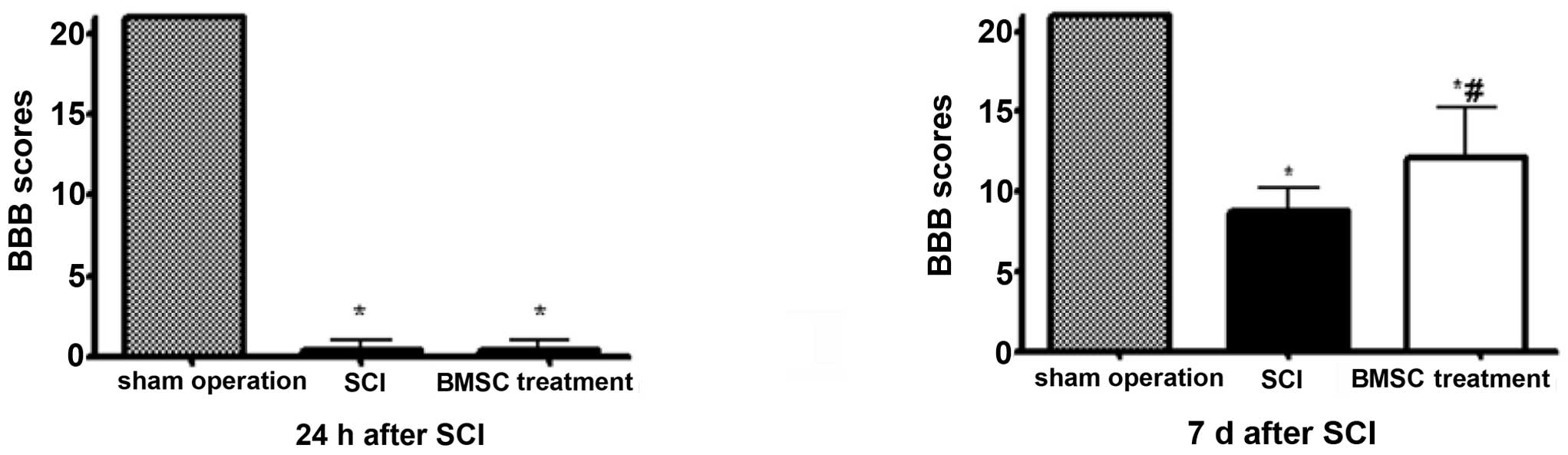Introduction
Spinal cord injury (SCI) often causes life-long
disability. Therefore, it is important to gain an understanding of
the pathophysiology underlying SCI in order to develop clinical
treatment strategies (1,2). Typically, there are two phases of
SCI; the primary injury, in which mechanical impact is afflicted
directly on the spine, and the secondary injury, which involves a
complex cascade of molecular events, including disturbances in
ionic homeostasis (3), local
edema, vascular abnormalities, ischemia-reperfusion, glutamate
excitotoxicity and inflammatory responses (4–6).
Previous studies have indicated that, following SCI, stress
responses of the endoplasmic reticulum (ER) drive neurons and
oligodendrocytes towards apoptosis (7). An ER-resident caspase, caspase-12,
has been shown to mediate apoptosis signaling induced by ER stress
(8). Furthermore, activation of
the unfolded protein response (UPR) upregulates the expression and
activity of caspase-12 and may be important in neuronal cell death
in ischemic brain injury (9). It
has also been observed that the activation of caspase-12 is
involved in the apoptosis of spinal cord neurons and
oligodendrocytes, which is induced by oxygen-glucose-serum
deprivation/restoration (10,11).
Therefore, the inhibition of caspase-12 expression following SCI
may potentially be used as a therapeutic target for neuron
protection.
