Introduction
Cardiac dysfunction, an important characteristic of
the acute phase response following severe burns (1), may trigger multiple organ failures
during the shock stage and lead to poor outcomes. The
pathophysiology of cardiac dysfunction following burn injury is not
yet understood. Animals are required in order to study the
mechanisms of action and develop new treatment strategies.
Anesthesia is also required for the development of animal models of
thermal injury and to perform other protocols.
Anesthetics may induce pathophysiological changes in
hemodynamics, inflammation, oxidative stress and adhesion, and may
even affect the progression of experimentally mimicked disease
(2). Therefore, suitable
anesthetics are important in animal experiments and allow adequate
differentiation in application. Various anesthetics have been used
in previous studies, including intraperitoneal injection of
ketamine and xylazine (K/X), avertin (AV; also named
tribromoethanol), chloral hydrate, barbiturates and
thiobutabarbital, and inhaled volatile anesthetics, such as
isoflurane and halothane (3–5).
Among these, the K/X combination is one of the most widely used
anesthetic approaches in animal experiments (6,7). In
this combination, each component is suggested to compensate for the
limitations of the other and to provide the most favorable
anesthetic effect. Several authors, however, have reported that K/X
may produce evident cardiac depression, including bradycardia and
hypotension, in mice (8,9). AV is a frequently used, short-acting,
alcohol-based anesthetic, which was demonstrated to induce only
modest effects on M-mode estimates of basal cardiac function and
have no effect on cardiac output (10). AV was used as the control
anesthetic in the current study because of its relative cardiac
advantages.
Echocardiography, a well-established non-invasive
procedure, has been widely used for the detection of cardiac
structure or cardiovascular effects (11–13).
To the best of our knowledge, there have been no studies concerning
its use in rats with burns to assess cardiac function. Although the
effects of K/X and AV on cardiac function have been compared in
detail by transthoracic echocardiography and closed-chest cardiac
catheterization in normal adult male Swiss Webster mice (14), there have been no studies regarding
the application of echocardiography in the assessment of
cardiovascular changes in severely burned rats.
Troponin I is present in heart muscle tissue and is
a highly specific and sensitive cardiac biomarker. Raised serum
levels indicate cardiac injury and are associated with worse
outcomes in numerous disease states (15). In trauma, raised cardiac troponin I
(cTnI) levels have been identified at relatively late time points
following injury and were observed to be associated with increased
risks of adverse cardiac events and mortality (16). In the current study, the serum cTnI
level was determined as a direct marker of myocardial injury.
Increased apoptosis of cardiomyocytes and the
possible effects of this have been documented previously (17). In addition, the
mitochondrial-mediated pathway of apoptosis may play a significant
role in in vivo cardiac ischemia/reperfusion (18).
As previously stated, anesthetics and thermal injury
may have synergistic impact on cardiac function. The present study
used echocardiography and the determination of cTnI and apoptosis
levels to assess the in vivo cardiac effects of K/X
injections in severely scalded rats and to explore the preliminary
mechanism.
Materials and methods
Animals
Forty male Wistar rats (Animal Research Laboratories
of the First Affiliated Hospital of Chinese PLA General Hospital,
Beijing, China) weighing between 200 and 220 g were kept under
controlled standard housing conditions with free access to standard
laboratory food and water for a 7-day adaptation period before
being randomly assigned to different groups. The groups were as
follows: KXB (scalds anesthetized with K/X, n=10), KXC (sham scalds
anesthetized with K/X, n=10), AVB (scalds anesthetized with AV,
n=10) and AVC (sham scalds anesthetized with AV, n=10).
Ethical approval
All experiments in this study were conducted in
accordance with the National Regulations for the Administration of
Affairs Concerning Experimental Animals (approved by the State
Council on October 31, 1988 and promulgated by Decree No. 2 of the
State Science and Technology Commission on November 14, 1988) and
the Beijing Regulations for the Administration of Affairs
Concerning Experimental Animals (approved by the Science and
Technology Committee of Beijing on October 17, 1996), and were
approved by the Animal Protocol Review Board of Agents and
Anesthesia Protocol of the ETM-2111
112383_Feng_08-03-2013-(E)-Jen/ce.
Anesthetics and anesthesia protocol
The following agents were purchased: xylazine
hydrochloride injection (1.5 ml:0.03 g, Sumianxin II; Shengda
Animal Medicine Co. Ltd., Dunhua, Jilin, China), ketamine
hydrochloride injection (2 ml:1 g; Fujian Gutian Pharmaceutical Co.
Ltd., Ningde, China), AV (25 g, Sigma-Aldrich, St. Louis, MO, USA),
t-amyl alcohol (100 ml, Sigma-Aldrich) and buprenorphine HCl (20
mg; Amresco LLC, Solon, OH, USA). AV was prepared as described
previously (19).
Food was withheld for 12 h prior to the experimental
procedures. On the day of the experiment, each rat was weighed and
clinically examined for behavior, respiration and cardiovascular
parameters. The experiments were conducted between 9:00 a.m. and
12:00 a.m. Two different regimens using intraperitoneal injections
of a K/X mixture (25/6 mg/kg) or AV (200 mg/kg s.c.) were used to
induce surgical-depth anesthesia in the rats. A standardized pain
protocol was used to uniformly assess discomfort following injury
and supplemental analgesics were administered accordingly.
Buprenorphine (0.025–0.05 mg/kg s.c.) was administered when the
animals showed signs of discomfort.
The rats then immediately underwent scalds or sham
scalds, followed by echocardiography 10, 20 and 30 min after the
scalds. The animals were sacrificed by decapitation using a
standard small-animal guillotine device 24 h after scalding. Blood,
lung and heart tissues were harvested.
Scald procedure and resuscitation
Each rat was shaved in preparation for the
experiments 1 day in advance. The animals were secured in a
constructed wooden template device. The dorsal and lateral skin
surfaces were exposed through an oval aperture in the template, and
the animals were immersed in 94°C water for 12 sec on the back and
upper sides as described previously (20).
The use of the template limited the burned area,
avoided injury to the abdominal organs and produced full-thickness
dermal scalds affecting 30% of the total body surface area (TBSA).
The rats with sham scalds were handled identically to the scalded
rats with the exception that they were immersed in room temperature
water and thus served as controls. Following immersion in water,
all rats were immediately dried, administered fluid (Ringer’s
lactate solution, 4 ml/kg by the Parkland formula) (21) during the post-burn period and
placed in individual cages awaiting echocardiography. The animals
were maintained under anesthesia for the duration of the
echocardiographic examination.
Echocardiography
Hair was removed from the chests of the rats using a
razor. The rats were then fixed in a left lateral position and
placed on a heating pad to maintain the body temperature at
37–38°C. Acoustic coupling gel warmed to room temperature was
applied to the chest prior to examination. A commercially available
ultrasound system was used for the echocardiographic examinations
(Sonos 7500; Philips, Andover, MA, USA). The depth was set to 2 cm
and zoomed to 1.2 cm. Wall thickness and left ventricular (LV)
dimensions were obtained from a short-axis view at the level of the
papillary muscles at a frame rate of 260 Hz. The ultrasound system
was used to measure the cardiac cycle (time), LV end-diastolic
chamber diameter (LVEDd), LV end-systolic chamber diameter (LVESd),
fractional shortening (FS%) and ejection fraction (EF). The
Teichholz formula (22) was used
to calculate the LV chamber volume [LV end-diastolic volume
(LVEDV)]: LVEDV=[7.0/(2.4 + LVEDd)] × LVEDd3, where the
heart rate (HR)=1/time × 60.
The gains were adjusted to eliminate background
noise and enable clear tissue signals to be obtained; 5–10 cycles
were recorded. The measurements and analyses were repeated by
different individuals.
