Introduction
Knee osteoarthritis (KOA) is a degenerative disease
that results in joint pain, stiffness and a reduction in knee
function (1). Regardless of
continued physiotherapy and the wide use of non-steroidal
anti-inflammatory drugs, the disease is of concern, as it affects
the mobility of individuals and quality of life. Degeneration and
loss of articular cartilage are characteristic features of
osteoarthritis. The appearance of fibrillations, matrix depletion,
cell clusters and changes in matrix composition reflect the
aberrant behavior of resident chondrocytes (2). It is generally believed that
degeneration of cartilage in osteoarthritis is characterized by two
phases: a biosynthetic phase, during which the cells resident in
cartilage, the chondrocytes, attempt to repair the damaged
extracellular matrix; and a degradation phase, in which the
activity of enzymes produced by the chondrocytes digests the
matrix, matrix synthesis is inhibited, and the consequent erosion
of the cartilage is accelerated (3,4,5,6). The
matrix metalloproteinases (MMPs) are considered important for the
chondrolytic processes that contribute to the degenerative changes
in osteoarthritis cartilage (7,8).
Currently, there is increasing interest in non-synthetic natural
drugs that are derived from plant or herbal sources, due to the
greater tolerance to such agents and the reduced levels of adverse
drug reactions (9). Eucommia
ulmoides Oliver is a native Chinese medicinal herb, the bark of
which has long been utilized for the treatment of arthritis in
China. However, the mechanisms of action of Eucommia remain
unclear. In the present study, the effects of an aqueous extract of
Eucommia on the articular cartilage were investigated in a
rat model of KOA. Mankin’s grade was evaluated and the serum and
synovial fluid levels of MMP-1, -3 and -13 were measured.
Materials and methods
Medicinal material
Eucommia bark (500 g; origin, Sichuan, China)
was purchased from Zhixin Chinese Herbal Co., Ltd. (batch no.
120701; Guangzhou, China). The aqueous extract of Eucommia
was prepared as described previously (10). Eucommia bark (500 g) was
soaked in distilled water for 30 min, then heated to boiling for 10
min, simmered for 30 min and the dregs were filtered. The procedure
was repeated twice, all decoction was collected which yielded a
final concentration of ~0.5 g crude extract/ml (1,000 ml).
Instruments
An inverted microscope (BX51TRF; Olympus Optical
Co., Ltd., Tokyo, Japan), a high-speed centrifuge (3k30; Sigma, St.
Louis, MO, USA), a microplate reader (MK352, Hercules, CA, USA), a
microtome (Leica RM2235; Leica Biosystems, Wetzlar, Germany) and a
Leica TP1020 Auto Processor System (Leica Biosystems) were used in
the study.
Animals and KOA model
A total of 54 Sprague-Dawley rats (weight, 180–220
g) comprising 27 males and 27 females, were obtained from the
Laboratory Animal Center, Guangzhou University of Traditional
Chinese Medicine [license no. scxk(Yue)2008–0020; Guangzhou,
China]. Rats were housed in a humidity-controlled room at 20°C with
access to fresh water and standard laboratory food ad
libitum. All experimental procedures were approved by the
Animal Care and Use Committee, Guangzhou University of Traditional
Chinese Medicine (2008C067). Osteoarthritis (OA) was induced in
rats by section of the anterior cruciate ligament of the right knee
through a stab incision (11), KOA
was not induced in the blank group.
