Introduction
The brain is a highly organized organ that is
responsible for learning, memory, emotion and social behavior. The
frequency of cerebellar damage as a complication of premature birth
is increasing (1). Cerebellar
hypoplasia is a developmental disorder characterized by the
incomplete development or underdevelopment of the cerebellum; this
disorder may be focal or diffuse/generalized (2). In infancy, the symptoms of cerebellar
hypoplasia include developmental delay, hypotonia, ataxia, seizures
and involuntary eye movements (nystagmus). At later ages, symptoms
include headache, vertigo, imbalance and hearing impairment. There
is no standard course of treatment for cerebellar hypoplasia, and
only symptomatic and supportive therapies are provided. Gestational
exposure to drugs (such as nicotine, cocaine, ethanol,
glucocorticoids, phenytoin and anticancer drugs) and radiation
(including X-rays) during gestation may induce cerebellar
abnormalities in animals and/or humans (1,3–5).
N-methyl-N-nitrosourea (MNU), an alkylating agent,
is a potent chemical genotoxic carcinogen (6). MNU induces cancers of the breast,
gastrointestinal tract, respiratory tract, lymphoreticular tissue,
skin, teeth, pancreas and kidney, depending on the route and timing
of exposure and the animal strain (7–10).
MNU has been widely used to induce neural toxicity and tumors in
animal models (11), due to the
fact that it crosses the blood-brain barrier (12,13).
MNU causes O6-methylguanine-induced point mutations,
which have been suggested to be responsible for the initiation of
carcinogenesis (14) and neuronal
damage during gestational exposure (15,16).
MNU exposure during the prenatal/neonatal period induces two types
of brain hypoplasia: Microcephaly (hypoplasia of the cerebral
cortex) is the result of fetal mouse exposure to MNU on day 13 or
15 of the gestation period (6,17),
while cerebellar hypoplasia is the result of neonatal rat exposure
to MNU (18–20).
Arachidonic acid (AA) is a polyunsaturated fatty
acid present in the phospholipids of cell membranes, and it is
particularly abundant in the retina and brain (21,22).
Neurological health requires sufficient levels of docosahexaenoic
acid (DHA) and AA (23). Early
infancy may be a critical period when visual and brain developments
are susceptible to the effects of inadequate stores or a deficient
intake of DHA and AA (24). AA
drives postnatal neurogenesis and elicits a beneficial effect on
prepulse inhibition in Pax6 knockout rats, characterized by
impaired postnatal neurogenesis (25,26).
Randomized clinical trials of supplemental DHA and AA have been
conducted in full-term infants, and infants who received the
supplementation demonstrated enhanced cognitive functions, as
compared with the control groups (27,28).
MNU has been demonstrated to induce retinal damage due to the
selective formation of the DNA adduct, 7-methyldeoxyguanosine, in
photoreceptor cell nuclei followed by photoreceptor cell apoptosis
(29,30), while AA supplementation during the
gestational, lactational and post-weaning periods has been shown to
prevent MNU-induced retinal degeneration in young rats (31).
The aim of the present study was to elucidate the
effect of prenatal and postnatal dietary AA supplementation on
MNU-induced cerebellar hypoplasia in young Lewis rats.
Materials and methods
Animal procedures
The study protocol and all animal procedures were
approved by the Animal Care and Use Committee of Kansai Medical
University (Hirakata, Japan) and were in accordance with the
guidelines for animal experimentation at Kansai Medical University.
Sixteen 10-week-old female SPF/VAF rats (LEW/CrlCrlj) that were
1-week pregnant were purchased from Charles River Japan (Yokohama,
Japan). The rats were maintained in specific pathogen-free
conditions and had free access to water and CE-2-modified diets
containing different doses of AA. Animals were housed in plastic
cages with paper-chip bedding (Paper Clean; Japan SLC Inc.,
Hamamatsu, Japan) in an air-conditioned room at 22±2ºC and 60±10%
relative humidity with a 12-h light/dark cycle. Offspring were
sacrificed to leave a maximum of 10 per dam, and the dams were
maintained on their respective diets during the 21-day lactation
period. During a post-weaning period of up to 60 days, the
offspring were maintained on a CE-2 diet. A total of 115 male and
female pups were used in this study. Four to ten rats were
sacrificed at each time point (7, 14, 21, 28, and 60 days), and
similar numbers of males and females in each dietary group were
included.
Chemical and dose formulation
MNU was obtained from Sigma-Aldrich (St. Louis, MO,
USA) and was kept at −80ºC in the dark. The MNU solution was
dissolved in physiological saline containing 0.05% acetic acid
immediately prior to use. MNU (35 mg/kg) or vehicle (physiological
saline containing 0.05% acetic acid) was administered by
intraperitoneal (i.p.) injection.
AA-supplemented diet
As in previous studies, the AA-supplemented diet was
formulated by CLEA Japan, Inc. (Tokyo, Japan) (9,10,31).
AA was purchased from Cargill Alking Bioengineering LLC (Wuhan and
Hubei, China). The diet with 2.0 w/w% AA was semi-purified based on
the modified CE-2 formulation (CLEA Japan), while the basal diet
consisted of modified CE-2. Gas chromatography analyses of the
fatty acid compositions of the diets have been previously reported
(10). The total fatty acid
volumes were 47.20, 86.75 and 126.63 μg/mg diet for the CE-2 diet
(0.006 w/w% AA), basal diet (0.008 w/w% AA), and 2.0% AA diet,
respectively. The diets were stored at 4ºC to prevent lipid
oxidation prior to use.
