Introduction
Depression is a psychiatric disorder that presents
as a reduction in self-confidence and a lack of confidence in the
world and the future (1). Its core
symptoms involve a depressed mood, anhedonia, irritability,
difficulties in concentrating and abnormalities in appetite and
sleep (2). Interestingly,
Gan-Zhu-Shuxie (GZSX) theory, derived from traditional Chinese
medicine (TCM), has been implicated in mood, digestion and
reproduction (3). The dysfunction
of GZSX may result in depression and a series of comorbidities,
such as functional gastrointestinal and reproductive disorders.
Chaihu-Shugan-San (CSS) has been demonstrated to act
as an antidepressant with polypharmacological mechanisms (4). CSS has been used for several
centuries to improve certain symptoms similar to depression
(5). Importantly, we previously
isolated meranzin hydrate (MH) from CSS by targeting the unknown
absorbed compound that was found in the blood of patients with
depression following oral CSS administration (6). Furthermore, we isolated MH from
Fructus Aurantii (FA) for the first time (Xi H, Ping R and Feng Q:
Application and preparation of bitter orange extract meranzin
hydrate for preparing enterocinetic kinetic edicaments. Filed
January 8th 2008; issued January December 1, 2011), and conducted
further studies with the compound (7,8).
To the best of our knowledge, there have been no
studies simultaneously investigating the pharmacokinetic parameters
of MH in a rat model of depression and in healthy rats following
CSS administration. Chronic mild stress (CMS) in rats is a model of
depression. The aim of this study was to evaluate and compare the
pharmacokinetics of MH in the two types of rats.
A rapid, sensitive, simple and accurate
ultra-performance liquid chromatography with photodiode array
(UPLC-PDA) method to determine MH levels in the plasma of CMS rats
and controls was developed and successfully applied in this
pharmacokinetic study.
Materials and methods
Crude drugs
The CSS decoction included seven crude drugs:
Bupleurum root, Pericarpium Citri Reticulatae, Chuanxiong Rhizoma,
Rhizoma Cyperi, Fructus Aurantii, Paeonia and Radix Glycyrrhizae in
a ratio of 8:5:5:5:5:3:2. All the dry herbs were purchased from
Xiangya Hospital, Central South University (Changsha, China) and
identified by the directing pharmacist, Xinzhong Li (Xiangya
Hospital, Central South University). The voucher specimen (no.
20120910) was deposited in the laboratory of ethnopharmacology
(Xiangya Hospital), prior to the herbs being immersed in distilled
water (1:8, g/ml) for 1 h and boiled twice for 30 min. The blended
supernatants were lyophilized to obtain a powdered form of CSS,
which then was stored at 4°C until use.
Chemicals and reagents
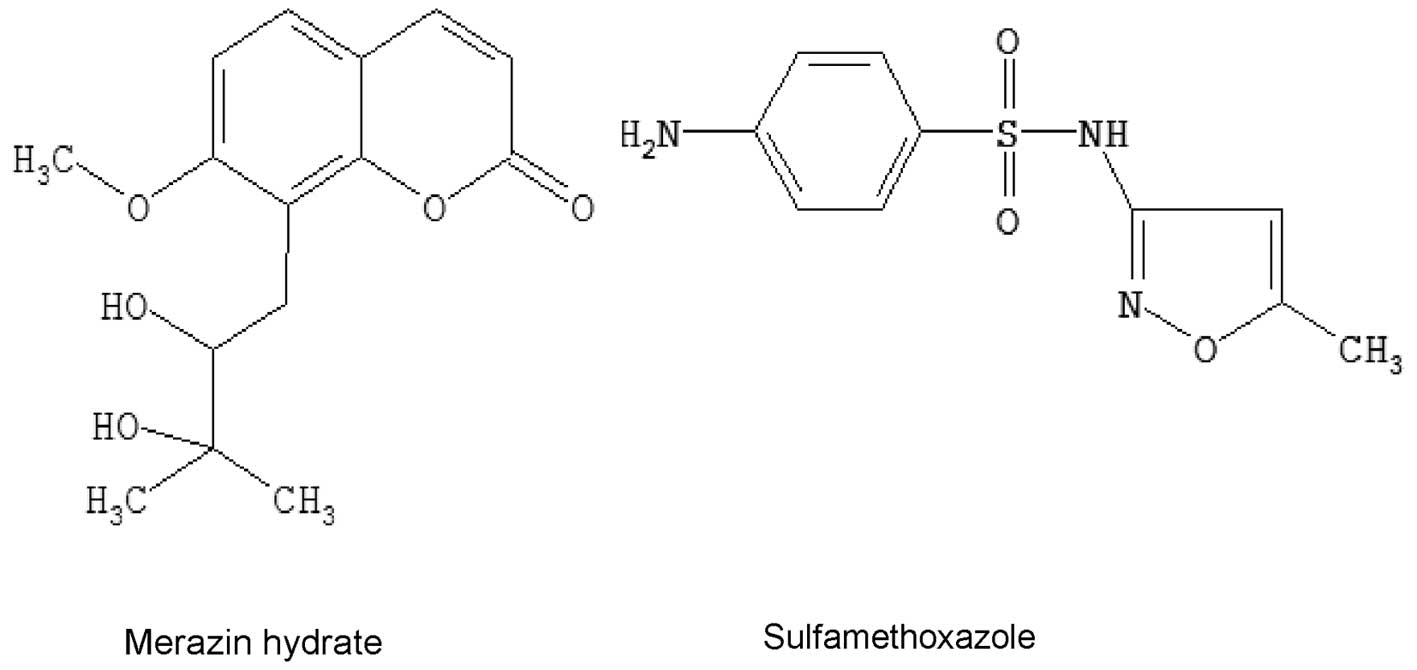
MH was purchased from the Huaxi Medical University
Medicine Factory (Chengdu, China), while sulfamethoxazole (SMZ; CAS
No. 723-46-6, USP 98%) was provided by the National Institute for
Food and Drug Control (Beijing, China) and was used as an internal
standard (IS). The chemical structures of MH and SMZ are shown in
Fig. 1. Acetonitrile and methanol
[high-performance liquid chromatography (HPLC) grade] were obtained
from Tedia Co., Inc. (Fairfield, OH, USA). All other reagents were
of analytical grade. House triple-distilled water from silica glass
equipment was always used.