Experimental procedures
The rats were randomly divided into three groups:
the blank, Eucommia and control groups. In the blank group,
18 normal rats were fed ad libitum; whereas in the
Eucommia group, an aqueous extract of Eucommia (6
ml/kg/day) was administered to each rat (n=22) for 4 weeks. The
Eucommia dosage was based on the surface area of the rats,
which was calculated by the Meeh-Rubner formula (12). In the control group, distilled
water (6 ml/kg/day) was administered to each rat (n=22) for 4 weeks
(Table I).
 | Table IRat groups and administration. |
Table I
Rat groups and administration.
| Groups | No. of rats | Treatment | Methods | Dosage
(ml/kg/day) |
|---|
| Blank | 18 | - | - | - |
| Control | 18 | Distilled water | Gavage | 6 |
| Eucommia | 18 | Eucommia
decoction | Gavage | 6 |
Matrix metalloproteinase-1 (MMP-1), MMP-3
and MMP-13 levels
Six rats were randomly selected from each group 1, 2
and 4 weeks following the initiation of treatment. Blood was
sampled from the retro-orbital plexus of the selected rats. In
order to obtain the synovial fluid, the right knee of each rat was
cut and the joint cavity was exposed under aseptic conditions. The
cavity was then lavaged with 1 ml saline and 0.5 ml synovial fluid
was aspirated. The synovial fluid specimens were centrifuged at
4,500 r/min for 10 min, then the supernatant was stored in
Eppendorf tubes at −80°C. The serum and synovial fluid levels of
MMP-1, -3 and -13 were measured by double-antibody sandwich
enzyme-linked immunosorbent assay (ELISA).
Histopathological findings
Samples of articular cartilage were obtained from
the lateral tibial condyle of each rat and histopathologically
graded (Table II) (13).
 | Table IIHistological and histochemical
grading. |
Table II
Histological and histochemical
grading.
| Variable | Grade |
|---|
| Structure |
| Normal | 0 |
| Surface
irregularities | 1 |
| Pannus and surface
irregularities | 2 |
| Clefts to
transitorial zone | 3 |
| Clefts to radial
zone | 4 |
| Clefts to calcified
zone | 5 |
| Complete
disorganization | 6 |
| Cells |
| Normal | 0 |
| Diffuse
hypercellularity | 1 |
| Cloning | 2 |
| Hypocellularity | 3 |
| Safranin-0
staining |
| Normal | 0 |
| Slight
reduction | 1 |
| Moderate
reduction | 2 |
| Severe
reduction | 3 |
| No dye noted | 4 |
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Quantitative
variables are expressed as the mean ± standard deviation. One-way
analysis of variance, the Student’s t-test and correlation analyses
were used, and the differences were determined by the t-test and
the least significant difference (LSD). P<0.05 was considered to
indicate a statistically significant difference.
Results
Macroscopic evaluation
In the blank group, joint swelling was not
identified and the articular cartilage of the knee appeared smooth,
lustrous, pale blue and translucent. However, in the control group,
examination indicated swelling of the right knee joint and
congestion. Furthermore, the synovial fluid was opaque and pale
yellow, the cartilage surface was dull and rough, significant
ulcers and fissures were observed, and the gross exposure of
subchondral bone and ulcerations was occasionally demonstrated. In
addition, congestion, hypertrophy and edema of the synovial
membrane were identified in this group. In the Eucommia
group, the knee articular cartilage was pale and close to normal in
appearance; however, the cartilage was softer compared with that of
the control group and an occasional crack was identified in the
surface. Moreover, mild hyperplasia of the synovial lining was
identified in the Eucommia group.
Histopathology
The articular cartilage samples from the rats in
each group were evaluated using Mankin’s grades (Table III) (13).
 | Table IIIMorphological changes in the articular
cartilage. |
Table III
Morphological changes in the articular
cartilage.