Lung wet/dry weight and heart
histopathological examination
The lung tissue samples from the study groups and
from non-survivors were analyzed. The lung tissue was analyzed to
determine the wet/dry weight ratio (W/D). Following the termination
of the experiments, the lungs were removed from the perfusion
chamber and weighed. The lungs were then heated in an oven at 80°C
for 48 h and reweighed.
For histopathological analysis, a small sample of
the heart was resected, fixed with 10% formaldehyde solution for 48
h, embedded in paraffin, cut into 4-μm pieces using a microtome,
and stained with hematoxylin and eosin.
cTnI analysis
The peritoneal cavity was opened to expose the
coeliac artery, and blood samples were collected and centrifuged
(2,000 × g, 4°C, 10 min) to separate the serum. The serum was
collected and stored at −80°C until analysis. Commercially
available ELISA kits (E02T0491; Shanghai BlueGene Biotech Co.,
Ltd., Shanghai, China) were used to determine serum cTnI levels
according to the manufacturer’s instructions. A multidetection
microplate reader (SynergyTM 2; Biotek, Winooski, VT,
USA) was used to detect cTnI activity at 460 nm after reacting the
serum sample on a cTnI antibody-coated plate.
Apoptosis (TUNEL) assay
The rat heart was excised and dissected
longitudinally to expose the endocardium. Tissues were quickly
fixed in 4% paraformaldehyde. The 5-μm thick paraffin-embeded
sections were prepared for the TUNEL (Keygen Biotech, Nanjing,
China) assay according to the manufacturer’s instructions. The
sections were examined with a microscope at ×400 magnification. A
total of 10 fields per section were examined by an investigator
blinded to the experimental procedure. The brown-stained nuclei
were regarded as cells undergoing apoptosis (positive cells). The
apoptosis index (AI) was calculated using the following formula: AI
(%)=Npositive cell/Ntotal cell×100%.
Western blot analysis
The muscle tissues were frozen and stored in liquid
N2 and total protein extracts were prepared using RIPA
lysis buffer plus protease inhibitors, as described previously
(20). Each protein lysate (40 mg)
was separated by 15% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a PVDF membrane using
a semi-dry system. Nonspecific sites were blocked with 5% nonfat
dry milk in TBS containing 0.1% Tween-20 (TBS-T). The blots were
incubated overnight with the appropriate dilution of the primary
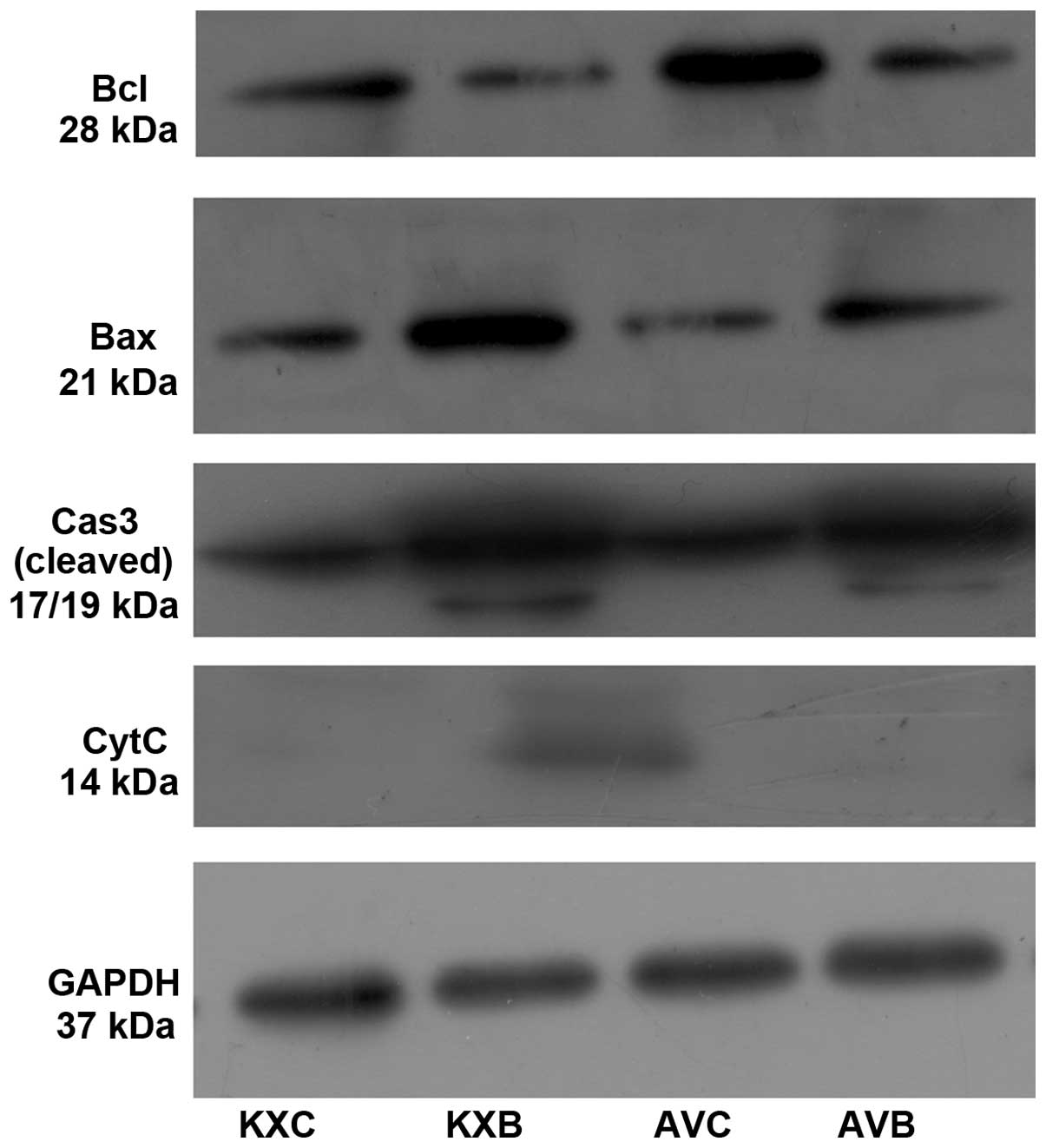
antibodies. Anti-bcl, bax, caspase 3 and cytochrome c (Cell
Signaling Technology, Inc., Danvers, MA, USA) antibodies were used
as primary antibodies at a dilution of 1:1,000. The membranes were
repeatedly washed with TBS-T prior to incubation with
HRP-conjugated anti-rabbit or anti-mouse IgG (Bioss, Beijing,
China) antibody at a dilution of (1:2,000). The ECL blot detection
kit (Thermo Scientific, Middletown, VA, USA) was used according to
the manufacturer’s instructions to visualize reactive products.
Statistical analysis
Statistical comparisons were performed only for
exploratory data analysis. All values are presented as the mean ±
SEM. The survival rates were evaluated using the Kaplan-Meier
method. Analysis of variance was performed to assess an overall
difference among the groups. The least significant difference
method was used for pairwise multiple comparisons. SPSS for Windows
(version 16.0; SPSS, Inc., Chicago, IL, USA) was used to perform
all analyses. P<0.05 was considered to indicate a statistically
significant result.