Chromatographic conditions
The Acquity UPLC system consisted of a binary
solvent manager, a sample manager, a column heater and a PDA
detector and was acquired from Waters Corp. (Milford, MA, USA). The
PDA optical detector was an ultraviolet spectrophotometer that
operated between 190 and 500 nm and the analytical column was a
Waters BEH C18 column (2.1×100 mm i.d.) with a particle size of 1.7
μm. The mobile phase comprised acetonitrile (A) and 0.5%
aqueous acetic acid (B) with a gradient mode of 0–1 min, 3–3% A;
1–3 min, 3–15% A; 3–5 min, 15–18% A; 5–7 min, 18–20% A and 7–9 min,
20–30% A, v/v. The column temperature was maintained at 40°C and
the autosampler was conditioned at 25°C. The flow rate was 0.4
ml/min and the injection volume was 6 μl.
Determination of MH in the CSS
decoction
The lyophilized powder of CSS was dissolved in
distilled water and an aliquot (1.0 ml) of the solution was
extracted with methanol (9.0 ml). The extract solution was vortexed
for 3 min and subsequently centrifuged for 10 min at 12,000 × g.
The supernatant solution was filtered through a 0.22 μM
filter unit (EMD Millipore Corporation, Billerica, MA, USA), prior
to UPLC analysis. The content of MH in CSS was measured under the
aforementioned chromatographic conditions.
Animals
Experiments were performed using male Sprague Dawley
rats (weight, 200–220 g) provided by the Animal Experimental Center
in Kaifu District (Changsha, China). All experiments conformed to
the Regulations for the Administration of Affairs Concerning
Experimental Animals (1988), and were approved by the Animal
Experimental Center for Central South University. Animals were
housed in a temperature-controlled facility with a 12-h light/dark
cycle. The animals were acclimatized to the facilities for one week
and were then randomly divided into two groups: i) the healthy
control group (n=8) and ii) the CMS model group (n=7).
CMS procedure
The CMS stressors were adapted from the procedure
described by First et al (9) and the procedure consisted of a
variety of unpredictable mild stressors, including: two periods (18
h) of grouped caging, with four rats per cage (usually CMS rats
were housed like the controls, one per cage); two periods (6 h) of
cage tilting (tilted by 30° on a wooden board); one period (24 h)
of food deprivation; two periods (24 and 46 h) of water
deprivation; one period (18 h) of a wet cage (200 ml water spilled
in each cage); two periods (3 h) of stroboscopic lightning (a
flashlight flickering at 300 flashes/min in a dark room); two
periods (3 h) of white noise (a non-tuned radio on high volume) and
one 48-h period of continuous light. These stressors were randomly
scheduled over a one-week period and repeated throughout the
five-week procedure.
The non-stressed control animals were housed in
constant conditions, with one rat per cage, i.e. similar to the
stressed animals, but without any manipulations. We were aware of
the fact that social isolation by itself is a stressor for rodents,
who are naturally social animals; however, we overcame this
limitation by exposing all groups to the same housing
conditions.
Open field test
The open field test was performed in accordance with
a previous study (10), in order
to measure spontaneous activity in rodents. Briefly, the apparatus,
consisting of a black square cage measuring 100×100×40 cm, was
divided into 25×25 cm equal squares on the floor of the arena. The
test room was dimly illuminated. A single rat was placed in the
center of the cage and, following 30 sec of adaptation, the
crossing number (CN, i.e. a rat stepping from one square to another
with its rear legs) was utilized as the measurement parameter. A
separate researcher, who was blind to the treatment group, scored
the behavior in the open field. Following each test the arena was
cleaned with 90% alcohol solution.