| Groups | No. of rats | Mankin’s grade |
|---|
| Blank | 18 | 0.25±0.21 |
| Control | 18 | 4.87±0.83a |
| Eucommia | 18 | 2.64±0.07a |
Morphological changes in the articular
cartilage after 4 weeks of treatment
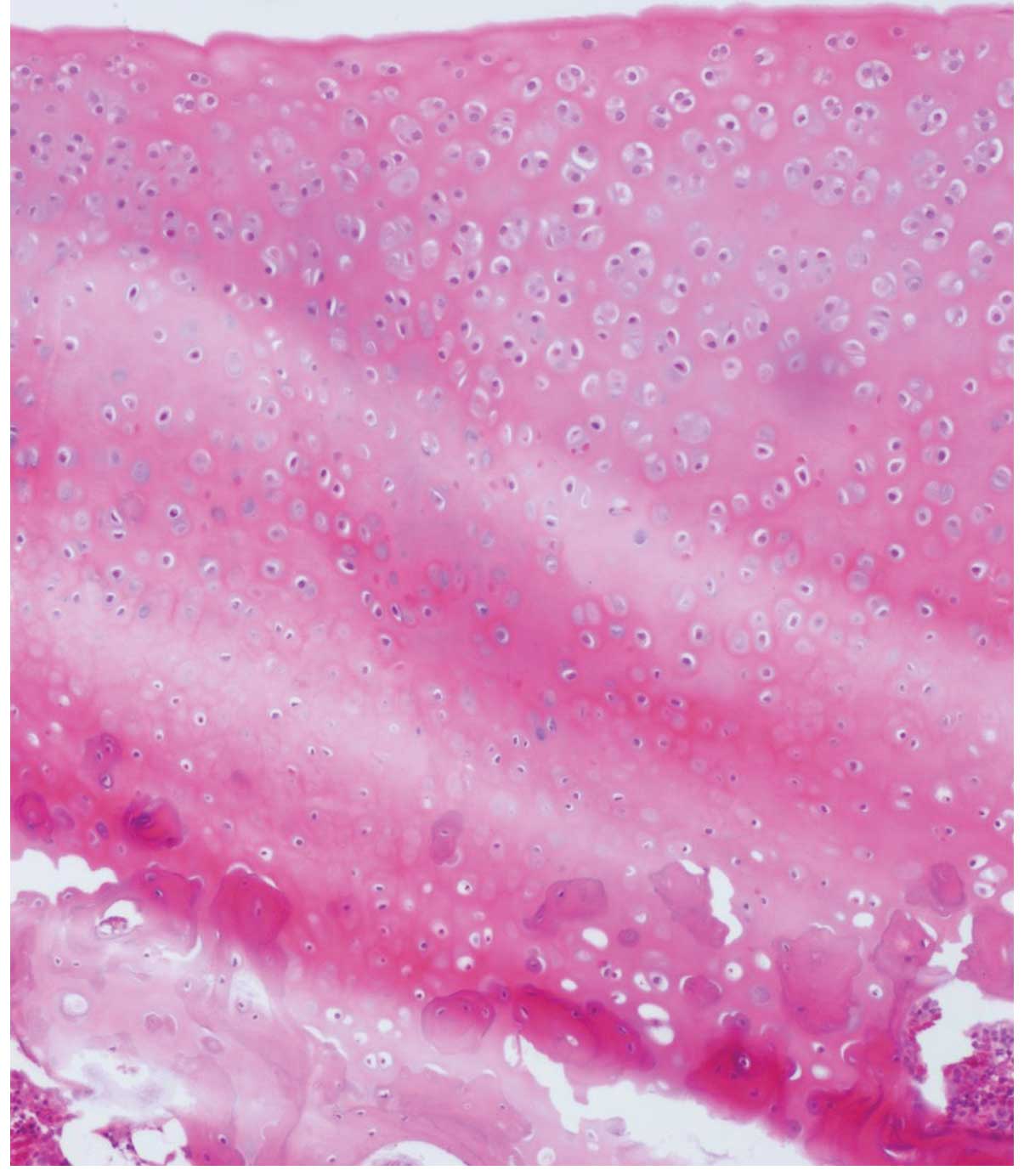
In the control group, the surface of the articular
cartilage was rough and indications of erosion and exfoliation, and
visible cracks across the surface, were observed. Chondrocyte
cluster formation was identified and the tide line was not
continuous. In addition, stromal staining appeared uneven,
decreased and was absent in intensity. Fibrous tissue proliferation
and clusers were observed (Fig.