Bone marrow stromal cells (BMSCs) have been
identified as potential candidates for the treatment of SCI
(12). BMSCs are a population of
heterogeneous mesenchymal cells in the bone marrow consisting of
pluripotent mesenchymal stem cells and murine BMSCs. Under suitable
conditions, they may be propagated in vitro for up to 50
passages with no signs of malignant transformation (13). The transplantation of BMSCs into
SCI rats to promote axonal regeneration, reduce lesion size and
improve functional outcome has been reported (14,15).
At present, the beneficial effects of BMSCs in several models of
CNS injury are considered to be due to the release of trophic
factors and the activation of endogenous survival signaling
pathways via secreted soluble factors in neurons and
oligodendrocytes, as opposed to a result of neuronal or glial
differentiation (16). However,
the exact mechanisms underlying the protective effects of BMSCs in
SCI remain unknown.
In the present study, BMSCs were injected into the
spinal cords of modified Allen’s weight-drop SCI model rats in
order to investigate their effect on apoptosis and the expression
of caspase-12. The aim of this study was to investigate the
protective role of BMSCs in the rats with SCI and to attempt to
develop an effective clinical method of treatment for SCI.
Materials and methods
BMSC culture and induction of SCI in the
rat model
Primary rat BMSCs were isolated and characterized
using methods described in our previous work (17). In brief, the tibias and femurs were
dissected from Sprague-Dawley adult male rats under sodium
pentobarbital (40 mg/kg, i.p.) anesthesia. Rats were purchased from
the Experiment Animal Center of Zhejiang University. After removing
the end of each bone, 5 ml BMSC culture medium, consisting of α-MEM
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 20% fetal bovine
serum and antibiotics, was injected into the central canal of the
bone in order to extrude the marrow. Whole marrow cells were
extracted and cultured at a density of 5–10×105
cells/cm2 in BMSC culture medium. The nonadherent cells
were removed after 24 h by changing the medium and the medium was
changed every other day until the cells became confluent.
Subsequently, when the cells were nearly confluent, they were
detached by trypsin and serially subcultured in the ratio 1:3,
passaged three times and washed twice with PBS to a concentration
of 106 cells/100 μl for transplantation.
A total of 45 Sprague-Dawley rats were randomly
divided into three groups (the sham-operation, SCI and BMSC
treatment groups; n=15 in each group). Under sodium pentobarbital
(40 mg/kg, i.p.) anesthesia, the vertebral columns of the rats were
exposed and a laminectomy at level T10 was carried out.
Subsequently, a contusion injury was produced using a weight-drop
device in the SCI and BMSC treatment groups. A weight of 10 g was
dropped from a height of 50 mm onto the exposed spinal cord and the
impounder was left for 20 sec before withdrawal in order to produce
a moderate contusion. Rats in the sham-operation group underwent
the same surgical procedure but without SCI. Twenty-four hours
after the operation, 5 μl labeled BMSCs were injected using an
electrode microneedle into the epicenter of the injured spinal
cords of the BMSC treatment group rats, while the same quantity of
PBS was injected into rats of the SCI and sham-operation groups.
Hind limb locomotor function was assessed using the Basso, Beattie
and Bresnahan (BBB) locomotor rating scale at 24 h and 7 days after
transplantation (18). The rats
were sacrificed for subsequent pathological, immunohistochemical
and quantitative (q)PCR analyses. All animal experiments were
conducted with the approval of the Animal Care Committee of the
School of Medicine, Zhejiang University (Hangzhou, China).
Immunohistochemical assay
Five rats per group were re-anesthetized with sodium
pentobarbital (60 mg/kg, i.p.) and perfused with 4%
paraformaldehyde in phosphate-buffered saline 24 h after
transplantation. The lesion epicenter (4 mm) of the spinal cord was
removed, post-fixed in the same fixative for 24 h and prepared for
cryostat sectioning.