Forced swimming test
In accordance with the study by Porsolt et al
(11), the forced swimming test
was conducted by placing the rat in a Plexiglas®
cylinder (40 cm tall, 30 cm in diameter) filled to a height of
21.5±1.5 cm with water at a temperature of 24±0.5°C. The rats were
left to swim in the cylinder under conditions where escape was not
possible. To avoid additional stress on the animals, the original
forced swimming test described by Porsolt et al (11) was modified by performing a single
test session lasting for 5 min, during which the animals were
videotaped from a vantage point above the cylinders in a dimly
illuminated room. The duration of immobility, which was defined as
the lack of motion of the whole body, except for small movement
necessary to keep the animal’s head above the water, was recorded.
Subsequent to each test, the cylinder was cleaned.
Preparation of standard and quality
control (QC) samples
Standard stock solutions were prepared by dissolving
MH and SMZ in methanol to yield nominal concentrations of 64
μg/ml and 100 μg/ml, respectively, for storage at
4°C. The solutions were subsequently further diluted in methanol to
produce working standards. Calibration samples of MH (2.5, 10, 40,
80, 160, 320 and 640 ng/ml) were prepared by spiking 1 ml blank
plasma with appropriate quantities of working standard solutions
and SMZ (IS, 50 μl). QC samples of MH were independently
prepared at three different concentration levels (10, 40 and 160
ng/ml) to determine the recovery, accuracy and the precision of the
method. All the plasma samples were stored at −20°C, prior to
analysis.
Plasma sample preparation
The blank plasma (1 ml) in a centrifuge tube (5 ml)
was added to different amounts of MH (2.5–640 ng, 50 μl), a
fixed quantity of SMZ (5,000 ng, 50 μl) and 850 μl
methanol. The mixture was mixed thoroughly by ultrasound and
vortexed for 30 sec. Following this, the denatured protein
precipitate was separated by centrifugation at 3,000 × g for 15 min
at 4°C and the supernatant was transferred to another tube and
evaporated to dryness in a water bath at 50°C under a stream of
nitrogen. The residues were reconstituted in methanol (50
μl), prior to being vortexed for 15 sec. The centrifugation
procedure was repeated as mentioned previously and then 6.0
μl supernatant solution was injected into the UPLC system
for analysis.
Calibration curve and limit of
quantification (LOQ)
Standard samples of MH (2.5–640 ng/ml) and 5,000 ng
SMZ (IS) in plasma were prepared as previously mentioned. Standard
curves were established following the extraction and UPLC analyses
of the spiked plasma samples. Following the determination of the
peak-area ratios of MH to SMZ in the UPLC chromatograms, the
calibration curve was established by least-squares linear fitting
of the peak-area ratios of MH to the IS. The LOQ was defined as the
lowest concentration.
Precision and accuracy
The intra-day accuracy and precision were assessed
by determining QC samples at three concentration levels of MH (10,
40 and 160 ng/ml) on the same day (n=6). The inter-day accuracy and
precision were also evaluated from the analysis of the QC samples
on three consecutive days (n=6). Precision was expressed as
relative standard deviation (RSD) and accuracy was expressed as
[(mean detected concentration-added concentration)/(added
concentration)] x 100.
Recovery
The relative recoveries of MH from rat plasma were
determined using the QC samples (n=6). The peak-area ratios (MH to
SMZ) of the UPLC chromatograms were compared with those of
reference solutions to calculate the relative recoveries of MH.
Stability in rat plasma
The short-term, long-term and freeze-thaw
stabilities of MH in plasma were assessed using QC samples (n=6).
Short-term stability was assessed by analyzing QC plasma samples
kept at room temperature for 4 h, which exceeded the routine
preparation time of the samples. Long-term stability was determined
by assaying QC plasma samples following storage at −20°C for 14
days. Freeze-thaw cycles (−20°C/room temperature) were also applied
to QC samples to investigate the freeze-thaw stability of MH. In
each freeze-thaw cycle, the samples were frozen and stored at −20°C
for 24 h, and subsequently thawed at room temperature.
Pharmacokinetic study
Following the behavior tests, the two groups were
fasted with free access to water for 12 h prior to the experiment.