Experimental procedures
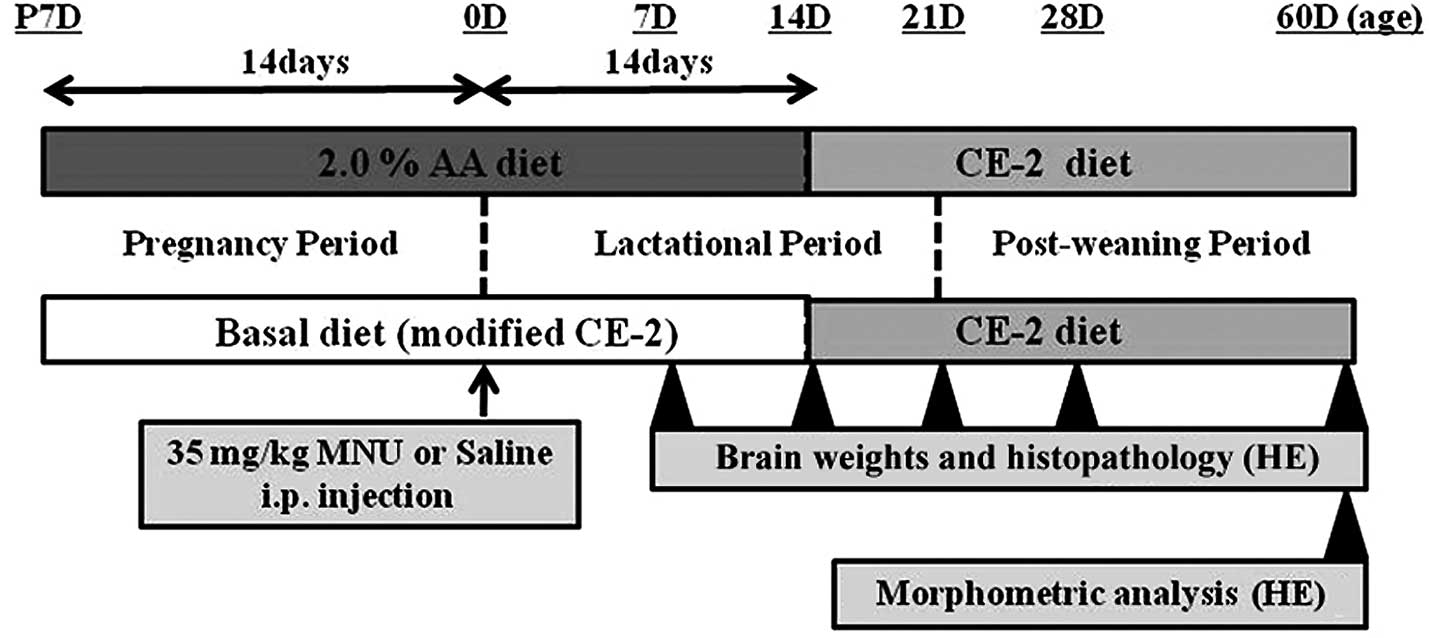
Male and female Lewis rats were fed with the basal
diet or an experimental diet (2.0% AA) from fertilization to
sacrifice. At birth (0 days of age), the rats received an i.p.
injection of vehicle (physiological saline) or 35 mg/kg MNU
(Fig. 1). At 7, 14, 21, 28, and 60
days following MNU or vehicle treatment, rats were anesthetized
with isoflurane (Forane®; Abbot Japan Co., Ltd., Tokyo,
Japan) and sacrificed by exsanguination from aortic transection.
The time-points were predominantly based on guidelines for
neuropathological assessment in developmental neurotoxicity testing
(32). During the experiment, all
pups were observed daily for clinical signs of toxicity and were
weighed at the time of MNU treatment and on the day of sacrifice.
Brains were quickly removed at the time of sacrifice, and complete
necropsies were conducted on all animals to check for systemic
toxicities induced by AA supplementation. Brain weights (cerebrum
and cerebellum with medulla oblongata) were measured separately
(Fig. 2A) by a method similar to a
previous study (4). The food
consumption and body weight of the dams were measured once per week
to estimate the actual dosage of AA during the pregnancy and
lactation periods.
Macro- and histopathological
examinations
Brain tissues were fixed overnight in 10% neutral
buffered formalin, embedded in paraffin, sectioned at a thickness
of 4 μm and stained with hematoxylin and eosin (HE). Following
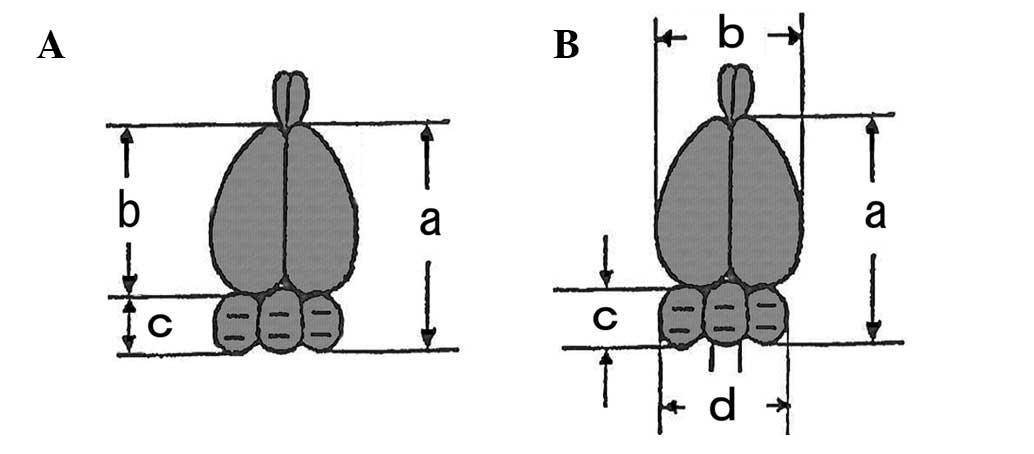
fixation, macroscopic photographs were taken of all brains, and the
total brain length (from the rostral border immediately lateral to
the most caudal border of the cerebellum), cerebral width,
cerebellar length (over the middle of the vermis) and cerebellar
width were measured with a ruler (Fig.
2B) by a method modified from previous studies (4,32).