DAB staining was performed on the cryostat sections
(thickness, 8 μm). Briefly, cryostat sections were first washed in
PBS and incubated with 1% hydrogen peroxidase
(H2O2) to block the non-specific reactivity
of endogenous peroxidase. After washing in phosphate buffered
saline (PBS), the sections were treated for antigen retrieval with
10.2 mmol/l sodium citrate buffer (pH 6.1) for 20 min at 95°C,
followed by incubation in medium of PBS with an anti-caspase-12
primary antibody (1:150; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) plus 1% BSA at 25°C for 2 h. Control sections were
incubated in PBS plus 1% BSA. After washing, the sections were
incubated for 1 h at 25°C in a biotinylated goat-anti-mouse
secondary antibody (diluted in 1:200 in PBS, Boster Biological
Technology, Ltd., Wuhan, China), and subsequently the sections were
incubated with a horseradish peroxidase (HRP)-conjugated
avidin-biotin complex (ABC) for 20 min, and the staining was
visualized with 0.05% DAB plus 0.3% H2O2 in
PBS. The sections were also stained with hematoxylin for 1 min, and
the stained sections were dehydrated with a graded series of
ethanol and xylene before using cover slips. Images of the ventral
area of the spinal cord were captured (magnification, ×40) using a
digital camera attached to a microscope (Nikon E600, Nikon Company,
Tokyo, Japan). Total numbers of caspase-12-positive cells were
manually counted in 10 fields in a section around the injured area.
The results were expressed as the average number of
caspase-12-positive cells per field in each group.
qPCR assay
The remaining 5 rats per group were re-anesthetized
and decapitated for RNA sample preparation. The lesion epicenter
corresponding to one intervertebral segment at the T10 cord level
was removed (~4 mm) and total RNA was obtained using an RNA
extraction kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions. The reaction mixture used for qPCR (40
μl) contained 4 μl cDNA, 35.2 ml SYBR-Green PCR mix, 5 units (0.5
μl) Taq DNA polymerase and 0.3 μl 20 pmol/ml caspase-12 primer. The
cDNA was denatured at 94°C for 3 min. The template was amplified
for 40 cycles (denaturation at 94°C for 10 sec, annealing at 57°C
for 30 sec and extension at 72°C for 30 sec), prior to detecting
fluorescence at 72°C. Meanwhile, primers for housekeeping gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used in PCR
to amplify GAPDH (forward: 5′-AGTTCAACGGCACAGTCAAG-3′ and reverse:
5′-TACTCAGCACCAGCATCACC-3′) as an internal control for caspase-12
(forward: 5′-CACTGCTGATACAGATGAGG-3′ and reverse:
5′-CCACTCTTGCCTACCTTCC-3′). Fold-change in gene expression was
estimated using the comparative C(T) method (19).
Statistical analysis
All values are presented as the mean ± SD.
Statistical comparisons in all groups were made using one-way
analysis of variance (ANOVA). A probability of 95% (P<0.05) was
considered to indicate a statistically significant difference.
Fold-changes in gene expression were estimated using the CT
comparative method normalizing to GAPDH CT values and relative to
control samples as follows: ΔCT = CT
caspase-12 - CT GAPDH; ΔΔCT = ΔCT
- ΔCT control; fold difference =
2−(ΔΔCT).
Results
BMSC transplantation improves limb
locomotion
Rats in the SCI and BMSC treatment groups exhibited
marked bilateral hind limb paralysis, with no movement or only
slight movement of the joints, when observed during open-field
walking. By contrast, all rats in the sham-operation group were
able to walk normally 24 h after injury. The animals were observed
for 7 days post-transplantation in order to measure their locomotor
activity according to the BBB scale. During the 7 days, the degree
of recovery of locomotor function, to a plateau below the range of
10 points, was observed in the SCI group compared with rats in the
BMSC treatment group, which exhibited greater recovery (BBB scores
>10, P<0.05; Fig. 1).