CSS was orally administered to the rats at a dose of 30 g/kg (for
raw medicinal materials). Blood samples (0.3 ml) were collected in
heparinized tubes at 0 (prior to administration), 5, 10, 15, 45,
60, 120, 240, 300, 360, 480, 720 and 1,440 min subsequent to
administration, left for 30 min at room temperature and then
centrifuged at 12,000 × g for 10 min at 4°C, in order to obtain the
plasma. The supernatant was transferred and stored in 0.5-ml
polypropylene tubes at −20°C prior to analysis. The next step was
performed following the plasma sample preparation. The
concentrations of MH in the rat plasma were determined at each
time-point. Data from those samples were used to construct the
pharmaco-kinetic profiles by plotting drug concentration versus
time curves. Pharmacokinetic parameters, including area under the
concentration-time curve (AUC(0-t)), maximum plasma
concentration (Cmax), time to reach the maximum
concentration (Tmax), clearance rate (CL/F) and mean
residence time (MRT(0-t)), were estimated using
statistical moment analysis with Drug and Statistics 2.0 (DAS 2.0)
software (Mathematical Pharmacology Professional Committee of
China, Shanghai, China).
Statistical analysis
All data are expressed as the mean ± standard
deviation. The database was set up with the SPSS 16.0 software
package from SPSS, Inc. (Chicago, IL, USA). Differences between the
two groups were analyzed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The content of MH in the CSS decoction was
calculated to be 113.16 μg/g. Typical chromatograms of
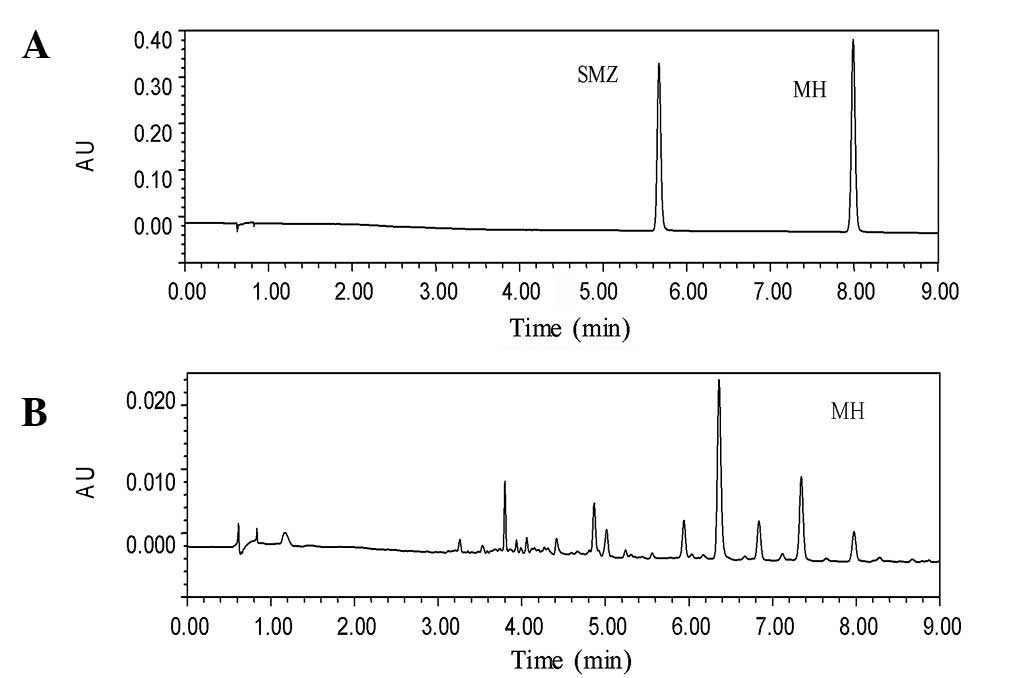
authentic standards and the CSS test sample are shown in Fig. 2, while representative chromatograms
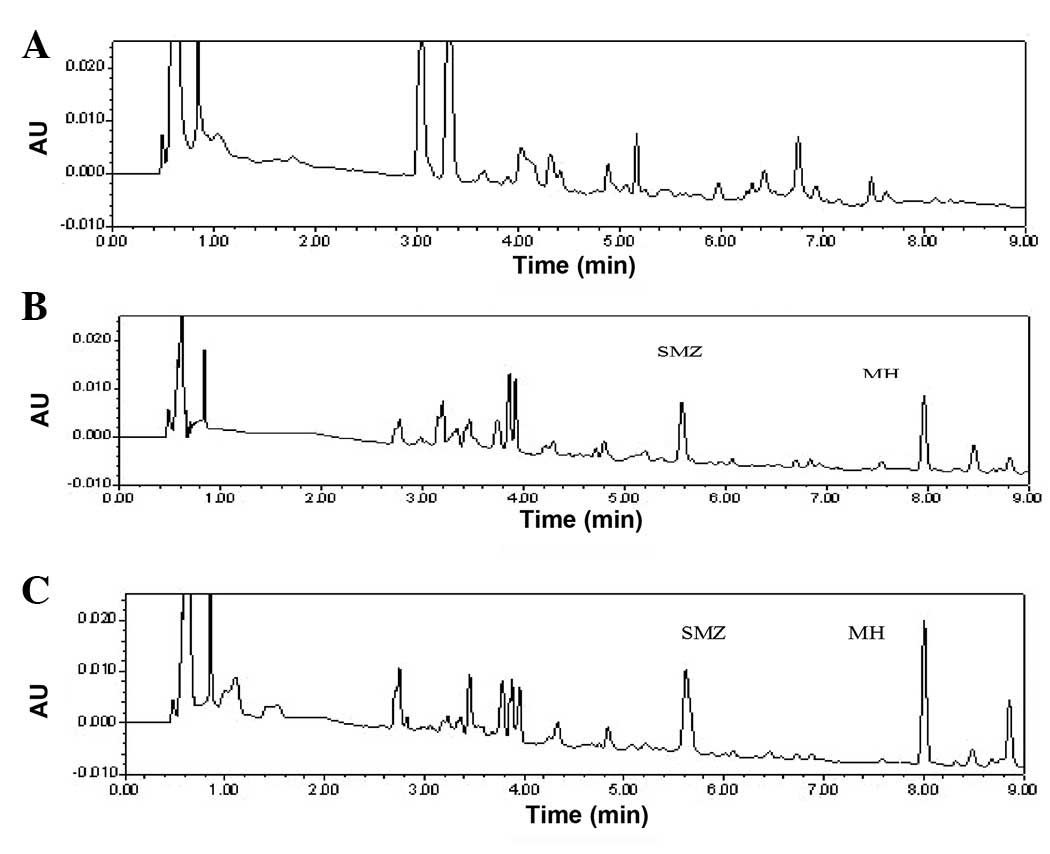
of a blank control sample, spiked plasmas and a subject sample are