Results
Mortality
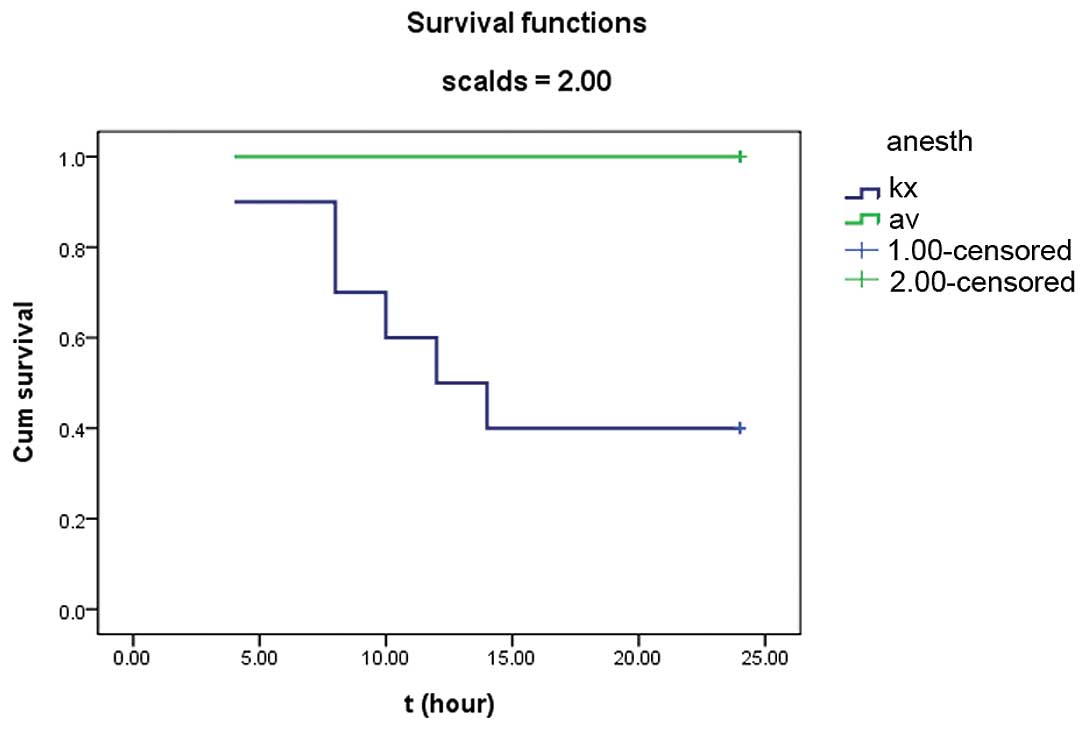
Survival rates were observed at 24 h after scalding.
All burned and control rats anesthetized with AV had uniform
recoveries, but those anesthetized with K/X were dispirited within
6–8 h after injury. Six rats (6/10, 60%) succumbed within 6–24 h
after injury in the KXB group and one rat succumbed 4 h after sham
injury in the KXC group. No rats anesthetized with AV died. As a
result, the survival of the KXB group was significantly different
(P=0.004) from that of the AVB group, as shown by the survival
curves (Fig. 1).
Comparison between the two anesthetic
regimes in normal rats
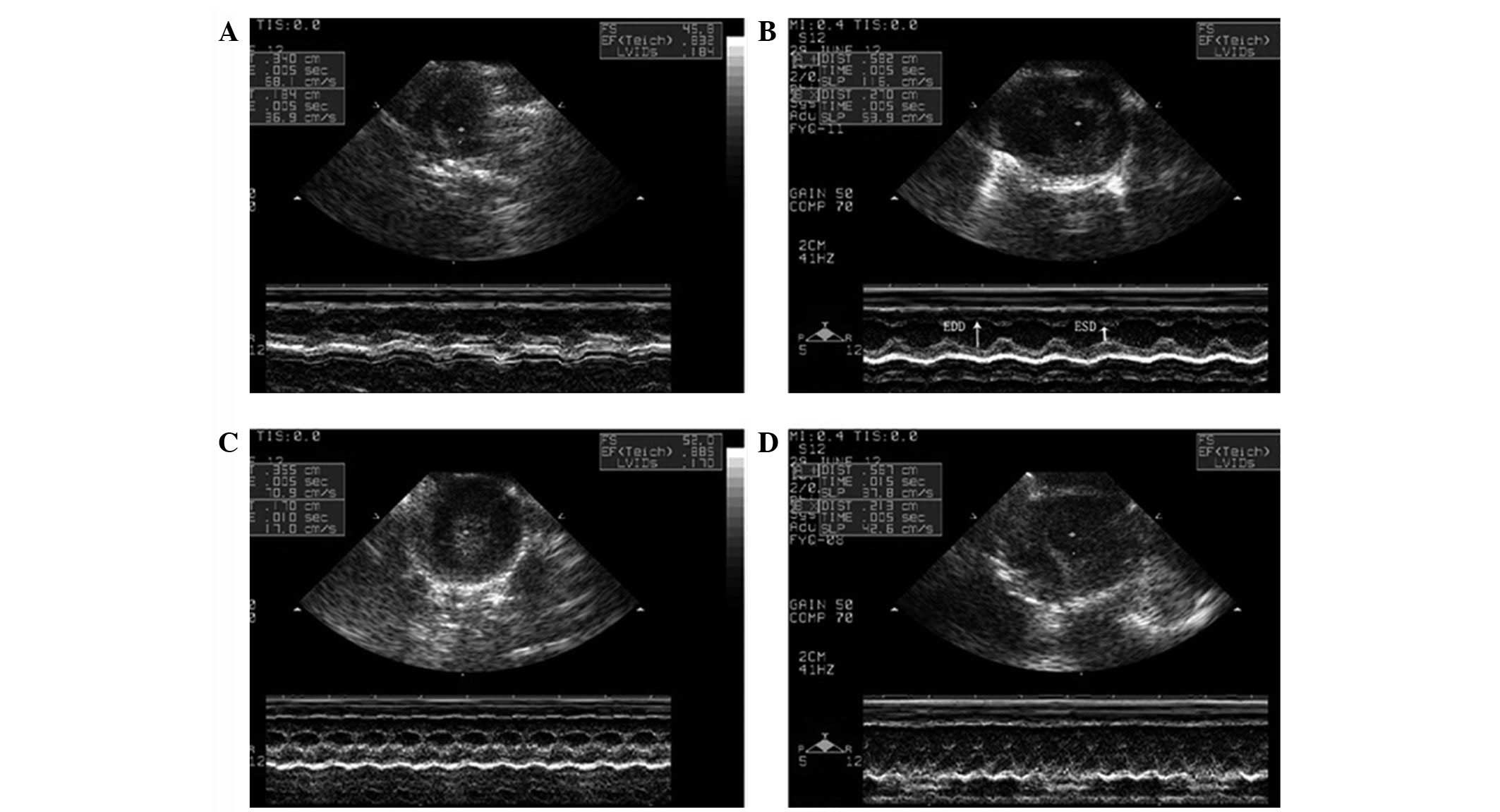
Ultrasonic cardiograms of the rats are shown in
Fig. 2. The effects and time
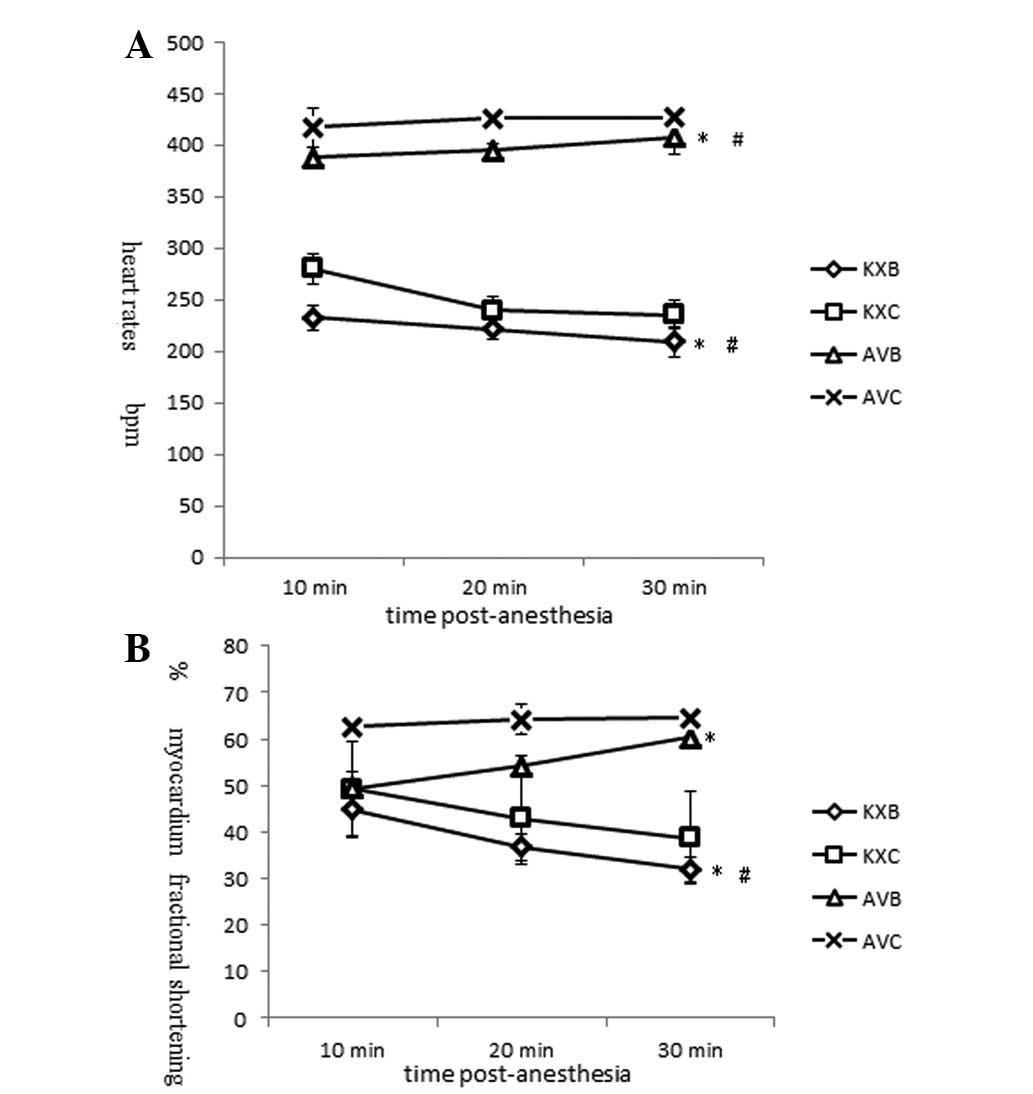
trends of K/X and AV on heart rates are shown in Fig. 3A. Rats anesthetized with K/X showed
a significant reduction in HR (233±18.4 to 210±8.7 beats/min,
P<0.05) over the 30-min period. The HR at each time point was
significantly lower than that in AV group (P<0.01). The HR in
the AV group was observed to increase slightly from 418±12 to
427±15 beats/min. The cardiac contractility results showed that FS%
was inhibited and dropped acutely with K/X anesthesia (49.3±3.6 to
38.8±2.4%, P<0.01), but it remained stable with AV anesthesia
(62.6±0.9 to 64.5±1.8%, P<0.05; Fig. 3B). Similar trends were observed for
the EF, LVEDV and left ventricular end-systolic volume (LVESV;
Fig. 4).