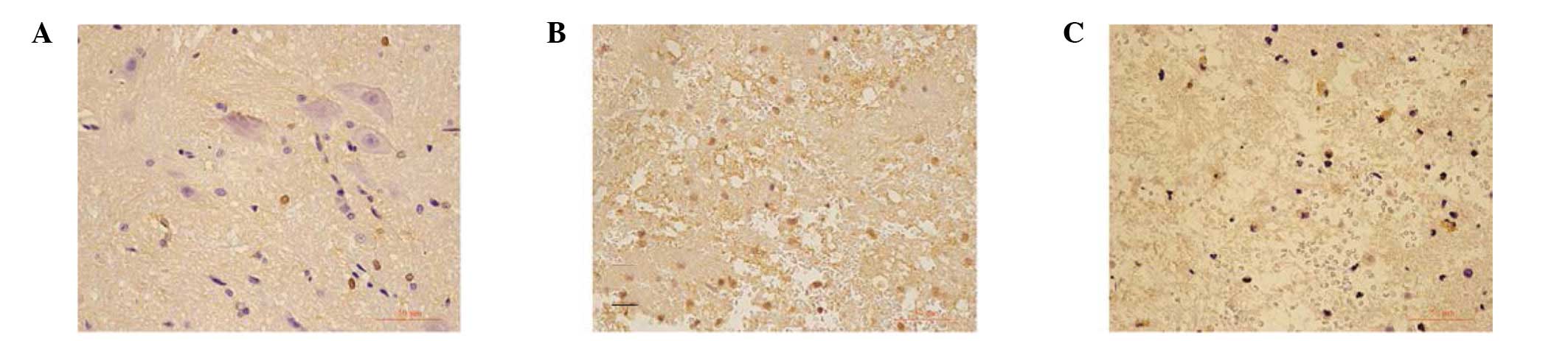
Immunohistochemistry and qPCR assay
Cells that stained positive for caspase-12 exhibited
buff-colored granules when stained with diaminobenzidine (DAB;
Fig. 2). Caspase-12 was located at
the neurons and oligodendrocytes. There were few caspase-12
positive cells in the sham-operation group. However, 24 h after
transplantation, the neurons and oligodendrocytes showed an
increased caspase-12-positive cell number (16.8±3.5/field) when
compared with the sham operation rats (5.3±0.7/field), whereas the
number of caspase-12-positive cells was decreased in the BMSC
treatment rats (11.2±2.6/field, P<0.05) 24 h after
transplantation.
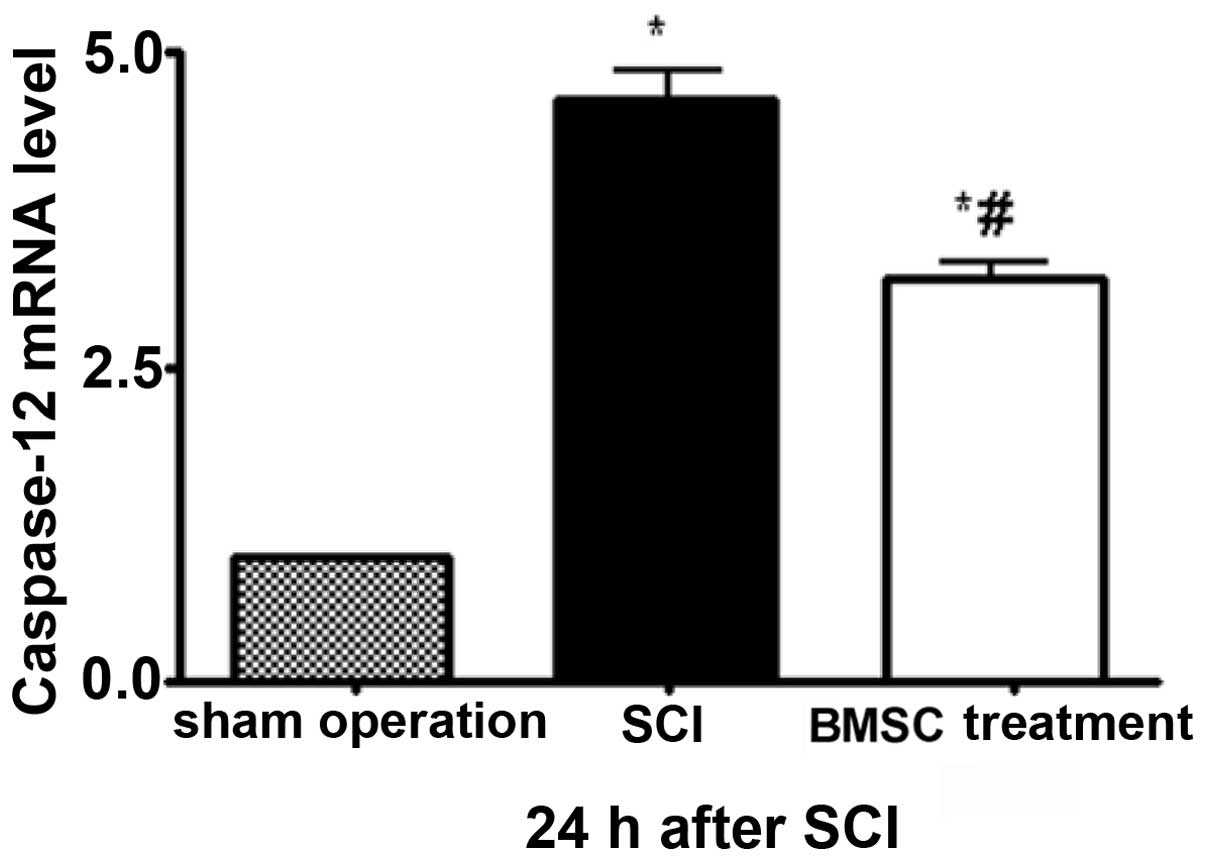
Moreover, compared with the sham-operation rats, the
mRNA expression levels of caspase-12 increased in the SCI group 24
h after SCI, whereas BMSC treatment reduced the caspase-12 mRNA
expression at the same time-point after transplantation (P<0.05;
Fig. 3).
Discussion
An understanding of the pathophysiology underlying
SCI is important for guiding research efforts and developing
clinical treatment strategies (20,21).
Upregulation of caspase-12 is important in ER stress-induced cell
death in brain ischemia (22),
Parkinson’s disease (23),
Alzheimer’s disease (24) and SCI
(11). Previous studies have
revealed that SCI triggers ER stress responses and that
therapeutically reducing ER stress-induced SCI may be an efficient
method of treatment for ischemic SCI (25,26).
Consistent with previous investigations (11,27),
the present study also demonstrated an increased production of the
pro-apoptotic factor caspase-12.
BMSCs, also known as mesenchymal stem cells, are a
mixed cell population that includes stem and progenitor cells.
These cells are easily obtained, available for autologous
transplantation, immune-privileged to allogeneic cells and able to
migrate to areas of inflammation (28,29).
There is increasing evidence to suggest that transplanted stem
cells operate as ‘small molecular factories’; following brain
attack, they secrete neurotrophins, growth factors and other
supportive substances that may have continual therapeutic benefits
in brain ischemia (30). Since
treating SCI involves repairing the initial injury of severed fiber
tracts, as well as fighting widespread secondary damage, stem cell
therapy may aid the regeneration of damaged tissue in the injured
area via provision of new cells. Furthermore, stem cells may
counteract factors in the lesion environment that inhibit axonal
regeneration (31). The results of
the present study demonstrated that BBB scores recovered rapidly
following BMSC transplantation and the highest density of BMSCs was
observed surrounding the SCI. Pathological examination also
revealed that lesioned tissues contained numerous cells, milder
scar formation and reduced cavities. Caspase-12 is known to have a
negative impact on SCI (11,27).