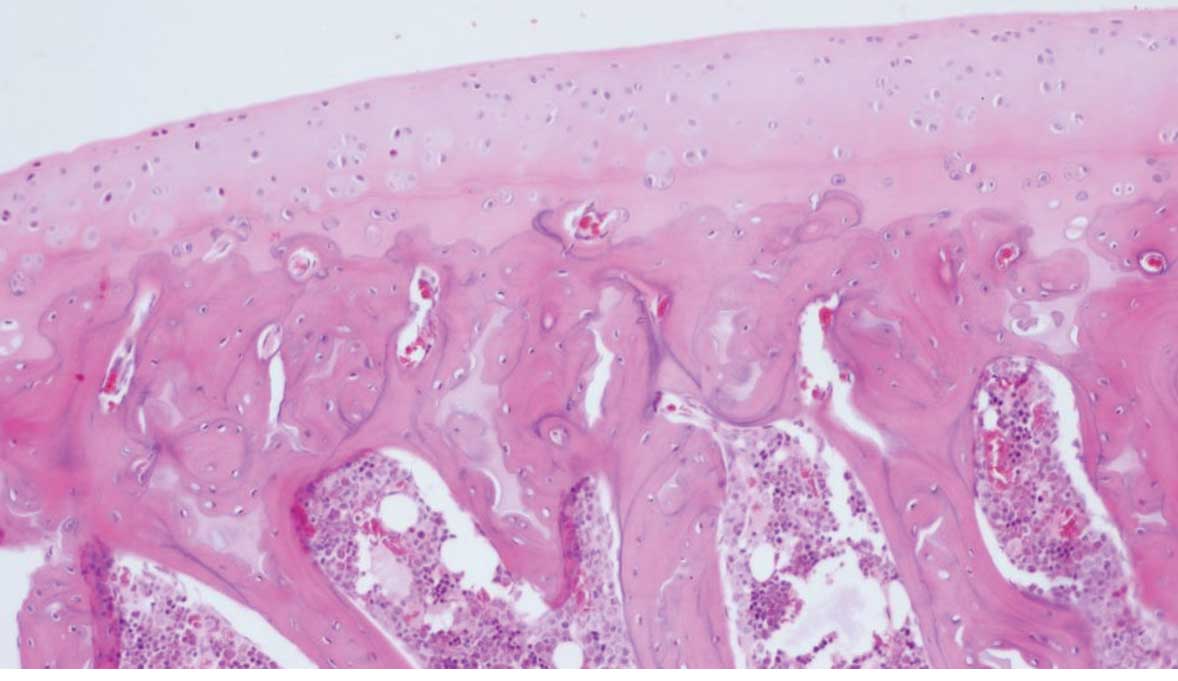
1). In the blank group, the surface of the cartilage was smooth
and the cell layers were clearly defined. In addition, the cells
were uniform and arranged neatly. Staining of the stroma appeared
even (Fig. 2). In the
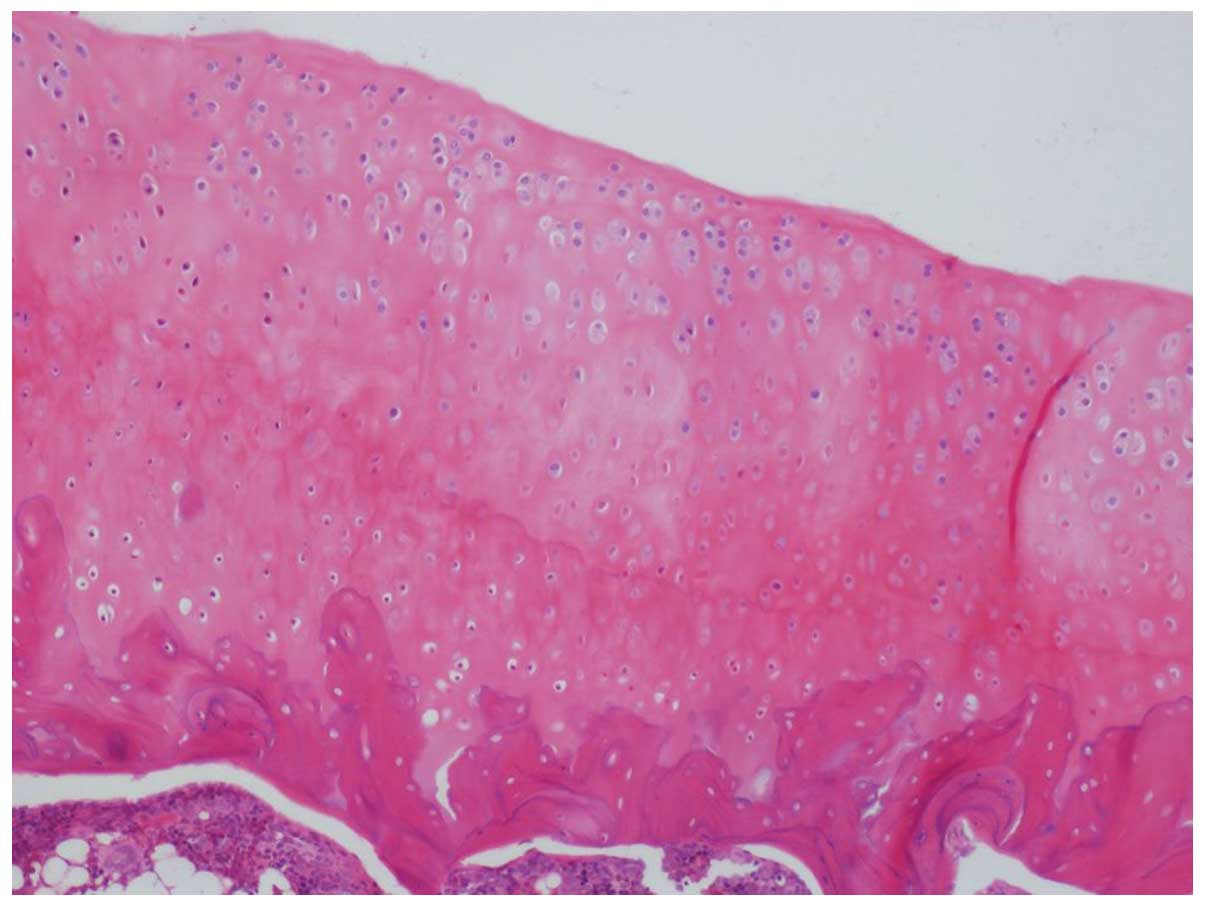
Eucommia group, the articular cartilage had structural
integrity. The staining of the stroma was occasionally uneven.
Furthermore, there was a decreased number of chondrocytes, and mild
to moderate damage of the articular cartilage was evident (Fig. 3).
Levels of MMP-1, -3 and -13
In the synovial fluid of the Eucommia group,
the levels of MMP-1, -3 and -13 were significantly lower at 4 weeks
compared with those of the control group (P<0.05; Table IV). In addition, the serum levels
of MMP-1, -3, and-13 in the Eucommia group were
significantly lower than those of the control group (P<0.05;
Table V).
 | Table IVLevels of MMP-1, -3 and-13 in the
synovial fluid at various time points. |
Table IV
Levels of MMP-1, -3 and-13 in the
synovial fluid at various time points.
| Groups | No. of rats | Time (weeks) | MMP-1 | MMP-3 | MMP-13 |
|---|
| Blank | 18 | - | - | - | - |
| Control | 18 | 1 | 3.79±0.18 | 3.17±0.44 | 0.39±0.11 |
| | 2 | 5.01±0.93 | 4.07±0.11 | 0.55±0.84 |
| | 4 | 5.89±0.65 | 5.12±0.34 | 0.67±0.21 |
|
Eucommia | 18 | 1 | 3.76±0.02 | 3.16±0.65 | 0.38±0.47 |
| | 2 | 3.76±0.77 | 4.01±0.29 | 0.31±0.61 |
| | 4 | 2.81±0.93a | 2.46±0.75a | 0.24±0.81a |
 | Table VLevels of MMP-1, -3 and -13 in the
serum following surgery at various time points. |
Table V
Levels of MMP-1, -3 and -13 in the
serum following surgery at various time points.