Comparison between the two anesthetic
regimes in severely burned rats
The HRs in the burned groups were significantly
lower than those in the sham groups treated with K/X or AV
(P<0.01). The HRs in the KXB group were fatally low, appeared to
decrease during the study period and were significantly lower than
those in the sham injury group. The FS% of the scalded rats in the
KXB and AVB groups decreased to a significantly lower level than
that in the AVC group rapidly within ten minutes after scalding
(P<0.01). During the anesthesia period, FS% showed opposite time
trends in the burned groups treated with K/X and AV. The FS% in the
KXB group decreased, whereas that in the AV group was restored
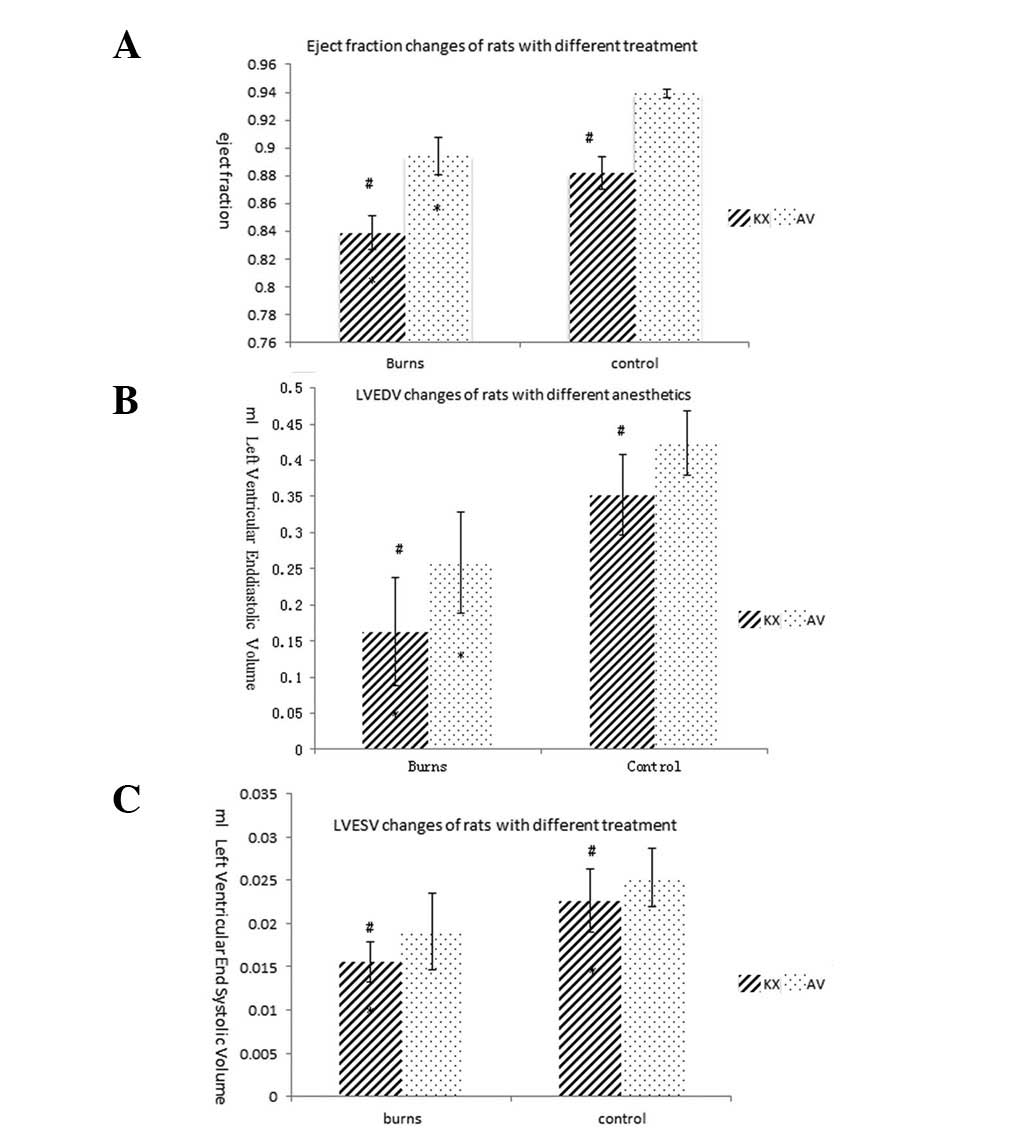
rapidly (Fig. 4). The EF in the
burned group anesthetized with K/X was extremely low initially and
remained low; the level was lower than that in the AVC and AVB
groups (0.8387±0.03032 vs. 0.8942±0.03279 and 0.9392±0.00665,
respectively). The scalded groups exhibited a smaller LVEDV and
LVESV, as estimated by the Teichholz formula, than the sham groups
(P<0.01). The LVESV (0.0156±0.0023 cm3) in the KXB
group reached lower levels compared with those in the other three
groups (KXC, 0.0226±0.0036; AVB, 0.0191±0.0044; and AVC,
0.0253±0.0033 cm3, P<0.05). The LVEDV in the KXB
group (0.163±0.0743 cm3) was significantly reduced
compared with those in the KXC, AVB and AVC groups (0.3517±0.05562,
0.2586±0.07048 and 0.4217±0.04411 cm3,
respectively).