The gross trimming levels of the brain were levels three and five
for the cerebrum and level seven for the cerebellum with medulla
oblongata, in accordance with the recommendation for
neuropathological assessment in developmental neurotoxicity testing
(32). HE-stained sections of the
brains were scanned with a high-resolution digital slide scanner
(NanoZoomer 2.0 Digital Pathology; Hamamatsu Photonics, Hamamatsu,
Japan) to prepare the digital images. The image files were opened
in color mode with NDP.view software (Hamamatsu Photonics).
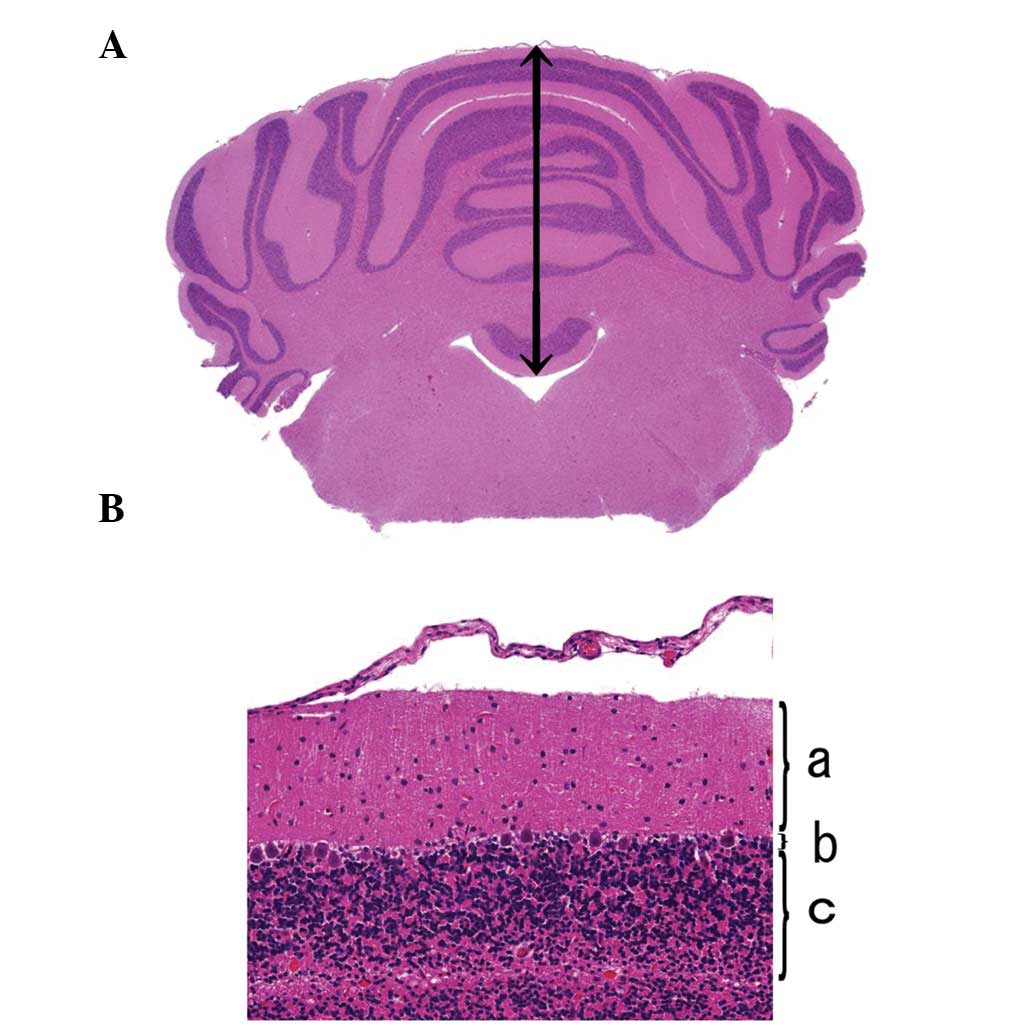
Qualitative linear measurements of the cerebellum were obtained in
order to determine the height of the cerebellum (Fig. 3A) and the widths of the molecular,
Purkinje and granular cell layers at the cerebellar vertex
(Fig. 3B), using methods modified
from previous studies (1,32).
Histopathological and morphometrical evaluations
were performed by a toxicologic pathologist (K.Y.) certified by the
Japanese Society of Toxicologic Pathology and the International
Academy of Toxicologic Pathology. The histopathological terminology
and diagnostic criteria of rodent nervous lesions were based on the
guidelines of the International Harmonization Nomenclature and
Diagnostic Criteria for Lesions in Rats and Mice Project (33).
Statistical analysis
All discrete values are expressed as the mean ±
standard error (SE) and were analyzed using the two-tailed
independent Student's t-test for unpaired samples, subsequent to
confirming the homogeneity of variances. The results include
comparisons between MNU- and saline-treated rats fed each diet and
between the basal diet-fed rats and rats fed an AA-supplemented
diet in the MNU-treated and vehicle-treated groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
General remarks
No deaths occurred, and no clinical signs or
symptoms were evident in any dams during the experimental period.
The AA diet did not affect the body weight gain (the growth rate)
in pups or result in weight changes in the dams, irrespective of
MNU treatment; however, the growth rate in the MNU-treated pups
tended to be lower than that in the vehicle-treated pups from the
age of 21 days (Table I).
Hypoactivity in the open field and poor neuromuscular ability in
pole climbing in the cages were observed only in the MNU-treated
rats fed a basal or AA diet (data not shown).
 | Table ISequential changes in body weight
(mg). |
Table I
Sequential changes in body weight
(mg).
| Days after
treatment |
|---|
|
|
|---|
| Group | 7 | 14 | 21 | 28 | 60 |
|---|
| Basal dieta + saline injection | 15.3 | 31.4 | 48.3 | 78.3 | 254.3 |
| Basal diet + MNU
injection | 13.1 | 28.1 | 29.3c | 44.4c | 133.4c |
| AA dietb + saline injection | 14.5 | 33.3 | 48.1 | 83.1 | 263.3 |
| AA diet + MNU
injection | 12.1 | 28.0 | 29.5c | 35.5c | 130.0c |
Estimated intake of AA
During the pregnancy and lactation periods, the AA
intake of the dams was 4.7 and 9.4 mg/kg/day in the basal diet
group, 77.7 and 242.6 mg/kg/day in the 0.1% AA group, 261.8 and
874.0 mg/kg/day in the 0.5% AA group and 1,075.1 and 3,058.5
mg/kg/day in the 2.0% AA group, respectively.