depicted in Fig. 3. MH and SMZ in
the plasma were completely separated, without significant
interference. The retention times of MH and SMZ were 7.9 and 5.6
min, respectively, and it was observed that the calibration curve
was linear over the concentration range of 2.5–640 ng/ml in the rat
plasma. The representative regression equation of the calibration
curve was y=315.3 and x=−2.589 (r2=0.9995, n=6) and the
LOQ and limit of detection (LOD) were 3 and 1 ng/ml, respectively.
The precision and accuracy of the method are summarized in Table I. The intra- and inter-day
precisions were ≤1.63 and ≤1.54%, respectively, and the mean
recovery ratios of MH at concentrations of 10, 40 and 160 ng/ml
were demonstrated to be 94.25±1.69, 95.67±1.57 and 97.76±1.37%,
respectively with all RSDs ≤1.79% (Table II). Table III summarizes the results of the
short-term, long-term and freeze-thaw stability of MH in rat
plasma. The stability of MH in rat plasma was acceptable in the
present study.
 | Table I.Precision and accuracy of the UPLC
method for MH in rat plasma (n=6). |
Table I.
Precision and accuracy of the UPLC
method for MH in rat plasma (n=6).
| Nominal concentration
(ng/ml) | Intra day (n=6) | Inter day (n=6) |
|---|
|
|
|---|
| Mean ± SD
(ng/ml) | RSD (%) | Mean ± SD
(ng/ml) | RSD (%) |
|---|
| 10 | 9.67±0.13 | 1.34 | 9.87±0.12 | 1.22 |
| 40 | 39.21±0.52 | 1.33 | 39.01±0.60 | 1.54 |
| 160 | 153.07±2.50 | 1.63 | 150.72±1.53 | 1.01 |
 | Table II.Recovery of MH from rat plasma. |
Table II.
Recovery of MH from rat plasma.
| Concentration
(ng/ml) | Recovery (%) (mean ±
SD) | RSD (%) |
|---|
| 10 | 94.25±1.69 | 1.79 |
| 40 | 95.67±1.57 | 1.64 |
| 160 | 97.76±1.37 | 1.40 |
 | Table III.Stability of MH in rat plasma at three
QC levels (n=6). |
Table III.
Stability of MH in rat plasma at three
QC levels (n=6).