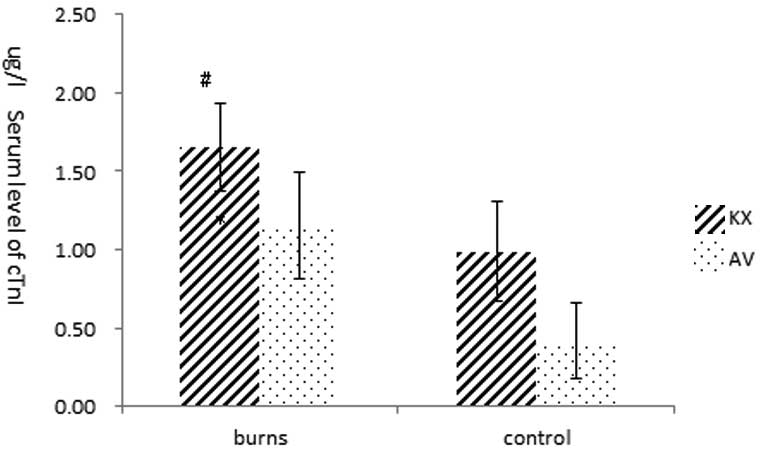
Level of cTnI
Significant differences in serum cTnI levels were
observed among the groups (P<0.01). The cTnI level in the KXB
group (1.66±0.28 ng/ml) were significantly increased and was higher
than the level in the other groups (KBC, 0.99±0.32; AVB, 1.16±0.34;
AVC, 0.42±0.24 ng/ml, P<0.01). The cTnI level in the AVB group
was slightly higher than that in the AVC group (P>0.05). No
significant difference in cTnI levels was observed between the KXC
and AVB groups (P>0.05; Fig.
5).
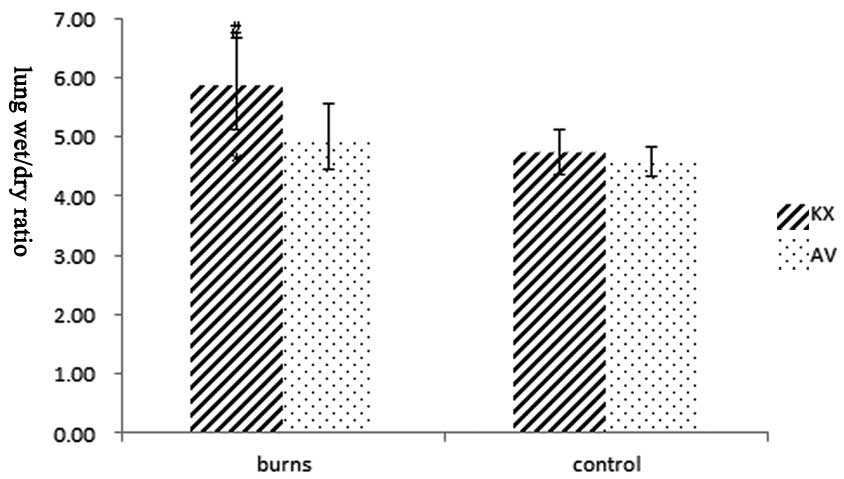
Lung wet/dry weight and heart
histopathological examination
To assay the index of pulmonary congestion of each
group, the W/D was calculated. The lung W/D (Fig. 6) increased in the burned groups.
The W/D in the KXB group (5.88±0.77) increased significantly and
was higher than those in the KXC, AVB and AVC groups (4.74±0.37,
5.00±0.55 and 4.59±0.26, respectively, P<0.01). No significant
difference in W/D was observed between the KXC and AVC groups
(P>0.05).
The histopathology of heart tissue from each group
is shown in Fig. 7. No
degeneration of the myocardium was observed in any group. A certain
amount of inflammatory cell infiltration was observed; however,
this is common in rodents.
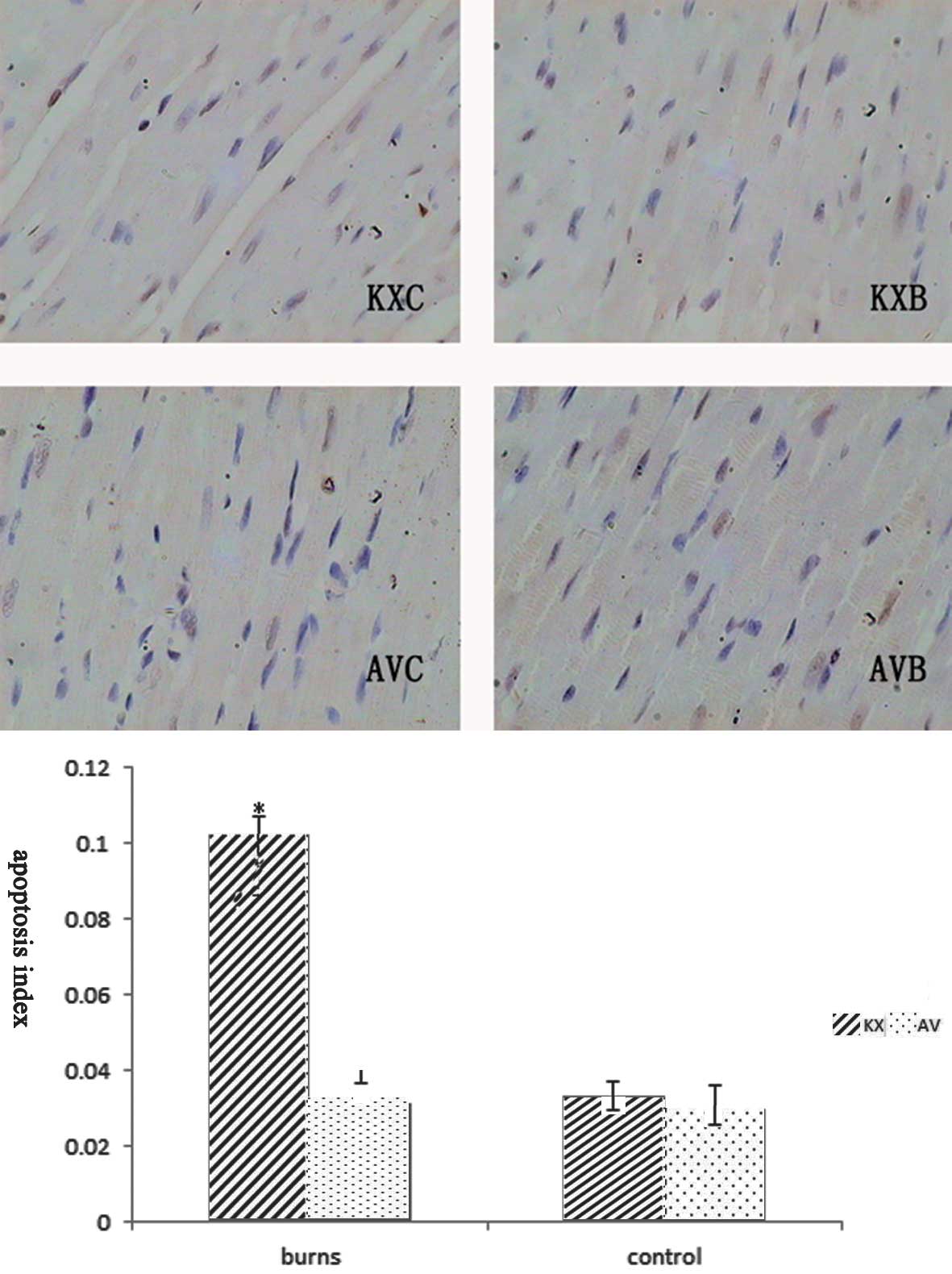
Apoptotic cell death by triggering the
mitochondrial pathway
To analyze the development of apoptosis following
scald injury, heart tissue was analyzed by the TUNEL technique
(Fig. 8). As the figure shows,
apoptotic nuclei were more evident with irregular contours in the
cardiac myocytes of scalded rats, although there was no significant
difference between the AVB and AVC groups (P>0.05). The
expression of mitochondrial apoptosis-related proteins is shown in
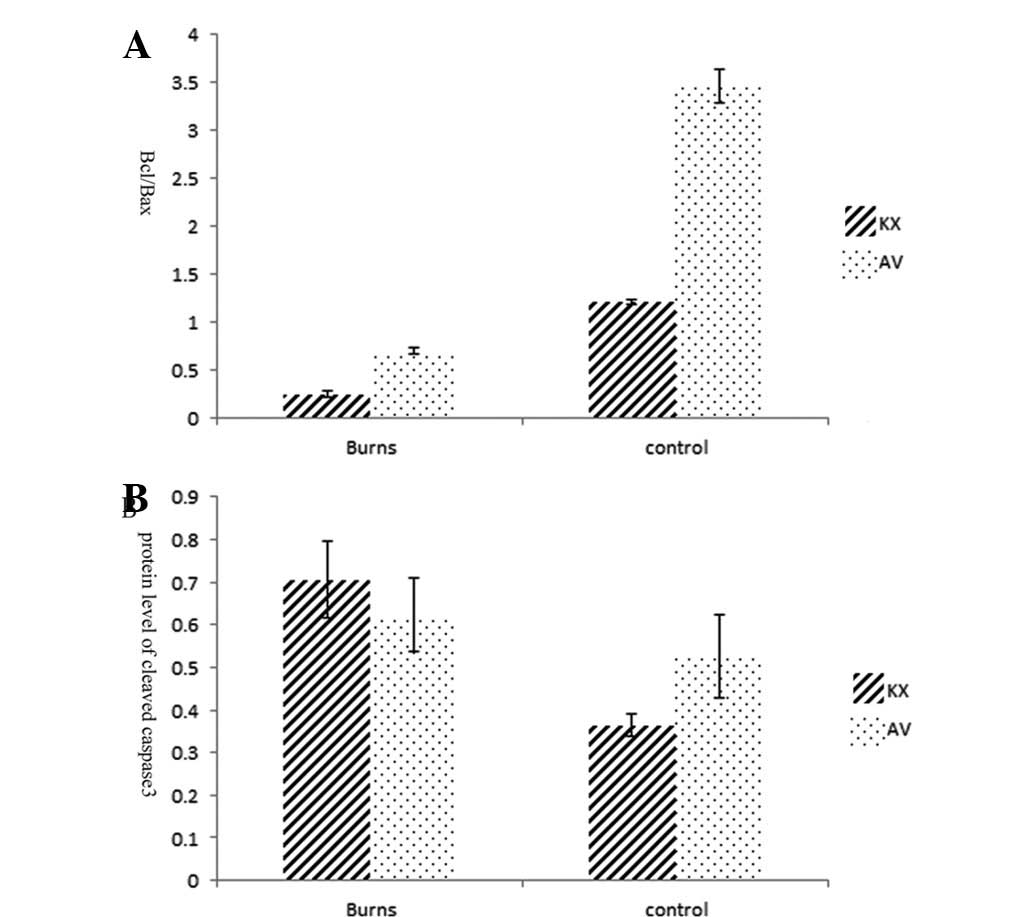
Fig. 9. There was a 3-fold
increase in the apoptotic index of myocardial cells in the KXB
group compared that in the AVB group (P<0.01). The activation of
the mitochondrial apoptosis pathway is indicated by the expression
of cytochrome c and the ratio of bcl/bax, the latter was much lower
in group KXB than in the other groups (P<0.01; Fig. 10A). The level of executioner
caspase 3 (cleaved caspase 3), increased in the scalded rats. There
was a more than 2-fold increase in the caspase 3 concentration in
the KXB group compared with that in the KXC group (P<0.01), and
a more than eightfold increase in the AVC group than that in the
AVB group (P<0.01; Fig.
10B).
Discussion
Cardiac dysfunction is one of the most common
complications of severe burns. Anesthetics, however, have an impact
on cardiac function. In the present study, the combined impact of
burn injury and anesthetics was explored. Echocardiographic
measurements and blood biochemical examinations were used to show
that anesthesia may have significant effects on cardiac function,
which are more harmful in individuals with severe burns. The
mitochondrial apoptosis pathway may be involved in this process.
These findings have important implications for the selection of an
