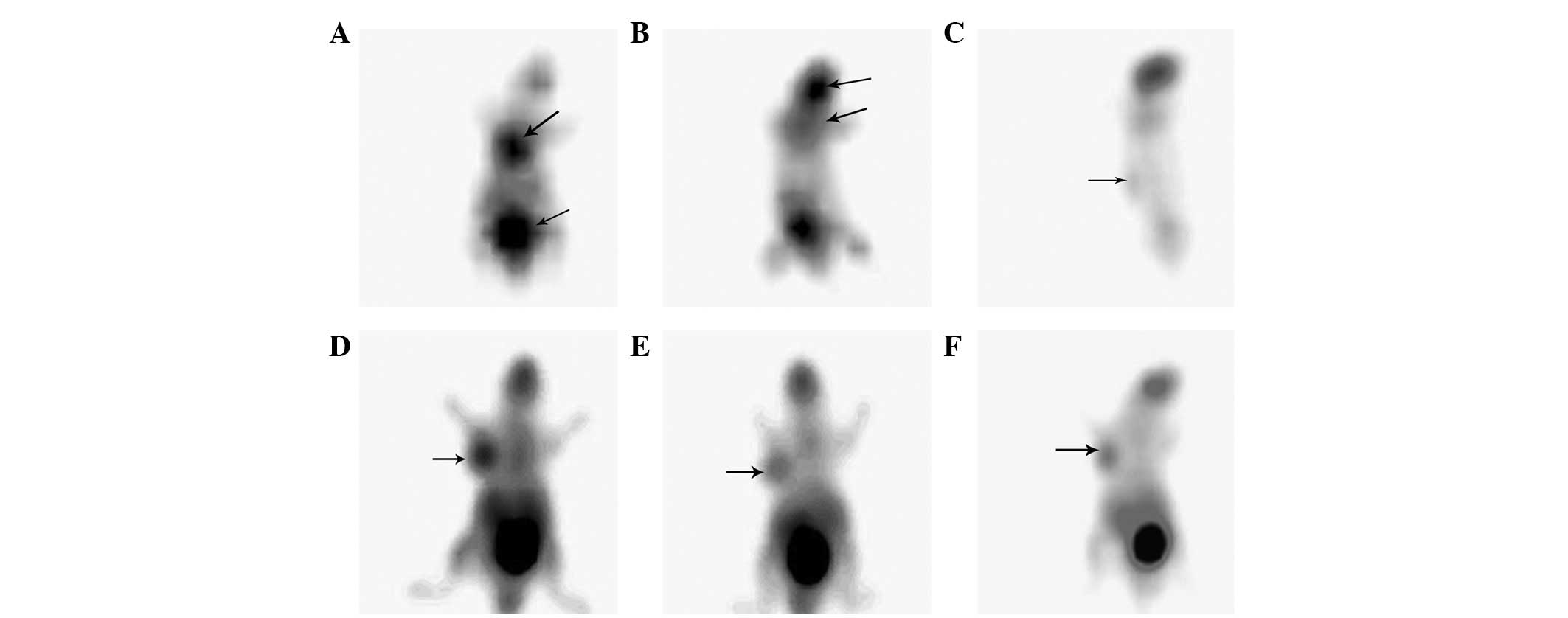
Fig. 1 shows
typical examples of the PET scans of the anesthetized animals
acquired under the various conditions. With no warming and no
fasting condition or fasting and no warming condition (Fig. 1A and B), the highest
18F-FDG uptakes were observed in the myocardium (T/N,
3.15±0.44), urinary bladder (T/N, 3.55±0.63), cerebral tissue (T/N,
3.1±0.60), skeletal muscle (T/N, 1.24±0.10) and brown fat (T/N,
2.55±0.34). There was no evident 18F-FDG uptake in the
tumor.
When the animals were fasted without being warmed,
PET images of the tumors were acquired. However, the images were
unstable (2/6) and of a poor quality (Fig. 1C). Fasting only increased the
18F-FDG uptake (T/N, 1.153±0.008) of the tumor to a
certain degree. When the mice were warmed and fasted, tumor images
of a satisfactory quality were acquired (12/12; Fig. 1D–F). The results showed that
warming and fasting in combination significantly increased the
18F-FDG uptake of the tumor (T/N, 2.0±0.29) and reduced
the 18F-FDG uptake of the brown fat and myocardium
compared with the mice that were only fasted (P<0.05).