| Stability | Nominal concentration
MH (ng/ml)
|
|---|
| 10 | 40 | 160 |
|---|
| Short term
stability | 9.49±0.61 | 38.61±0.70 | 147.43±3.05 |
| Long term
stability | 9.28±0.75 | 38.44±0.65 | 147.25±2.21 |
| Freeze thaw
stability | 9.18±0.47 | 38.35±0.59 | 146.63±2.06 |
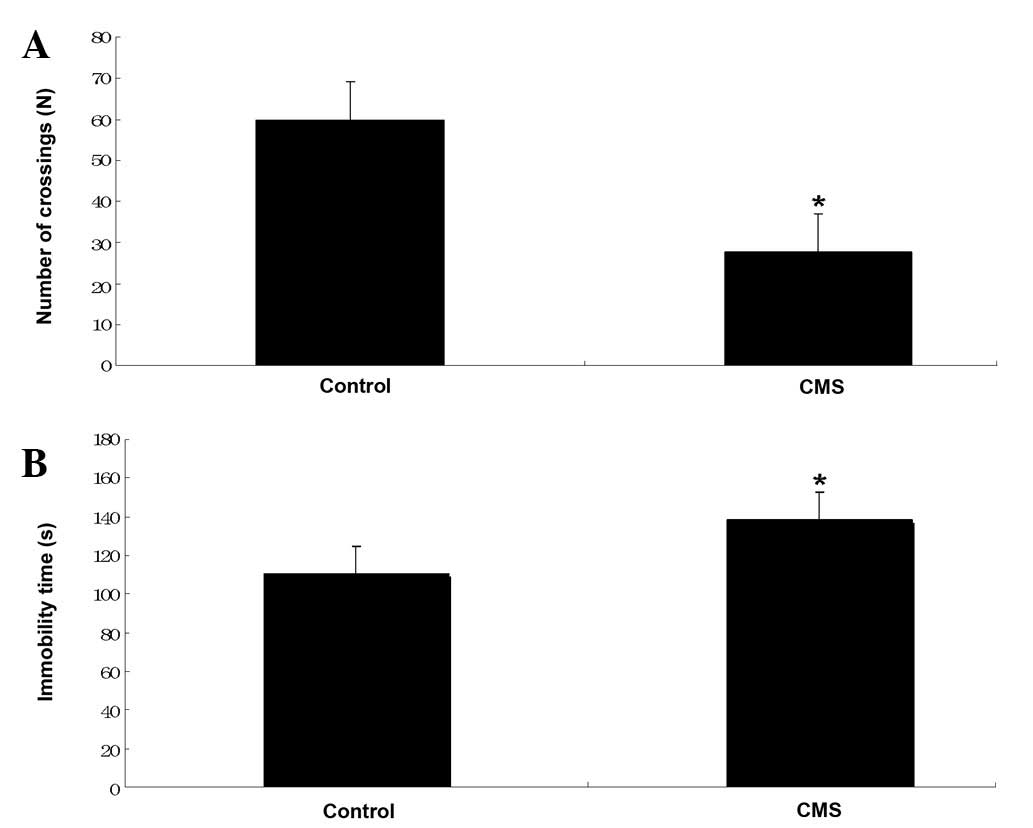
The immobility time (sec) and the number of
crossings (n) observed for the rats with CMS were compared with the
values obtained for the control rats in Fig. 4. The immobility time was shown to
increase significantly in the CMS rats (138.4±11.4 versus 110.5±7.2
sec for the control), while the number of crossings was reduced
(27.7±11.0 versus 59.8±26.4 for the control). These data indicated
the success of the model of chronic depression.
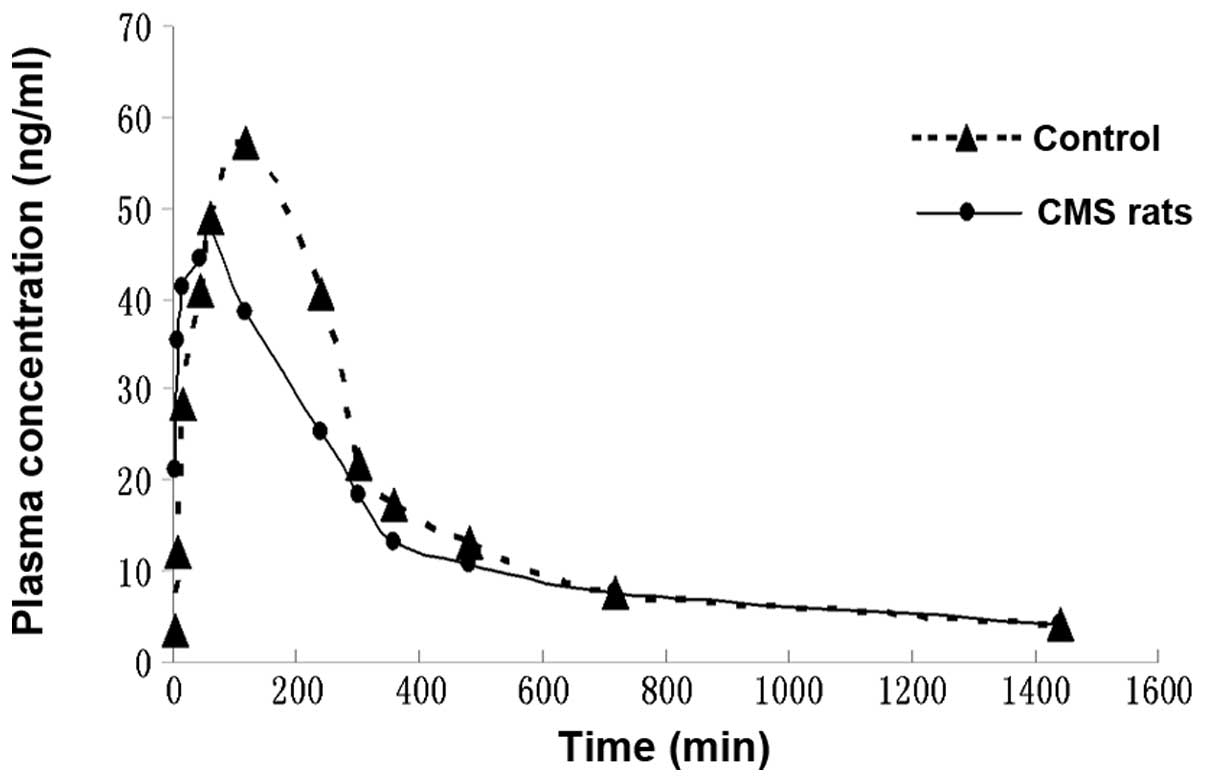
The plasma MH concentration-time curves were
analyzed using DAS software (Mathematical Pharmacology Professional
Committee of China) on a personal computer to determine the
compartment model, and the plasma concentration-time curve of MH
was fitted with a two-compartment model. The mean plasma
concentration versus time profiles of MH in the CMS rats and the
controls, following the oral administration of CSS, are illustrated
in Fig. 5. The main
pharmacokinetic parameters, including the Tmax,
Cmax, half-life (T1/2), Ka,
AUC0-1440, MRT0-1440 and CL/F were calculated
for each group and are listed in Table
IV.