appropriate anesthetic during study of severe burns in rats.
Ketamine, which is a phencyclidine derivative, is
commercially available in a racemic form or as an S(+) purified
isomer. The racemic form consists of a mixture of S(+) and R(−)
isomers. Ketamine activates the sympathetic system, resulting in an
increase in HR, cardiac output and oxygen consumption. Initially,
ketamine is distributed to highly perfused tissues. Due to these
significant side-effects, ketamine may be combined with
tranquilizers or sedatives, such as xylazine, to provide a
relatively safe anesthesia that may be administered without
specialized equipment. Xylazine counterbalances the undesirable
effects of ketamine, however, its use may cause cardiovascular
abnormalities arising from a reduction in sympathetic tonus
(23).
In the current study, we observed that the K/X
combination markedly suppresses cardiac function, characterized by
reductions in HR, FS% and LVEDV, even in normal rats. These results
are consistent with those of a previous study which identified that
K/X anesthesia resulted in the most prominent negative inotropic
and chronotropic responses compared with pentobarbital and
isoflurane anesthesia (24). Our
data showed that K/X had a greater HR inhibitory effect than that
reported in the previous study (240±7 vs. 326±4 beats/min). AV
showed slight effects on the HR, which was similar to that observed
for the unanesthetized rats (427±10 vs. 421±26 beats/min). In
addition, other parameters, including FS%, EF and LVEDV, also
changed only slightly, which indicated that AV produced only a
slight cardiac inhibitory effect. This result was validated by the
W/D and the cTnI level.
Reduced cardiac function as a major component of
multi-organ failure following burn injury was recognized as early
as 1931 (25). In the present
study, the contractility and diastolic functions, including FS%,
EF, LVESV and LVEDV, also indicated there was a depressed cardiac
state in scalded rats. No definite signal pathway has been
confirmed to be responsible for cardiac dysfunction following
injury. Two major theories have been proposed for the pathogenesis
of myocardial dysfunction following burns. One involves decreased
myocardial perfusion due to hypovolemia following burns, which
leads to ischemic injury and results in direct cardiomyocyte
damage. The second theory involves the inflammatory or toxic
response, which mainly induces reversible intrinsic myocardial
depression, by cytokines or lipopolysaccharides (26,27).
In the adult mammalian heart, cardiac myocyte apoptosis has been
identified as a mechanism of cell death in acute myocardial
infarction and ischemia-reperfusion injury. Lightfoot et
al(17) reported that there
was a 10-fold increase in the number of apoptotic cells in the
subendomyocardium of the left ventricular tissue harvested 24 h
post burn compared with the number of apoptotic cells in sham burn
controls. We observed that the number of apoptotic nuclei increased
and was accompanied by activation of the mitochondrial pathway.