Impact of 18F-FDG uptake
duration on image quality
In this study, the effect of the 18F-FDG
acquisition time was analyzed. Intriguingly, the duration of
18F-FDG uptake had no impact on the tumor image quality.
Following intraperitoneal injection of 18F-FDG for 1,
1.5 and 2 h, the PET images of the tumors were displayed. There
were no statistical differences in the T/N radios among the three
groups (1.904±0.246, 2.114±0.354 and 1.978±0.312, respectively;
P>0.05).
Discussion
When PET scanning was introduced at the end of the
1970s, its metabolic/functional image qualities led to an immediate
interest. The available tracers made it possible to study blood
flow, regional oxygen consumption, the main metabolic pathways and
ligand-receptor interactions in the brain, heart and numerous types
of tumor (4). At present, PET
scanning is predominantly used in oncology, due to its noninvasive,
quantitative and reproducible characteristics. During the diagnosis
of a number of different types of cancer, including lung, breast
and thyroid carcinomas, ovarian cancer, brain, head and neck and
bone tumors and lymphoma, PET has demonstrated significant
advantages. PET scanning, with the glucose analog FDG, is also
increasingly used to study murine models of human diseases. It
exhibits a powerful evaluation modality in experimental research
for monitoring the progression and transformation of tumors
(5), the biological
characterization of tumor tissue (6) and for determining the efficacy of
therapeutic agents (7). However,
due to the different types of tumors and tumor heterogeneity, there
is a diversity among the images produced. In order to understand
the imaging characteristics of PET scanning for LSCC xenografts,
the present study used 18F-FDG as a tracer for tumor
imaging. However, the mode of anesthesia, the dietary conditions
and ambient temperature have been shown to influence the
18F-FDG uptake of the tumor. Therefore, the present
study investigated the effects of these factors on tumor imaging,
and a handling protocol that optimized the quality of the image of
the tumor was developed.
The type of anesthesia used was a critical factor in
the process of the study. Chloral hydrate, isoflurane and
pentobarbital are the most commonly used anesthetics in rodent
animal experiments. In this study, the effects of these three types
of anesthetics on the mice were compared. The results showed that 7
ml/kg 5% chloral hydrate (the highest limit for common usage) was
lethal for the nude mice, while a dosage of 5 ml/kg was only able
to induce anesthesia for 30–40 min. The administration of an
additional dosage (2 ml/kg) of chloral hydrate was dangerous for
the nude mice. The effects of isoflurane inhalation were induced
rapidly (≥10 sec); however, the duration of the effects was too
short (≥1 min). When combining isoflurane inhalation with chloral
hydrate injection, a synergistic effect was observed in the course
of the anesthesia. However, the combination of the drugs was shown
to be more dangerous. The administration of 1% pentobarbital (5
ml/kg) was relatively safe for the nude mice. The drug acted for a
longer duration (40–50 min), and it was possible to administer an
additional dose (2 ml/kg) to the mice according to the experimental
requirements.
Due to the limitations in spatial resolution, the
volume of the tumor may directly affect the quality of the imaging
(8). Wu and Yu (9) observed that a tumor diameter between
0.5 and 0.8 cm was suitable for the conduction of interventional
research. When the tumor diameter exceeded 1.0 cm, necrosis
occurred in the center of the tumor, which had a detrimental effect
on the tumor image. However, in a study on the use of clinical PET
scanning for human nasopharyngeal carcinoma xenografts, Yuan et
al(10) observed that a
maximum tumor diameter of between 1 and 1.4 cm was more appropriate
for clinical PET imaging. With regard to these studies, we selected
tumor diameters between 0.8 and 1.5 cm as the objects of
18F-FDG PET study. The results showed that tumor
diameters in this range were suitable for PET imaging. There was no
significant correlation between tumor size and PET image
quality.
The conditions in which the animals are kept have a
significant impact on the 18F-FDG uptake of the tumor.