Brain weights
In the saline-treated rats fed with or without AA,
the total weight, cerebrum weight and cerebellum weight increased
as the age of the rats increased, which was suggestive of a normal
growth rate. Fourteen days subsequent to the MNU treatment, the
total weight, cerebrum weight and/or cerebellum weight were
significantly decreased compared with those in the saline-treated
rats (Table II). There were no
significant differences in any parameters between the MNU-treated
rats fed with or without AA at the age of 60 days. The decreased
growth rates in the cerebrum and cerebellum at the age of 60 days
resulted in those structures comprising 80 and 20% of total brain
weight in the saline-treated rats fed a basal diet, 83 and 17% in
the MNU-treated rats fed a basal diet, 79 and 21% in the
saline-treated rats fed an AA diet and 84 and 16% in the
MNU-treated rats fed an AA diet, respectively (Table II). These results suggest that the
change in brain weight in the MNU-treated rats was due to the
significantly reduced weight of the cerebellum.
 | Table IISequential changes in brain absolute
weight in rats following 35 mg/kg MNU treatment. |
Table II
Sequential changes in brain absolute
weight in rats following 35 mg/kg MNU treatment.
| A. Basal
dieta + saline injection |
|---|
|
|---|
| Days after
treatment |
|---|
|
|
|---|
| Brain region | 7 | 14 | 21 | 28 | 60 |
|---|
| Total [mg
(%)c] | 744.0 | 1402 (100) | 1597.8 (100) | 1930.2 (100) | 2297.3 (100) |
| Cerebrum [mg
(%)] | NE | 1073.7 (76) | 1272.6 (78) | 1537.4 (80) | 1826.6 (80) |
|
Cerebellumd [mg (%)] | NE | 328.3 (24) | 325.2 (22) | 392.8 (20) | 470.7 (20) |
|
| B. Basal diet + MNU
injection |
|
| Days after
treatment |
|
|
| Brain region | 7 | 14 | 21 | 28 | 60 |
|
| Total [mg (%)] |
628.0f | 1203.0 (100) | 1243.8
(100)f | 1706.6
(100)f | 2054.3
(100)e |
| Cerebrum [mg
(%)] | NE | 963.3 (80) | 993.2
(80)f | 1419.6 (83) | 1711.0
(83)e |
| Cerebellum [mg
(%)] | NE | 239.7
(20)f | 250.6
(20)f | 287
(17)f | 343.3
(17)f |
|
| C. AA
dietb + saline injection |
|
| Days after
treatment |
|
|
| Brain region | 7 | 14 | 21 | 28 | 60 |
|
| Total [mg (%)] | 730.5 | 1362.7 (100) | 1734.8
(100)g | 1997.5 (100) | 2421 (100) |
| Cerebrum [mg
(%)] | NE | 1053.0 (77) | 1377.6
(79)g | 1598.8 (80) | 1907 (79) |
| Cerebellum [mg
(%)] | NE | 309.7 (23) | 357.2 (21) | 398.8 (20) | 514 (21) |
|
| D. AA diet + MNU
injection |
|
| Days after
treatment |
|
|
| Brain region | 7 | 14 | 21 | 28 | 60 |
|
| Total [mg (%)] |
593.8f | 1006.2
(100)f | 1240.2
(100)f | 1604.2 (100) | 1978.9
(100)f |
| Cerebrum [mg
(%)] | NE | 806.3
(80)e | 974.2
(79)f | 1363.6
(85)f | 1655.3
(84)f |
| Cerebellum [mg
(%)] | NE | 199.8
(20)f | 266.0
(21)f | 240.6
(15)e | 323.6
(16)f |
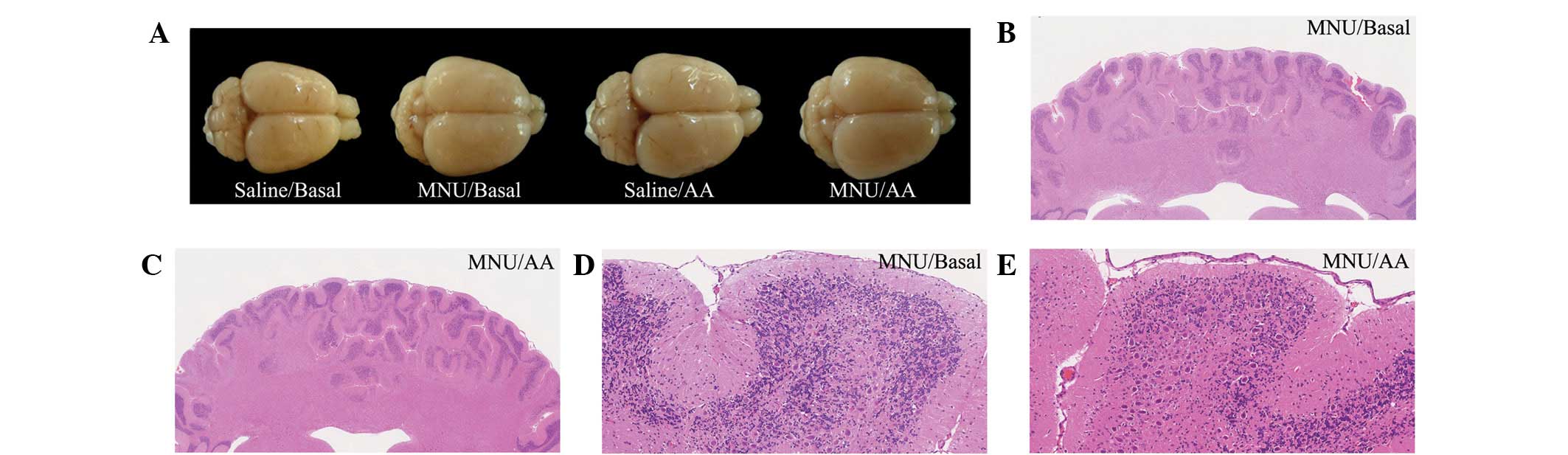
Macroscopic analysis of the brains
In the saline-treated rats, irrespective of whether
the rats had been fed AA, no brain abnormalities (including in the
cerebellum) were observed at any time-point. By contrast,
macroscopic abnormalities of the cerebellum were identified in the
MNU-treated rats from 21 days subsequent to treatment, irrespective
of whether the rats had been fed AA. These abnormalities were
characterized by a reduction of the cerebellar vermis tubercle,
followed by the altered appearance of quadrigeminal bodies
(Fig. 4A).