However, it is not known whether the occurrence of apoptosis
actually contributes to the development of the heart
dysfunction.
In preliminary experiments, we observed that rats
anesthetized with K/X (25/6 mg/kg) had high mortality following
severe burns, such as those covering 30% TBSA. Pleural effusion was
observed in the rats that died within 24 h. Therefore, we designed
the current study to explore the cardiac impacts of anesthesia and
burns. We also used echocardiography and the cTnI assay to identify
abnormal cardiac parameters that have been used for the diagnosis
of myocardial damage in other models (28). Echocardiography was used to show
that the burned rats had lower HRs, FS%, EF and LVEDV than the
unburned rats. Several authors have described the association
between cardiac dysfunction and severe burns during the shock stage
in various models (29,30).
We observed that cardiac function in the burned rats
anesthetized with K/X was inhibited more severely compared with
that in the burned rats anesthetized with AV. The same procedure of
scalding and resuscitation was applied in the KXB and AVB groups.
Therefore, the difference between these two groups likely resulted
from the anesthetics, ketamine and xylazine, which produced
negative impacts on these rats. Under normal conditions, ketamine
stimulates the sympathetic system by increasing circulating
catecholamine concentrations (31). Ketamine, however, exhibits
potentially negative cardiovascular effects in patients with
catecholamine-dependent heart failure (32) or other critical illnesses in which
catecholamine is excessively mobilized. Therefore, it may be that
the stress of severe burns weakened the sympathetic stimulation and
therefore, the cardiac inhibition effect of ketamine became
dominant in severe thermal injury.
It has been clinically confirmed that there is a
close correlation between echocardiography and leakage of troponin
(33). Cardiac troponin T and cTnI
are now recognized as the most tissue-specific and sensitive
biomarkers associated with cardiac damage and have been included as
a diagnostic criterion for several cardiac-related pathologies
(34,35). A high level of troponin may result
from increased permeability of the myocytes or degradation of
native troponin into smaller fragments due to ischemia (36). Franco et al reported that
changes in creatine kinase isoenzyme fraction MB (CK-MB) serum
activity observed in dogs treated with atropine, xylazine and
ketamine S(+) were higher than the baseline values 6 h after the
experiment (37). These data
indicate that there is likely to be a definite change in cTnI
levels. The cTnI level 24 h after burns has been reported to be
significantly higher in patients with burns covering >30% TBSA
and cTnI has been regarded as a marker for post-burn cardiac injury
(38). Therefore, we measured the
changes in cTnI levels to assess the impact of anesthetics on burn
injury. It was observed that the cTnI levels increased in the
burned groups and were particularly high in the rats anesthetized
with K/X, a result that indicated greater heart injury. A possible
explanation for these findings is that K/X depressed cardiac
function and reduced cardiac output, which exacerbated the
hypoperfusion of coronary circulation caused by severe burns. Thus,
ischemia and oxygen deficiency may cause myocardial damage.
Pulmonary edema is one of the most significant
syndromes of acute heart failure. The simplest way to evaluate
edema formation in the lung is to use gravimetric approaches, such
as W/D. A much higher W/D was observed in the KXB group.
As previously mentioned, burns triggered significant
apoptosis in the myocardium, which indicated that the apoptosis
signaling pathway and the caspase family proteases may be
significantly involved in the development of myocardial dysfunction
following thermal injury. In the present study, results indicative
of apoptosis were observed and suggest that mitochondrial apoptosis
may be involved as the parameters of apoptosis, such as the bcl/bax
ratio and cytochrome c level, changed significantly; these
effects were worsened by anesthetics such as the K/X
combination.
At first, we hypothesized that differences in the
experimental systems may have caused this outcome; for example, the
proportion of xylazine in the K/X combination in the present study
(25/6 mg/kg) was higher than those in other studies [37/7 mg/kg
(23), 80/10 mg/kg (39) and 100/4 mg/kg (40)], which may be an additional cardiac
inhibitory factor. The K/X combination, however, has been used
widely and safely, although K/X has been reported to be associated
with more highly elevated levels of cytokines, such as IL-6, than
are associated with isoflurane in rats with burn injury (39). In a recent study, a high dose of
K/X (200/60 mg/kg) was even observed to significantly reduce
myocardial infarct size compared with the low dose, and may
introduce unwanted variability in ischemia-reperfusion studies
(41). Therefore, the evident
inhibitory result of K/X in this study is considered to be mainly
due to the synergistic effect of anesthetic and burn injury.
Although certain serious side-effects have been
reported, the safety and efficacy of AV in mice are
well-documented. AV caused only a modest reduction in LV FS%, and
was similar to that of the conscious rats.
In summary, we recommend that particular attention
be given to the choice of anesthetic drugs used in experiments
studying burn injury. Although it remains difficult to recommend an
optimal choice of drugs and anesthesia technique for different
models and animal species, our results suggest that the K/X mixture
causes evident cardiac inhibition in severely scalded rats. AV may
be the optimal choice for animal models with severe burns.
Acknowledgements
The authors thank Dr. Luo Fuliang for his
echocardiographic support from the State Key Laboratory of
Cardiovascular Disease, Fuwai Hospital, National Center for
Cardiovascular Diseases, Chinese Academy of Medical Sciences. This
study was supported by the National Natural Science Foundation of
China (81120108014, 81201466).
References
|
1
|
Williams FN, Herndon DN, Suman OE, Lee JO,
Norbury WB, Branski LK, Mlcak RP and Jeschke MG: Changes in cardiac
physiology after severe burn injury. J Burn Care Res. 32:269–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frithiof R, Mats R, Johan U, Stefan E and
Hans H: Comparison between the effects on hemodynamic responses of
central and peripheral infusions of hypertonic NaCl during
hemorrhage in conscious and isoflurane-anesthetized sheep. Shock.
26:77–86. 2006. View Article : Google Scholar
|
|
3
|
Yang XP, Liu YH, Rhaleb NE, Kurihara N,
Kim HE and Carretero OA: Echocardiographic assessment of cardiac
function in conscious and anesthetized mice. Am J Physiol.
277:H1967–H1974. 1999.PubMed/NCBI
|
|
4
|
Takeishi Y, Ping P, Bolli R, Kirkpatrick
DL, Hoit BD and Walsh RA: Transgenic overexpression of
constitutively active protein kinase C epsilon causes concentric
cardiac hypertrophy. Circ Res. 86:1218–1223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka N, Dalton N, Mao L, Rockman HA,
Peterson KL, Gottshall KR, Hunter JJ, Chien KR and Ross J Jr:
Transthoracic echocardiography in models of cardiac disease in the
mouse. Circulation. 94:1109–1117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner AE, Mama KR, Steffey EP and Hellyer
PW: Evaluation of infusions of xylazine with ketamine or propofol
to modulate recovery following sevoflurane anesthesia in horses. Am
J Vet Res. 73:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Struck MB, Andrutis KA, Ramirez HE and
Battles AH: Effect of a short-term fast on ketamine-xylazine
anesthesia in rats. J Am Assoc Lab Anim Sci. 50:344–348.
2011.PubMed/NCBI
|
|
8
|
Kober F, Iltis I, Cozzone PJ and Bernard
M: Cine-MRI assessment of cardiac function in mice anesthetized
with ketamine/xylazine and isoflurane. MAGMA. 17:157–161. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roth DM, Swaney JS, Dalton ND, Gilpin EA
and Ross J Jr: Impact of anesthesia on cardiac function during
echocardiography in mice. Am J Physiol Heart Circ Physiol.
282:H2134–H2140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kiatchoosakun S, Kirkpatrick D and Hoit
BD: Effects of tribromoethanol anesthesia on echocardiographic
assessment of left ventricular function in mice. Comp Med.