| Groups | No. of rats | Time (weeks) | MMP-1 | MMP-3 | MMP-13 |
|---|
| Blank | 18 | - | - | - | - |
| Control | 18 | 1 | 10.95±0.66 | 11.11±0.29 | 1.01±0.12 |
| | 2 | 11.38±0.79 | 12.26±0.33 | 1.56±0.74 |
| | 4 | 13.56±0.17 | 12.38±0.89 | 1.96±0.22 |
|
Eucommia | 18 | 1 | 8.26±0.12 | 10.22±0.31 | 0.89±0.46 |
| | 2 | 6.10±0.54 | 7.18±0.82 | 0.76±0.33 |
| | 4 | 5.16±0.42a | 5.01±0.27a | 0.41±0.77a |
Discussion
Approximately 10% of individuals >55 years of age
are affected by osteoarthritis in the UK and the Netherlands,
one-quarter of whom are severely disabled (14). The condition is characterized by
degeneration of the articular cartilage and subsequent changes to
the subchondral bone. The underlying mechanisms remain unknown, but
the glycosaminoglycan-proteoglycan matrix is proposed to be
important in the progression of the disease (15). X-ray examinations of osteoarthritis
do not indicate early cartilage abnormalities. However, the
detection of potential biomarkers in the synovial fluid may be a
precise method that would facilitate the early diagnosis of the
disease.
Among the various biological markers associated with
OA, MMPs are important in cartilage degradation in human joint
diseases, and they function downstream of the OA signaling pathways
(16,17). Following excretion from the cell as
inactive pro-forms, MMPs are converted into the active enzymes,
which are inhibited by the reversible binding of MMPs with specific
inhibitors, including tissue inhibitors of metalloproteinases
(TIMPs) (18). The activation of
MMPs is closely associated with cartilage degradation. One or more
MMPs may digest the majority of the matrix components in
vitro(19). In addition,
elevated levels of MMPs are identified in OA cartilage at the site
of cartilage destruction, and specific digested parts of MMPs may
be identified in synovial fluid samples taken from patients with OA
(20). MMP-2 and -9 are
particularly important in cartilage degradation as they degrade a
variety of collagens, including basement membrane type V collagen
and denatured fibrillar type I collagen (21,22).
Therefore, MMPs may potentially be utilized as a promising
biological markers of OA.
The stem bark of Eucommia ulmoides Oliver (a
member of the Eucommiaceae family), which is also known as
Du-Zhong, is commonly utilized in traditional Chinese medicine for
the treatment of rheumatoid arthritis (23,24).
Furthermore, studies have shown that crude flavonoids and
polysaccharides from Eucommia extracts are the major
components that contribute to the anti-bacterial, -inflammatory,
-oxidation, -aging and -cancer activities, among numerous other
physiological functions, of the extracts (25,26).
Previous studies have suggested that
Eucommia, combined with various plant-derived medicines, is
effective in decreasing the rate of apoptosis in chondrocytes
(27). However, the effect of
Eucommia treatment on articular cartilage degeneration
remains unclear. The present study observed the effects of an
aqueous extract of Eucommia on the articular cartilage in a
rat model of KOA. The histopathology and MMP-1, -3 and -13 levels
in the serum and synovial fluid were investigated to identify the
possible involvement of Eucommia in the protection of
articular cartilage.