Various handling conditions, such as fasting and warming, may lead
to different tumor image qualities, with the difference of image
quality between warming and no warming being approximately
two-fold. Fueger et al(11), who studied the impact of animal
handling on the result of 18F-FDG PET imaging using
micro-PET, observed that fasting and warming resulted in over a
three-fold increase in tumor 18F-FDG uptake. This may
have been associated with glucose levels. When animals were allowed
free access to food, the insulin levels of the mice were increased,
which induced the elevation of blood sugar. Glucose may compete
with 18F-FDG for intracellular uptake and
phosphorylation and thereby reduce the 18F-FDG uptake of
the tumor. Furthermore, the elevated insulin levels may result in
an increased 18F-FDG uptake by skeletal muscle and the
myocardium (12,13), in addition to enhancing the
metabolic activity of brown adipose tissue (14). These factors may result in the
enhancement of the images of these tissues, but not the tumor.
However,, sufficient fasting may increase the 18F-FDG
uptake of the tumor and reduce the activity of muscle, heart and
brown adipose, thereby improving the tumor image quality.
Ambient temperature is also an important factor with
regard to 18F-FDG uptake. Data have shown that ambient
temperature has a pronounced effect on 18F-FDG
biodistribution in mice; for mice, the optimal ambient temperature
lies between 30 and 34ºC (15). At
this temperature, body temperature is maintained by heat convection
and no additional activity is required. However, when the
temperature is decreased, brown adipose tissue and muscle activity
are required to generate heat, in order to maintain a constant body
temperature. High temperature may increase the heart rate, leading
to dehydration of the animal, hypoglycemia and renal injury
(16). Therefore 30ºC was adopted
as the ambient temperature in this study. The results showed that
this temperature was appropriate and resulted in the production of
clear tumor images.
The image acquisition time was an additional
important factor. The transformation rate from hexokinase (HK) to
glucose-6-phosphate (G6P) has been shown to be different among
malignant and benign tumors and inflammatory lesions, while the
18F-FDG uptake rates have also been shown to differ
accordingly (17). According to
the Warburg effect, the 18F-FDG uptake of a tumor is
high; therefore, conducting PET scanning too early or too late may
influence the tumor imaging. In clinical practice, patients usually
undergo FDG-PET following the intravenous injection of
18F-FDG for ~1 h. To distinguish between tumors and
inflammation, delayed imaging may be performed in patients with
tumors, by injection with 18F-FDG for 2 h (18). According to clinical procedure, the
present study adopted 1, 1.5 and 2 h of 18F-FDG
injection as the initiation time for the PET scanning. The results
showed that the acquisition time did not have any impact on the
quality of the image of the tumor. This was due to the fact that
following 1 h of 18F-FDG injection, the uptake of the
tumor was high and was maintained for ~1 h.
In conclusion, clinical 18F-FDG PET
scanning may be applied to LSCC xenografts in a nude mouse animal
model. The results of the present study showed that a tumor
diameter of 0.8–1.5 cm is appropriate for PET scanning; that
overnight fasting and warming are critical conditions for tumor
imaging and that the animals should be quiet and relaxed, which may
be achieved by the administration of 1% pentobarbital. In addition,
the results showed that injection of 18F-FDG for 1 h is
an appropriate injection duration for PET scanning.
Acknowledgements
The authors would like to thank Chief Nurse Tao for
coordinating the work and performing the injection of FDG in this
experiment. The authors are also grateful to engineer Zhenzhong
Wang for his help in producing the warming cabinet. The
investigation was supported in part by the National Natural Science
Research Fund of China (grant no. 30572029) and the Wu Jieping
Medical Research Foundation (China; grant no. 320.6750.10121).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Kyzas PA, Evangelou E and Denaxa-Kyza D:
18F-fluorodeoxyglucose positron emission tomography to
evaluate cervical node metastases in patients with head and neck
squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst.