 | Table IV.Pharmacokinetic parameters of MH in
plasma following the oral administration of CSS to CMS and control
rats. |
Table IV.
Pharmacokinetic parameters of MH in
plasma following the oral administration of CSS to CMS and control
rats.
| Parameter | CMS rats | Control rats |
|---|
| Tmax
(min) | 54.000±8.216a | 108.000±26.830 |
| Cmax
(μg/l) | 57.544±12.673 | 58.664±6.640 |
| AUC0-1440
(μg·min/l) |
18401.317±4332.648 |
19896.758±1041.950 |
| T1/2
(min) | 145.635±75.671 | 87.338±31.145 |
| Ka
(1/min) | 0.083±0.074 | 0.021±0.009 |
| CL/F (l/min/kg) | 1442.188±391.815 | 1445.447±77.808 |
| MRT0-1440
(min) | 409.953±43.412 | 378.751±13.028 |
Following the oral administration of CSS to the
control rats, MH was absorbed and reached a Cmax value
of 58.66±6.64 μg/l within 108.00±26.83 min. The plasma
concentration of MH declined with a T1/2 value of
87.34±31.15 min. However, following the oral administration of CSS
to the CMS rats, the Cmax value of MH was 57.54±12.67
μg/l within 54.00±8.22 min, and the plasma concentration of
MH declined with a T1/2 value of 145.64±75.67 min.
Discussion
Previous studies have implicated the usage of MH in
anti-cancer (12), antibacterial
and anticoagulation (13)
treatments. However, we have identified additional effects
exhibited by MH. In vivo, MH (28 mg/kg) significantly
accelerated gastric emptying and intestinal transit in rats, and
also directly increased the amplitude of rat ileum contraction
in vitro (8). Furthermore,
in vivo MH (1–100 μM) dose-dependently increased the
amplitude of contractility in the longitudinal and circular jejunum
muscles of rats, and this was, at least partially, mediated by the
stimulation of H1 histamine receptors (14). Therefore, MH was selected as the
target to compare the pharmacokinetic profiles in CMS rats and
control rats.
The pharmacokinetic profile of MH in rat plasma was
fitted with a two-compartment model, detected by a simple, rapid
and accurate UPLC method. SMZ was selected as the appropriate IS,
as it was stable and did not exist in rat blank control plasma. The
total analysis time was 9.0 min and the retention time of MH was
7.9 min, which was significantly shorter than that achieved in a
previous method, with better resolution (6–8).
The acceptable peak shape and satisfactory
separation of MH and SMZ from endogenous components were achieved
in rat plasma under the previously mentioned chromatographic
conditions. The method was validated for linearity, accuracy,
precision, LOQ and recovery and was successfully applied to the
pharmacokinetic study of MH in CMS rats and controls.
The pharmacokinetic parameters determined in the
current study showed that CMS accelerated the absorption of MH in
rats following oral administration of CSS. There were differences
between our study and others. A previous study investigated the
potential effect of MH and a decoction of the herb, FA, on rat gut
motility, in addition to investigating the prokinetic mechanism of
MH (14). The study utilized
normal rats, not CMS rats, in contrast to the present study
(14). Another study aimed to
identify an antidepressive compound found in TCM by a novel
approach known as ethnopharmaco-kinetic- and activity-guided
isolation (EAGI) (6). In this
study, MH, a compound whose antidepressive effect is similar to FA
and CSS, was isolated for the first time from FA (6). This was achieved by targeting its
corresponding unknown chromatographic peak, and its antidepressive
effect was compared with FA or CSS (6). Similar to the present study, UPLC was
used, as a development of the chromatographic technique, in the
quality control of the TCM, CSS. However, the study did not
describe the pharmacokinetic parameters of MH in a rat model of
depression, in addition to healthy rats, following CSS
administration (6). Thus, to the
best of our knowledge, the present study has shown for the first
time that CMS accelerated the absorption of MH in rats following
the oral administration of CSS.