OA was surgically induced in the knee joint as
described previously (11). One
week following surgery, the control group demonstrated joint
capsule adhesions and the cartilage did not exhibit a glossy
surface, but was smooth and yellow. Two weeks following surgery,
the cartilage was yellow, but the color had darkened and fissures
of varying size were identified. Four weeks following surgery, the
structural damage of the cartilage was severe, and the femoral
external condyle and tibial plateau edge markedly demonstrated
hyperplasia. These results were consistent with the
pathomorphological changes that occur in osteoarthritis; therefore,
in the present study, the OA model was successfully established.
According to Mankin’s criteria (28), the grading of cartilage in the
control group was significantly higher compared with that of the
Eucommia group (P<0.05).
There is only a small volume of synovial fluid in
the knee joints of rats, which is difficult to extract. Therefore,
in the present study, saline was injected into the rat joint
cavity. The joint irrigation fluid was then extracted and the
levels of MMPs in the fluid were measured. The results of the
present study demonstrated that the aqueous Eucommia extract
significantly reduced the levels of MMP-1, -3 and -13 in the joint
irrigation fluid and in the blood of the rats. Therefore,
Eucommia may be important in the inhibition of inflammatory
factors and in preventing the degradation of the cartilage matrix
in rats with OA.
An increase in the number of studies demonstrating
the effect of Eucommia in the progression of OA has resulted
in an interest in drugs that affect bone metabolism, and drugs that
may slow down or even halt the process of joint degeneration.
However, the degradation of the cartilage extracellular matrix that
is observed in OA is a complex process. The present study explored
the effects of Eucommia on the levels of MMP-1, -3 and -13
in rats with osteoarthritis; however, the underlying mechanisms
remain unclear.
References
|
1
|
Felson DT: Clinical practice.
Osteoarthritis of the knee. N Engl J Med. 354:841–848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poole AR: Cartilage in health and disease.
Arthritis and Allied Conditions: A Textbook of Rheumatology.
Koopman WJ: 13th edition. Baltimore: Williams and Wilkins; pp.
279–333. 1997
|
|
3
|
Meachim G and Brooke G: The pathology of
osteoarthritis. Osteoarthritis: Diagnosis and Management. Moskowitz
RW, Howell DS, Goldberg VM and Mankin HJ: Philadelphia: WB
Saunders; pp. 29–42. 1984
|
|
4
|
Howell DS: Pathogenesis of osteoarthritis.
Am J Med. 80:24–28. 1986. View Article : Google Scholar
|
|
5
|
Adams ME: Pathobiology of knee
osteoarthritis. Clinical Concepts in Regional Musculoskeletal
Illness. Hadler NM: Orlando: Grune and Stratton; pp. 137–167.
1987
|
|
6
|
Hamerman D: The biology of osteoarthritis.
N Engl J Med. 320:1322–1330. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woessner JF Jr and Gunja-Smith Z: Role of
metalloproteinases in human osteoarthritis. J Rheumatol Suppl.
27:99–101. 1991.PubMed/NCBI
|
|
8
|
Shlopov BV, Lie WR, Mainardi CL, Cole AA,
Chubinskaya S and Hasty KA: Osteoarthritic lesions: involvement of
three different collagenases. Arthritis Rheum. 40:2065–2074. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimmatkar N, Thawani V, Hingorani L and
Khiyani R: Efficacy and tolerability of Boswellia serrata
extract in treatment of osteoarthritis of knee - a randomized
double blind placebo controlled trial. Phytomedicine. 10:3–7.
2003.
|
|
10
|
Liu XD, Xue YY, Xie L, et al:
Pharmacokinetics of ferric acid in rat after oral administration of
Radix Angelica Sinensis, Rhizoma Chuanxiong and their compound
preparations. Journal of China Pharmaceutical University.
34:448–451. 2003.(In Chinese).
|
|
11
|
Hayami T, Funaki H, Yaoeda K, Mitui K,
Yamagiwa H, Tokunaga K, et al: Expression of the cartilage derived
anti-angiogenic factor chondromodulin-I decreases in the early
stage of experimental osteoarthritis. J Rheumatol. 30:2207–2217.