Morphometrical analysis of the macroscopic brain
lesions comprised assessment of the total brain length (from the
rostral border immediately lateral to the most caudal border of the
cerebellum), cerebral width, cerebellar length (over the middle of
the vermis) and cerebellar width at 60 days subsequent to MNU
treatment (Table III). In the
saline-treated rats fed the AA-rich diet, every parameter examined
was consistent with that in the saline-treated rats fed a basal
diet. In the MNU-treated rats, the total brain length, cerebellar
length and cerebellar width were significantly decreased compared
with those in the saline-treated rats (Table III), with measurements of 17,669,
2,534 and 10,804 μm in the MNU-treated rats fed the basal diet and
17,758, 2619 and 11,499 μm in the MNU-treated rats fed the AA diet,
respectively. There were no significant differences in any
parameters between the MNU-treated rats fed with or without AA.
These results suggest that the reduction in the total brain length
of the MNU-treated rats was due to the significantly decreased
length and width of the cerebellum.
 | Table IIISequential changes in brain length in
rats 60 days after 35 mg/kg MNU treatment. |
Table III
Sequential changes in brain length in
rats 60 days after 35 mg/kg MNU treatment.
| Brain length
(μm) |
|---|
|
|
|---|
| Group | Total brain
length | Cerebral width | Cerebellar
length | Cerebellar
width |
|---|
| Basal
dieta + saline injection | 18981 | 14614 | 4986 | 11539 |
| Basal diet + MNU
injection |
17669d | 13999 |
2534d |
10804c |
| AA dietb
+ saline injection | 19232 | 14756 | 5367 | 12030 |
| AA diet + MNU
injection |
17758d | 13882 |
2619d | 11499 |
Histopathological examination of the
cerebellum
The histological studies revealed no abnormal
changes in the brain (including the cerebellum) at any time-point
in the vehicle-treated rats fed with basal or AA diets (data not
shown). The external (embryonic) granular cell layer was located on
the surface area of the cerebellum in the two groups until the age
of 14 days. In the cerebellum of the 21-day-old rats, the external
granular cell layer disappeared, followed by the occurrence of the
normal development of three cell layers: the molecular, Purkinje
and granular cell layers. This suggests the mature development at
this age to be a suitable substrate for the majority of the routine
methods used in neuropathological evaluation (32). MNU-treated rats fed a basal or AA
diet from the age of 7 days exhibited disorganization of the
cerebellar cortex, including disarrangement of external granular,
Purkinje and inner granular cells (data not shown). A reduced
cellularity of the inner granular cell layer and a disperse
deposition of Purkinje cells in the inner granular cell layer were
observed, followed by thinning of the cerebellar cortex due to loss
and/or disturbance of the molecular, Purkinje and granular cell
layers, diagnosed as hypoplasia of the cerebellar cortex. At the
age of 60 days, the severity of the hypoplasia of the cerebellar
cortex in the MNU-treated rats fed a basal diet (Fig. 4B and D) was similar to that in the
MNU-treated rats fed an AA-rich diet (Fig. 4C and E).
To confirm the qualitative differences among the
treated groups at the age of 60 days, the cerebellar height and the
widths of the molecular, Purkinje and granular cell layers at the
cerebellar vertex were measured (Table IV). In the saline-treated rats fed
an AA-rich diet, every parameter examined was consistent with that
in the saline-treated rats fed a basal diet. In the MNU-treated
rats, the total height and all parameters of the cortical width
(molecular, Purkinje and granular cell layers) were significantly
decreased as compared with those in the saline-treated rats
(Table IV), with measurements of
1,997.3, 98.2, 9.0 and 137.2 μm in the MNU-treated rats fed a basal
diet and 2,062.9, 106.9, 9.3 and 145.6 μm in the MNU-treated rats
fed an AA diet, respectively. There were no significant differences
in any parameters examined among the MNU-treated rats, irrespective
of whether the rats had been fed with AA.
 | Table IVMorphometrical changes in the
cerebellar cortex in rats 60 days subsequent to 35 mg/kg MNU
treatment. |
Table IV
Morphometrical changes in the
cerebellar cortex in rats 60 days subsequent to 35 mg/kg MNU
treatment.
| Groups | Cerebellar vertex
(μm) |
|---|
|
|---|
| Total height | Cortex width |
|---|
|
|---|
| Molecular cell
layer | Purkinje cell
layer | Granular cell
layer |
|---|
| Basal
dieta + saline injection | 4136.0 | 145.1 | 20.8 | 298.3 |
| Basal diet + MNU
injection |
1997.3d |
98.2d |
9.0d |
137.2d |
| AA dietb
+ saline injection | 4262.5 | 153.3 | 22.9 | 314.8 |
| AA diet + MNU
injection |
2062.9d |
106.9d |
9.3c |
145.6d |
Furthermore no changes in macroscopic or
histopathological characteristics were observed in the cerebrum at
any time-point in the MNU-treated rats fed a basal diet or AA diet
(data not shown).