Our experiments have compared the pharmacokinetics
of MH in a rat model of chronic depression and control rats
following the oral administration of CSS. CSS is one of many
traditional Chinese antidepressant drugs. The Chinese herbal
formula, Mood Smooth (Jia Wei Xiao Yao Wan), has been in use for
six hundred years in China as a treatment for depression. The
Chinese refer to this remedy as ‘the happy pill’, due to its
well-known antidepressant effect. It has been used by millions of
people over the centuries, and is particularly popular with
females. Other common remedies for depression include: Spleen tonic
herbal formula, Chi Spleen Tonic (Bu Zhong Yi Qi Wan); kidney
nourishing herbal formula, Kidney Yang Tonic (Jin Gui Shen Qi Wan)
and numerous other remedies that are widely used for different
patterns of depression. Further studies to investigate the
pharmacokinetic effects of other orally administered traditional
Chinese antidepressant drugs in rat models of chronic depression
and control rats are required.
Acknowledgements
This study was supported by the
key-discipline construct programs of Hunan province and SATCM, by
grant nos. 81072967 and 30572339 from the Natural Science
Foundation of China, and by the Key New Drug Creation Fund of the
Important National Science and Technology Specific Projects of the
Ministry of Science and Technology and Ministry of Public Health,
the twelve five-year plan of state (no. 2011ZX09101009-03).
References
|
1.
|
Lavergne F and Jay TM: A new strategy for
antidepressant prescription. Front Neurosci. 4:1922010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nestler EJ, Barrot M, DiLeone RJ, Eisch
AJ, Gold SJ and Monteggia LM: Neurobiology of depression. Neuron.
34:13–25. 2002. View Article : Google Scholar
|
|
3.
|
Clerbaux T, Detry B, Geubel A, Veriter C,
Liistro G, Horsmans Y and Frans A: The oxyhemoglobin dissociation
curve in liver cirrhosis. Chest. 129:438–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Xiong XY and Wang GX: The holistic
treatment for depression. Medicine and Philosophy. 28:66–67.
2007.(In Chinese).
|
|
5.
|
Kim SH, Han J, Seog DH, Chung JY, Kim N,
Hong PY and Lee SK: Antidepressant effect of Chaihu-Shugan-San
extract and its constituents in rat models of depression. Life Sci.
76:1297–1306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Fan R, Huang X, Wang Y, et al:
Ethnopharmacokinetic- and activity-guided isolation of a new
antidepressive compound from Fructus Aurantii found in the
traditional chinese medicine Chaihu-Shugan-San: a new approach and
its application. Evid Based Complement Alternat Med.
2012:6075842012.PubMed/NCBI
|
|
7.
|
Hu SH, Huang X, Liang QH, et al:
Simultaneously qualitative determination of multiple compounds in
Chaihu-Shugan-San (CSS) and in rat intestine by UPLC-PDA. Journal
of Medical Research. 39:42–45. 2010.(In Chinese).
|
|
8.
|
Qiu XJ, Huang X, Chen ZQ, et al:
Pharmacokinetic study of the prokinetic compounds meranzin hydrate
and ferulic acid following oral administration of Chaihu-Shugan-San
to patients with functional dyspepsia. J Ethnopharmacol.
137:205–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
First M, Gil-Ad I, Taler M, Tarasenko I,
Novak N and Weizman A: The effects of fluoxetine treatment in a
chronic mild stress rat model on depression-related behavior, brain
neurotrophins and ERK expression. J Mol Neurosci. 45:246–255. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Blokland A, Lieben C and Deutz NE:
Anxiogenic and depressive-like effects, but no cognitive deficits,
after repeated moderate tryptophan depletion in the rat. J
Psychopharmacol. 16:39–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Porsolt RD, Bertin A and Jalfre M:
Behavioral despair in mice: a primary screening test for
antidepressants. Arch Int Pharmacodyn Ther. 229:327–336.
1977.PubMed/NCBI
|
|
12.
|
Riviere C, Goossens L, Pommery N, Fourneau
C, Delelis A and Henichart JP: Antiproliferative effects of
isopentenylated coumarins isolated from Phellolophium
madagascariense Baker. Nat Prod Res. 20:909–916. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Rosselli S, Maggio A, Bellone G, et al:
Antibacterial and anticoagulant activities of coumarins isolated
from the flowers of Magydaris tomentosa. Planta Med.
73:116–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Huang W, Huang X, Xing Z, et al: Meranzin
hydrate induces similar effect to Fructus Aurantii on intestinal
motility through activation of H1 histamine receptors. J
Gastrointest Surg. 15:87–96. 2011. View Article : Google Scholar : PubMed/NCBI
|