100:712–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gambhir SS, Czernin J, Schwimmer J,
Silverman DH, Coleman RE and Phelps ME: A tabulated summary of the
FDG PET literature. J Nucl Med. 42(Suppl): 1S–93S. 2001.PubMed/NCBI
|
|
5
|
Abbey CK, Borowsky AD, McGoldrick ET,
Gregg JP, Maglione JE, Cardiff RD and Cherry SR: In vivo
positron-emission tomography imaging of progression and
transformation in a mouse model of mammary neoplasia. Proc Natl
Acad Sci USA. 101:11438–11443. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dearling JL, Flynn AA, Sutcliffe-Goulden
J, Petrie IA, Boden R, Green AJ, Boxer GM, Begent RH and Pedley RB:
Analysis of the regional uptake of radiolabeled deoxyglucose
analogs in human tumor xenografts. J Nucl Med. 45:101–107.
2004.PubMed/NCBI
|
|
7
|
Waldherr C, Mellinghoff IK, Tran C,
Halpern BS, Rozengurt N, Safaei A, Weber WA, Stout D, Satyamurthy
N, Barrio J, Phelps ME, Silverman DH, Sawyers CL and Czernin J:
Monitoring antiproliferative responses to kinase inhibitor therapy
in mice with 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl
Med. 46:114–120. 2005.PubMed/NCBI
|
|
8
|
Hatt M, Cheze-le Rest C, van Baardwijk A,
Lambin P, Pradier O and Visvikis D: Impact of tumor size and tracer
uptake heterogeneity in 18F-FDG PET and CT non-small
cell lung cancer tumor delineation. J Nucl Med. 52:1690–1697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu SH and Yu CJ: The establishment of
nasopharyngeal carcinoma (NPC) animal model and observation of
biologic characteristics from NPC. Journal of Qiqihar Medical
College. 28:2183–2184. 21872007.(In Chinese).
|
|
10
|
Yuan JW, Feng YL, Xian WJ, Fan LX, He XH,
Yuan BH, Huang KM, Su SD and Liu Y: Application of clinical PET-CT
scanner for human nasopharyngeal carcinoma (NPC) transplantation
tumor animal model in nude mice. Anatomy Research. 31:378–381.
2009.
|
|
11
|
Fueger BJ, Czernin J, Hildebrandt I, Tran
C, Halpern BS, Stout D, Phelps ME and Weber WA: Impact of animal
handling on the results of 18F-FDG PET studies in mice.
J Nucl Med. 47:999–1006. 2006.PubMed/NCBI
|
|
12
|
Mossberg KA and Taegtmeyer H: Time course
of skeletal muscle glucose uptake during euglycemic
hyperinsulinemia in the anesthetized rabbit: a
fluorine-18–2-deoxy-2-fluoro-D-glucose study. J Nucl Med.
33:1523–1529. 1992.PubMed/NCBI
|
|
13
|
Huitink JM, Visser FC, van Leeuwen GR, van
Lingen A, Bax JJ, Heine RJ, Teule GJ and Visser CA: Influence of
high and low plasma insulin levels on the uptake of fluorine-18
fluorodeoxyglucose in myocardium and femoral muscle, assessed by
planar imaging. Eur J Nucl Med. 22:1141–1148. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Himms-Hagen J: Thermogenesis in brown
adipose tissue as an energy buffer: implications for obesity. N
Engl J Med. 311:1549–1558. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon C: Temperature Regulation in
Laboratory Rodents. New York, NY: Cambridge University Press; 1993,
View Article : Google Scholar
|
|
16
|
Leon LR, Blaha MD and DuBose DA: Time
course of cytokine, corticosterone, and tissue injury responses in
mice during heat strain recovery. J Appl Physiol. 100:1400–1409.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhuang H, Pourdehnad M, Lambright ES,
Yamamoto AJ, Lanuti M, Li P, Mozley PD, Rossman MD, Albelda SM and
Alavi A: Dual time point 18F-FDG PET imaging for
differentiating malignant from inflammatory processes. J NuclMed.
42:1412–1417. 2001.PubMed/NCBI
|
|
18
|
Abe Y, Tamura K, Sakata I, Ishida J, Ozeki
Y, Tamura A, Uematsu K, Sakai H, Goya T, Kanazawa M and Machida K:
Clinical implications of 18F-fluorodeoxyglucose positron
emission tomography/computed tomography at delayed phase for
diagnosis and prognosis of malignant pleural mesothelioma. Oncol
Rep. 27:333–338. 2012.
|