Discussion
The present study examined the effects of dietary AA
supplementation during the gestational, lactational and
post-weaning periods on MNU-induced cerebellar hypoplasia in young
rats. Irrespective of whether the rats had been fed an AA diet, the
brain weights of the MNU-treated rats, particularly the weights of
the cerebellum, were decreased compared with those of the
MNU-untreated rats from the 14th day following the MNU injection.
Macroscopic reductions in the cerebellar length and/or width and
histologically observed reductions in the cerebellar vertex height
and/or cortical width were also detected in the MNU-treated rats,
irrespective of whether the rats had been fed with AA.
Histopathologically, the MNU-treated rats (irrespective of AA
supplementation) exhibited disorganization of the cerebellar cortex
and disarrangement of the cortical layers (loss and/or disturbance
of the molecular, Purkinje and granular cell layers). There were no
significant differences in any parameters of the MNU-treated rats
fed with or without AA.
MNU exposure during the prenatal period induces two
types of brain hypoplasia: microcephaly and cerebellar hypoplasia.
Microcephaly (cerebral cortex hypoplasia) has been shown to occur
in the offspring of mice intraperitoneally injected with 10 mg/kg
MNU on day 13 or 15 of the gestation period (6,17).
MNU induces excessive cell death of neural precursor/stem cells and
the defective development of the cerebral cortex, resulting in
cerebral abnormalities. Embryos during the organogenetic periods of
the central nervous system are sensitive to temporal and spatial
environmental factors, since these factors affect critical
developmental processes, such as proliferation, migration,
differentiation, synaptogenesis, myelination and apoptosis
(34). Late-onset cerebellar
degeneration followed by hypoplasia has been shown to occur in the
offspring of mice exposed to 1 mg/kg MNU on day 16 of gestation
(19,20). In additional, daily subcutaneous
injections of 12.9 mg/kg MNU in rats at the ages of 4–7 days have
been demonstrated to induce cerebellar hypoplasia with reduced
cellularity of the internal granular cell layer and a disperse
deposition of Purkinje cells in the granular cell layer at 14 days
subsequent to birth; however, no lesions in the cerebrum were
induced (18). Cerebellar
hypoplasia is associated with MNU-induced cell death and inhibited
cell mitosis in the developing brain, particularly in the
cerebellum at the mitotic stage (35). Motor dysfunctions are induced by
imbalanced output activities from Purkinje cells to motor neurons.
Cerebellar neurons are generated in two germinative neuroepithelia
in two waves of proliferation and migration in rats (1). The development stage at day 0 in rats
shows the differentiation of Purkinje cells and the second wave
genesis and migration of granular cells (1). As indicated in the previously
mentioned studies, the target position of brain abnormalities
induced by MNU exposure may depend on the exposure period at fetal
or neonatal life. Cerebral hypoplasia occurs with MNU exposure at
the developmental period of cerebral neurons, while cerebellar
hypoplasia occurs with MNU exposure at the period with the most
proliferative activity of cerebellar neurons (1,34).
Therefore, the present experimental protocol with exposure at birth
was a reasonable strategy for MNU to induce cerebellar hypoplasia,
but not cerebral anomalies, in rats.
AA, together with DHA, is a fatty acid that is
important in central nervous system development; AA is commonly
added as a functional food ingredient to commercial infant formula
worldwide, in accordance with the international standards of Codex
Alimentarius (36). AA and DHA
have a critical function in neurodevelopment and the response to
neural injury in the neonatal stage (24). The levels of fatty acids in brain
tissue may be modified by dietary fatty acid intake (21,37).
AA directly affects neural stem/progenitor cells and promotes
postnatal neurogenesis (38).
Furthermore, AA ameliorates the prepulse inhibition relevant to
psychiatric disorder models, such as methylazoxymethanol-treated
rats and Pax6 knockout rats, through augmented postnatal
neurogenesis (25,26). By contrast, AA exhibits biphasic
actions in cultured brain neurons within a narrow concentration
range, with induction of cell death on one hand and promotion of
cell survival and enhancement of neurite extension on the other
(39). The neurotoxic action is
mediated by free radicals generated by AA metabolism, whereas the
neurotrophic actions are exerted by AA itself (39,40).
Dietary AA supplementation may be beneficial as a potential means
to delay the onset and/or progression of neural disease by the
inhibition of neuronal cell death at narrow windows of
susceptibility (in the developmental phase) for neuronal rescue.
Although the present strategy of AA supplementation during the
gestational, lactational and post-weaning periods has been shown to
prevent retinal degeneration in young rats (31), an identical therapeutic approach
did not rescue MNU-induced cerebellar hypoplasia in the present
study.
In the neurotoxicity model induced by MNU,
significant increases in the levels of lipid peroxidation, peroxide
products and reactive oxygen species production in the brain
(11) have been observed. MNU
enhances cellular oxidative stress and induces apoptosis. The
antioxidant, butylated hydroxytoluene, is capable of retarding the
cerebellar degeneration induced transplacentally by a single
injection of 1 mg/kg MNU on day 16 of pregnancy (20), while curcumin, another antioxidant,
is capable of rescuing functional and structural changes in the
cerebrum of young mice treated with 10 mg/kg MNU (11). An AA-rich diet may have low potency
to inhibit or protect the production of cellular oxidative stress
in the brain induced by MNU.
The AA intake by Japanese infants via breast milk is
~14.3 mg/kg/day (41). The 2.0% AA
diets in the present study provided an AA dose of 1,477 mg/kg/day
during pregnancy and 1,876 mg/kg/day during lactation, which
represented ~103- and 131-fold, respectively, the quantities
consumed by human infants. In combination, the results of the
present study indicated that an AA-enriched diet in the prenatal
and postnatal periods was unlikely to prevent cerebellar hypoplasia
in human infants, despite the importance of AA in brain
development. Further studies with other animal models are required
in order to understand any effects of AA on cerebellar
hypoplasia.