Although the caspase-12 enzyme was not analyzed via western
blotting, this study provided direct evidence that stem cells
suppress caspase-12 expression. These results suggested that BMSCs
may facilitate the recovery of neurons and oligodendrocytes from
SCI by attenuating caspase-12 expression, thus aiding remyelination
and functional recovery processes (32,33).
Further investigation is required to elucidate the direct mechanism
underlying the anti-apoptotic effect of BMSCs on neurons and
oligodendrocytes.
In conclusion, the present study provided further
insight into treatment methods for SCI by demonstrating that BMSC
transplantation attenuates the expression of caspase-12. However,
successful development of a BMSC therapy for SCI requires an
improved understanding and further biochemical assessments of the
long-term positive effects of BMSCs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81000535 and 81272158) and
the Department of Education of Zhejiang Province (Y201225157).
References
|
1
|
Bramlett HM and Dietrich WD: Progressive
damage after brain and spinal cord injury: pathomechanisms and
treatment strategies. Prog Brain Res. 161:125–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu C, Wu W, Zhang B, Xiang J and Zou J:
Temporospatial expression and cellular localization of glutamine
synthetase following traumatic spinal cord injury in adult rats.
Mol Med Rep. 7:1431–1436. 2013.PubMed/NCBI
|
|
3
|
Liu WM, Wu JY, Li FC and Chen QX: Ion
channel blockers and spinal cord injury. J Neurosci Res.
89:791–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong Z, Hong H, Chen H, Wang Z and Hong D:
Investigation of the protective effect of erythropoietin on spinal
cord injury in rats. Exp Ther Med. 2:837–841. 2011.PubMed/NCBI
|
|
5
|
Lin HS, Ji ZS, Zheng LH, et al: Effect of
methylprednisolone on the activities of caspase-3, -6, -8 and -9 in
rabbits with acute spinal cord injury. Exp Ther Med. 4:49–54.
2012.PubMed/NCBI
|
|
6
|
Baptiste DC and Fehlings MG:
Pharmacological approaches to repair the injured spinal cord. J
Neurotrauma. 23:318–334. 2006. View Article : Google Scholar
|
|
7
|
Penas C, Verdú E, Asensio-Pinilla E, et
al: Valproate reduces CHOP levels and preserves oligodendrocytes
and axons after spinal cord injury. Neuroscience. 178:33–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez JA, Zhang Z, Svetlov SI, Hayes
RL, Wang KK and Larner SF: Calpain and caspase processing of
caspase-12 contribute to the ER stress-induced cell death pathway
in differentiated PC12 cells. Apoptosis. 15:1480–1493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang A, Zhang J, Sun P, et al: EIF2alpha
and caspase-12 activation are involved in oxygen-glucose-serum
deprivation/restoration-induced apoptosis of spinal cord
astrocytes. Neurosci Lett. 478:32–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan S, Shi P, Zhang X, Gu C and Fan S:
Stronger expression of CHOP and caspase 12 in diabetic spinal cord
injury rats. Neurol Res. 31:1049–1055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sasaki H, Tanaka N, Nakanishi K, et al:
Therapeutic effects with magnetic targeting of bone marrow stromal
cells in a rat spinal cord injury model. Spine (Phila Pa 1976).
36:933–938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gou S, Wang C, Liu T, et al: Spontaneous
differentiation of murine bone marrow-derived mesenchymal stem
cells into adipocytes without malignant transformation after
long-term culture. Cells Tissues Organs. 191:185–192. 2010.
View Article : Google Scholar
|
|
14
|
Chiba Y, Kuroda S, Maruichi K, et al:
Transplanted bone marrow stromal cells promote axonal regeneration
and improve motor function in a rat spinal cord injury model.