2003.PubMed/NCBI
|
|
12
|
Hoşnuter M, Babucçu O, Kargi E, et al: Rat
skin surface measurement: a practical formula. Plast Reconstr Surg.
112:1486–1487. 2003.
|
|
13
|
Mankin HJ, Dorfman H, Lippiello L, et al:
Biochemical and metabolic abnormalities in articular cartilage from
osteo-arthritic human hips. The Journal of Bone and Joint Surgery.
53:523–537. 1971.PubMed/NCBI
|
|
14
|
Peat G, McCarney R and Croft P: Knee pain
and osteoarthritis in older adults: a review of community burden
and current use of primary health care. Ann Rheum Dis. 60:91–97.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lajeunesse D, Delalandre A,
Martel-Pelletier J and Pelletier JP: Hyaluronic acid reverses the
abnormal synthetic activity of human osteoarthritic subchondral
bone osteoblasts. Bone. 33:703–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benedetti S, Canino C, Tonti G, Medda V,
Calcaterra P, Nappi G, Salaffi F and Canestrari F: Biomarkers of
oxidation, inflammation and cartilage degradation in osteoarthritis
patients undergoing sulfur-based spa therapies. Clin Biochem.
43:973–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad R, El Mabrouk M, Sylvester J and
Zafarullah M: Human osteoarthritic chondrocytes are impaired in
matrix metalloproteinase-13 inhibition by IFN-gamma due to reduced
IFN-gamma receptor levels. Osteoarthritis Cartilage. 17:1049–1055.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beekman B, Drijfhout JW, Bloemhoff W,
Ronday HK, Tak PP and te Koppele JM: Convenient fluorometric assay
for matrix metalloproteinase activity and its application in
biological media. FEBS Lett. 390:221–225. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith GN Jr: The role of collagenolytic
matrix metalloproteinases in the loss of articular cartilage in
osteoarthritis. Front Biosci. 11:3081–3095. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta K, Shukla M, Cowland JB, Malemud CJ
and Haqqi TM: Neutrophil gelatinase-associated lipocalin is
expressed in osteoarthritis and forms a complex with matrix
metalloproteinase 9. Arthritis Rheum. 56:3326–3335. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makowski GS and Ramsby ML: Zymographic
analysis of latent and activated forms of matrix
metalloproteinase-2 and -9 in synovial fluid: correlation to
polymorphonuclear leukocyte infiltration and in response to
infection. Clin Chim Acta. 329:77–81. 2003. View Article : Google Scholar
|
|
22
|
Cawston TE, Weaver L, Coughlan RJ, Kyle MV
and Hazleman BL: Synovial fluids from infected joints contain
active metalloproteinases and no inhibitory activity. Br J
Rheumatol. 28:386–392. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie WZ, Fan CS and Zhu ZY: Quan Guo Zhong
Cao Yao Hui Bian. People’s Medical Publishing House; Beijing: pp.
423–424. 1996
|
|
24
|
Ge JR, Wang HM, Yang LZ, Yin HB, Feng XH,
Jiang Q, et al: II Period clinical trial of compound Du Zhong Jian
Gu grain treating knee osteoarthritis. Chinese Journal of
Traditional Medical Traumatology & Orthopedics. 10(5): 19–23.
2002.(In Chinese).
|
|
25
|
Tang X, Xu D, Mei X and Xu S: Advances in
pharmacological study of 26 kinds of natural active flavones. Zhong
Yao Cai. 26:46–54. 2003.(In Chinese).
|
|
26
|
Zhou CY, Wang M, Zhen Y, et al:
Anti-inflammatory and analgesic effects of Eucommia. Journal of
China Coal Industry Medicine. 12:1613–1615. 2009.(In Chinese).
|
|
27
|
Du JW, Zhong QZ and Xie ML: Effects of
Gushu Tang on chondrocyte apoptosis in experimental knee
osteoarthritis rabbits. Current Chinese Medicine. 30:57–58.
2010.(In Chinese).
|
|
28
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|