Acknowledgements
This study was supported in part by a Health and
Labour Sciences Research Grant (H22-Shokuhin-Ippan-002) and a
Grant-in-Aid for Scientific Research (C) from the Japan Society for
the Promotion of Science (JSPC25462740). The authors would like to
thank Ms. T. Akamatsu for her technical assistance and Dr T. Sasaki
(Maruho Co. Ltd, Osaka, Japan) and Dr N. Uehara (Kyusyu University,
Fukuoka, Japan) for their scientific advice.
Abbreviations:
|
AA
|
arachidonic acid
|
|
DHA
|
docosahexaenoic acid
|
|
MNU
|
N-methyl-N-nitrosourea
|
References
|
1
|
Biran V, Verney C and Ferriero DM:
Perinatal cerebellar injury in human and animal models. Neurol Res
Int. 2012:8589292012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safronova MM, Barbot C and Resendepereira
J: Hipoplasias cerebelosas. Acta Med Port. 23:841–852. 2010.(In
Portuguese).
|
|
3
|
Altman J: Morphological and behavioral
markers of environmentally induced retardation of brain
development: an animal model. Environ Health Perspect. 74:153–168.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogura H, Mikami T, Takamura N, Suzuki Y
and Chiba T: Development of behavioral function of cerebellar
hypoplasia rats as induced by cytosine arabinoside (ara-C). Nihon
Yakurigaku Zasshi. 76:33–44. 1980.(In Japanese).
|
|
5
|
Ramaekers VT, Heimann G, Reul J, Thron A
and Jaeken J: Genetic disorders and cerebellar structural
abnormalities in childhood. Brain. 120:1739–1751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kodama M, Fujiyama F, Yamada S, Shiota K
and Nagao T: Methylnitrosourea induces neural progenitor cell
apoptosis and microcephaly in mouse embryos. Birt Defects Res B Dev
Reprod Toxicol. 89:213–222. 2010.PubMed/NCBI
|
|
7
|
Kimura A, Yoshizawa K, Sasaki T, Uehara N,
Kinoshita Y, Miki H, Yuri T, Uchida T and Tsubura A:
N-methyl-N-nitrosourea-induced changes in epithelial rests of
Malassez and the development of odontomas in rats. Exp Ther Med.
4:15–20. 2012.PubMed/NCBI
|
|
8
|
Tsubura A, Lai YC, Miki H, Sasaki T,
Uehara N, Yuri T and Yoshizawa K: Animal models of
N-methyl-N-nitrosourea-induced mammary cancer and retinal
degeneration with special emphasis on therapeutic trials. In Vivo.
25:11–22. 2011.PubMed/NCBI
|
|
9
|
Yoshizawa K, Emoto Y, Kinoshita Y, Kimura
A, Uehara N, Yuri T, Shikata N, Hamazaki T and Tsubura A:
Arachidonic acid supplementation does not affect
N-methyl-N-nitrosourea-induced renal preneoplastic lesions in young
Lewis rats. Oncol Lett. 5:1112–1116. 2013.PubMed/NCBI
|
|
10
|
Yoshizawa K, Uehara N, Kimura A, Emoto Y,
Kinoshita Y, Yuri T, Takada H, Moriguchi T, Hamazaki T and Tsubura
A: Promoting effect of arachidonic acid supplementation on
N-methyl-N-nitrosourea-induced pancreatic acinar cell hyperplasia
in young Lewis rats. Oncol Lett. 5:76–82. 2013.PubMed/NCBI
|
|
11
|
Singla N and Dhawan DK:
N-methyl-N-nitrosourea induced functional and structural
alterations in mice brain - role of curcumin. Neurotox Res.
22:115–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleihues P and Patzschke K: Distribution
of N-(14C) methyl-N-nitrosourea in the rat after its
systemic administration. Z Krebsforsch. 75:193–200. 1971.(In
German).
|
|
13
|
Shibutani M, Maekawa A, Okeda R, Mitsumori
K, Imazawa T, Yoshiza J, Onodera H and Hayashi Y: An experimental
model for anaplastic astrocytomas and glioblastoma using adult F344
rats and N-methyl-N-nitrosourea. Acta Pathol Jpn. 43:464–474.
1993.PubMed/NCBI
|
|
14
|
Becker K, Dosch J, Gregel CM, Martin BM
and Kaina B: Targeted expression of human
O6-methylguanine-DNA ethyl transferase (MGMT) in
transgenic mice protects against tumor initiation in two-stage skin
carcinogenesis. Cancer Res. 56:3244–3249. 1996.PubMed/NCBI
|
|
15
|
Kidney JK and Faustman EM: Modulation of
nitrosourea toxicity in rodent embryonic cells by O6-benzylguanine,
a depletory of O6-methylguanine-DNA
methyltransferase. Toxicol Appl Pharmacol. 133:1–11. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schleifer S and Tempel K: Formation and
persistence of N7- and O6-methyl-guanine in
DNA of chick embryo brain cells in ovo following
administration of N-nitroso-N-methylurea. Zentralbl Veterinarmed A.
43:589–598. 1996.
|
|
17
|
Fujiyama F, Saito Y and Nagao T: Effects
of prenatal exposure to methyl nitrosourea on the developing brains
of mouse offspring. Congenit Anom (Tokyo). 47:A292007.
|
|
18
|
Fujimori K, Inoue K, Nakazawa K, Maekawa
A, Shibutani M and Takanaka A: Neurochemical and histological
analysis of motor dysfunction observed in rats with
methylnitrosourea-induced experimental cerebellar hypoplasia.
Neurochem Res. 17:223–231. 1992. View Article : Google Scholar
|
|
19
|
Smith SB, Brown CB, Wright ME and Yielding
KL: Late-onset cerebellar degeneration in mice induced
transplacentally by methylnitrosourea. Teratog Carcinog Mutagen.