Neurosurgery. 64:991–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koda M, Okada S, Nakayama T, et al:
Hematopoietic stem cell and marrow stromal cell for spinal cord
injury in mice. Neuroreport. 16:1763–1767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu Y, Wang J, Ding F, Hu N, Wang Y and Gu
X: Neurotrophic actions of bone marrow stromal cells on primary
culture of dorsal root ganglion tissues and neurons. J Mol
Neurosci. 40:332–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou XH, Zhi YL, Chen X, et al: Mesenchymal
stem cell seeded knitted silk sling for the treatment of stress
urinary incontinence. Biomaterials. 31:4872–4879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JW, Ha KY, Molon JN and Kim YH: Bone
marrow derived mesenchymal stem cell transplantation for chronic
spinal cord injury in rats: comparative study between intralesional
and intravenous transplantation. Spine (Phila Pa 1976). Apr
26–2013.(Epub ahead of print).
|
|
19
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008.PubMed/NCBI
|
|
20
|
Rahimi-Movaghar V, Yazdi A, Karimi M, et
al: Effect of decompression on complete spinal cord injury in rats.
Int J Neurosci. 118:1359–1373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen L, Zheng X, Zhang C, Zeng B and Hou
C: Influence of different urination methods on the urinary systems
of patients with spinal cord injury. J Int Med Res. 40:1949–1957.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakka VP, Gusain A and Raghubir R:
Endoplasmic reticulum stress plays critical role in brain damage
after cerebral ischemia/reperfusion in rats. Neurotox Res.
17:189–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holtz WA and O’Malley KL: Parkinsonian
mimetics induce aspects of unfolded protein response in death of
dopaminergic neurons. J Biol Chem. 278:19367–19377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quiroz-Baez R, Ferrera P,
Rosendo-Gutiérrez R, Morán J, Bermúdez-Rattoni F and Arias C:
Caspase-12 activation is involved in amyloid-β protein-induced
synaptic toxicity. J Alzheimers Dis. 26:467–476. 2011.
|
|
25
|
Wang Z, Zhang C, Hong Z, Chen H, Chen W
and Chen G: C/EBP homologous protein (CHOP) mediates neuronal
apoptosis in rats with spinal cord injury. Exp Ther Med. 5:107–111.
2013.PubMed/NCBI
|
|
26
|
Mizukami T, Orihashi K, Herlambang B, et
al: Sodium 4-phenylbutyrate protects against spinal cord ischemia
by inhibition of endoplasmic reticulum stress. J Vasc Surg.
52:1580–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HY, Zhang X, Wang ZG, et al:
Exogenous basic fibroblast growth factor inhibits ER stress-induced
apoptosis and improves recovery from spinal cord injury. CNS
Neurosci Ther. 19:20–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei A, Tao H, Chung SA, Brisby H, Ma DD
and Diwan AD: The fate of transplanted xenogeneic bone
marrow-derived stem cells in rat intervertebral discs. J Orthop
Res. 27:374–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bae JS, Han HS, Youn DH, et al: Bone
marrow-derived mesenchymal stem cells promote neuronal networks
with functional synaptic transmission after transplantation into
mice with neurodegeneration. Stem Cells. 25:1307–1316. 2007.
View Article : Google Scholar
|
|
30
|
Gu SH, Xu WD, Xu L, et al: Regenerated
host axons form synapses with neurons derived from neural stem
cells transplanted into peripheral nerves. J Int Med Res.
38:1721–1729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nandoe Tewarie RD, Hurtado A, Levi AD,
Grotenhuis JA and Oudega M: Bone marrow stromal cells for repair of
the spinal cord: towards clinical application. Cell Transplant.
15:563–577. 2006.PubMed/NCBI
|
|
32
|
Mekhail M, Almazan G and Tabrizian M:
Oligodendrocyte-protection and remyelination post-spinal cord
injuries: a review. Prog Neurobiol. 96:322–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman
M and Whittemore SR: Attenuating the endoplasmic reticulum stress
response improves functional recovery after spinal cord injury.
Glia. 59:1489–1502. 2011. View Article : Google Scholar : PubMed/NCBI
|