51:26–29. 2001.PubMed/NCBI
|
|
11
|
Hayward R and Lien CY: Echocardiographic
evaluation of cardiac structure and function during exercise
training in the developing Sprague-Dawley rat. J Am Assoc Lab Anim
Sci. 50:454–461. 2011.PubMed/NCBI
|
|
12
|
Martinez PF, Okoshi K, Zornoff LA,
Oliveira SA Jr, Campos DH, Lima AR, Damatto RL, Cezar MD, Bonomo C,
Guizoni DM, Padovani CR, Cicogna AC and Okoshi MP:
Echocardiographic detection of congestive heart failure in
postinfarction rats. J Appl Physiol. 111:543–551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoit BD: New approaches to phenotypic
analysis in adult mice. J Mol Cell Cardiol. 33:27–35. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hart CY, Burnett JC Jr and Redfield MM:
Effects of avertin versus xylazine-ketamine anesthesia on cardiac
function in normal mice. Am J Physiol Heart Circ Physiol.
281:H1938–H1945. 2001.PubMed/NCBI
|
|
15
|
Quenot JP, Le Teuff G, Quantin C, Doise
JM, Abrahamowicz M, Masson D and Blettery B: Myocardial injury in
critically ill patients: relation to increased cardiac troponin I
and hospital mortality. Chest. 128:2758–2764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lagi A, Meucci E and Cencetti S: Outcome
of patients with elevated cardiac troponin I level after mild
trauma. Am J Emerg Med. 26:248.e3–e5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lightfoot E Jr, Horton JW, Maass DL, White
DJ, McFarland RD and Lipsky PE: Major burn trauma in rats promotes
cardiac and gastrointestinal apoptosis. Shock. 11:29–34. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lundberg KC and Szweda LI: Initiation of
mitochondrial-mediated apoptosis during cardiac reperfusion. Arch
Biochem Biophys. 432:50–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meyer RE and Fish RE: A review of
tribromoethanol anesthesia for production of genetically engineered
mice and rats. Lab Anim (NY). 34:47–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu WL, Chai JK, Feng YQ, Ma L, Zhu GY,
Zhang HJ and Hu C: Expressions of endoplasmic reticulum stress
associated proteins in livers of severely burned rats. Zhonghua Yi
Xue Za Zhi. 92:853–856. 2012.(In Chinese).
|
|
21
|
Horton JW: Oxygen free radicals contribute
to postburn cardiac cell membrane dysfunction. J Surg Res.
61:97–102. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teichholz LE, Kreulen T, Herman MV and
Gorlin R: Problems in echocardiographic volume determinations:
echocardiographic-angiographic correlations in the presence of
absence of asynergy. Am J Cardiol. 37:7–11. 1976.
|
|
23
|
Boutureira J, Trim CM and Cornell KK:
Acute pulmonary edema after diazepam-ketamine in a dog. Vet Anaesth
Analg. 34:371–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein AB, Tiwari S, Thomas P, Hunt G,
Levent C, Stoddard MF, Tang XL, Bolli R and Dawn B: Effects of
anesthesia on echocardiographic assessment of left ventricular
structure and function in rats. Basic Res Cardiol. 102:28–41. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blalock A: Experimental Shock. VII The
importance of the local loss of fluid in the production of the low
blood pressure after burns. Arch Surg. 22:610–617. 1931. View Article : Google Scholar
|
|
26
|
Niederbichler AD, Hoesel LM, Ipaktchi K,
Olivarez L, Erdmann M, Vogt PM, Su GL, Arbabi S, Westfall MV, Wang
SC and Hemmila MR: Burn-induced heart failure: lipopolysaccharide
binding protein improves burn and endotoxin-induced cardiac
contractility deficits. J Surg Res. 165:128–135. 2011. View Article : Google Scholar
|
|
27
|
Huang YS, Yang ZC, Yan BG, Yang JM, Chen
FM, Crowther RS and Li A: Pathogenesis of early cardiac myocyte
damage after severe burns. J Trauma. 46:428–432. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alpert JS, Thygesen K, Antman E and
Bassand JP: Myocardial infarction redefined - a consensus document
of The Joint European Society of Cardiology/American College of
Cardiology Committee for the redefinition of myocardial infarction.
J Am Coll Cardiol. 36:959–969. 2000. View Article : Google Scholar
|
|
29
|
Horton J, Maass D, White J and Sanders B:
Effect of aspiration pneumonia-induced sepsis on post-burn cardiac
inflammation and function in mice. Surg Infect (Larchmt).
7:123–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reynolds EM, Ryan DP, Sheridan RL and
Doody DP: Left ventricular failure complicating severe pediatric
burn injuries. J Pediatr Surg. 30:264–269. 269–270. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bergman SA: Ketamine: review of its
pharmacology and its use in pediatric anesthesia. Anesth Prog.
46:10–20. 1999.PubMed/NCBI
|
|
32
|
Christ G, Mundigler G, Merhaut C,
Zehetgruber M, Kratochwill C, Heinz G and Siostrzonek P: Adverse
cardiovascular effects of ketamine infusion in patients with
catecholamine-dependent heart failure. Anaesth Intensive Care.
25:255–259. 1997.PubMed/NCBI
|
|
33
|
Bak Z, Sjöberg F, Eriksson O, Steinvall I
and Janerot-Sjoberg B: Cardiac dysfunction after burns. Burns.
34:603–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horwich TB, Patel J, MacLellan WR and
Fonarow GC: Cardiac troponin I is associated with impaired
hemodynamics, progressive left ventricular dysfunction, and
increased mortality rates in advanced heart failure. Circulation.
108:833–838. 2003. View Article : Google Scholar
|
|
35
|
Salomaa V, Ketonen M, Koukkunen H,
Immonen-Räihä P, Lehtonen A, Torppa J, Kuulasmaa K, Kesäniemi YA
and Pyörälä K; FINAMI Study Group. The effect of correcting for
troponins on trends in coronary heart disease events in Finland
during 1993–2002: the FINAMI study. Eur Heart J. 27:2394–2399.
2006.PubMed/NCBI
|
|
36
|
Wu AH: Increased troponin in patients with
sepsis and septic shock: myocardial necrosis or reversible
myocardial depression? Intensive Care Med. 27:959–961. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Franco LG, Fioravanti MC, Damasceno AD,
Borges AC, Soares LK, Rabelo RE and Silva LA: Assessment of serum
enzymatic markers of cardiomyocytes injury in female dogs submitted
to ketamine S(+), atropin and xylazine association. Acta Cir Bras.
24:36–42. 2009.PubMed/NCBI
|
|
38
|
Chen YN, Luo ZR, Zeng LJ, Wu MY, Wu YZ and
Lin ZY: Cardiac troponin I: a marker for post-burn cardiac injury.
Ann Clin Biochem. 37(Pt 4): 447–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Al-Mousawi AM, Kulp GA, Branski LK, Kraft
R, Mecott GA, Williams FN, Herndon DN and Jeschke MG: Impact of
anesthesia, analgesia, and euthanasia technique on the inflammatory
cytokine profile in a rodent model of severe burn injury. Shock.
34:261–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahrami S, Benisch C, Zifko C, Jafarmadar
M, Schöchl H and Redl H: Xylazine-/diazepam-ketamine and isoflurane
differentially affect hemodynamics and organ injury under
hemorrhagic/traumatic shock and resuscitation in rats. Shock.
35:573–578. 2011. View Article : Google Scholar
|
|
41
|
Sloan RC, Rosenbaum M, O’Rourke D, Oppelt
K, Frasier CR, Waston CA, Allan AG and Brown DA: High doses of
ketamine-xylazine anesthesia reduce cardiac ischemia-reperfusion
injury in guinea pigs. J Am Assoc Lab Anim Sci. 50:349–354.
2011.PubMed/NCBI
|