7:449–463. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith SB, Cooke CB and Yielding KL: The
antioxidant butylated hydrotoluene can retard cerebellar
degeneration induced transplacentally by a single low dosage of
N-methyl-N-nitrosourea. Teratog Carcinog Mutagen. 9:15–27. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arterburn LM, Hall EB and Oken H:
Distribution, interconversion, and dose response of n-3 fatty acids
in humans. Am J Clin Nutr. 83(Suppl): 1467S–1476S. 2006.PubMed/NCBI
|
|
22
|
Semba RD: Essential fatty acids and visual
development in infants. Handbook of Nutrition and Ophthalmology.
Humana Press; New Jersey: pp. 415–441. 2007
|
|
23
|
Davis-Bruno K and Tassinari MS: Essential
fatty acid supplementation of DHA and ARA and effects on
neurodevelopment across animal species: a review of the literature.
Birth Defects Res B Dev Reprod Toxicol. 92:240–250. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saste MD, Carver JD, Stockard JE, Benford
VJ, Chen LT and Phelps CP: Maternal diet fatty acid composition
affects neurodevelopment in rat pups. J Nutr. 128:740–743.
1998.PubMed/NCBI
|
|
25
|
Maekawa M, Takashima N, Matsumata M,
Ikegami S, Kontani M, Hara Y, Kawashima H, Owada Y, Kiso Y,
Yoshikawa T, Inokuchi K and Osumi N: Arachidonic acid drives
postnatal neurogenesis and elicits a beneficial effect on prepulse
inhibition, a biological trait of psychiatric illnesses. PLoS ONE.
4:e50852009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osumi N: Fatty acid signal, neurogenesis,
and psychiatric disorders. Nihon Shinkei Seishin Yakurigaku Zasshi.
30:141–148. 2010.(In Japanese).
|
|
27
|
Hoffman DR, Boettcher JA and
Diersen-Schade DA: Toward optimizing vision and cognition in term
infants by dietary docosahexaenoic and arachidonic acid
supplementation: a review of randomized controlled trials.
Prostaglandins Leukot Essent Fatty Acids. 81:151–158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uauy R, Hoffman DR, Peirano P, Birch DG
and Birch EE: Essential fatty acids in visual and brain
development. Lipids. 36:885–895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshizawa K, Nambu H, Yang J, Oishi Y,
Senzaki H, Shikata N, Miki H and Tsubura A: Mechanisms of
photoreceptor cell apoptosis induced by N-methyl-N-nitrosourea in
Sprague-Dawley rats. Lab Invest. 79:1359–1367. 1999.PubMed/NCBI
|
|
30
|
Yoshizawa K and Tsubura A: Characteristics
of N-methyl-N-nitrosourea-induced retinal degeneration in animals
and application for the therapy of human retinitis pigmentosa.
Nippon Ganka Gakkai Zasshi. 109:327–337. 2005.(In Japanese).
|
|
31
|
Yoshizawa K, Sasaki T, Kuro M, Uehara N,
Takada H, Harauma A, Ohara N, Moriguchi T and Tsubura A:
Arachidonic acid supplement during gestational, lactational and
post-weaning periods prevents retinal degeneration induced in a
rodent model. Br J Nutr. 109:1424–1432. 2013. View Article : Google Scholar
|
|
32
|
Bolon B, Garman R, Jensen K, Krinke G and
Stuart B: A ‘best practices’ approach to neuropathologic assessment
in developmental neurotoxicity testing - for today. Toxicol Pathol.
34:296–313. 2006.
|
|
33
|
Kaufmann W, Bolon B, Bradley A, Butt M,
Czasch S, Garman RH, George C, Groters S, Krinke G, Little P, McKay
J, Narama I, Rao D, Shibutani M and Sills R: Proliferative and
nonproliferative lesions of the rat and mouse central and
peripheral nervous systems. Toxicol Pathol. 40(Suppl): 87S–157S.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rice D and Barone S Jr: Critical periods
of vulnerability for the developing nervous system: evidence from
humans and animal models. Environ Health Perspect. 108(Suppl 3):
S511–S533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujimori K, Sunouchi M, Inoue K, Nakadate
M, Takanaka A and Omori Y: Cytotoxic effects of methylnitrosourea
on developing brain. Neurochem Res. 8:193–206. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Report of the 28th session of the Codex
Committee on Nutrition and Foods for Special Dietary Uses. Joint
FAO/WHO Food Standards Programme, Codex Alimentarus Commission;
2007
|
|
37
|
Moriguchi T, Loewke J, Garrison M, Catalan
JN and Salem N Jr: Reversal of docosahexaenoic acid deficiency in
the rat brain, retina, liver, and serum. J Lipid Res. 42:419–427.
2001.PubMed/NCBI
|
|
38
|
Sakayori N, Maekawa M, Numayama-Tsuruta K,
Katura T, Moriya T and Osumi N: Distinctive effects of arachidonic
acid and docosahexaenoic acid on neural stem/progenitor cells.
Genes Cells. 16:778–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katsuki H and Okuda S: Arachidonic acid as
a neurotoxic and neurotrophic substance. Prog Neurobiol.
46:607–636. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HY, Akbar M and Kim KY: Inhibition of
neuronal apoptosis by polyunsaturated fatty acids. J Mol Neurosci.
16:223–278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Imai N, Kawabe M, Tamano S, Doi Y,
Nakashima H, Suguro M, Numano T, Hara T, Hagiwara A, Furukawa F,
Kaneda Y, Tateishi N, Fujii W, Kawashima H, Shibata H and
Sakakibara Y: Arachidonate-enriched triglyceride oil does not
promote tumor development in a rat medium-term multi-organ
carcinogenesis model. Food Chem Toxicol. 50:2780–2791. 2012.
View Article : Google Scholar : PubMed/NCBI
|