Introduction
Percutaneous coronary intervention (PCI), also known
as coronary angioplasty, is a non-surgical method used to treat
narrowed coronary arteries that supply the heart muscle with blood
(1). PCI has been clinically
applied for almost 30 years and has become one of the main
treatments for coronary heart disease (CHD) (2). PCI with coronary stent implantation
has been demonstrated to consistently reduce the symptoms of
coronary artery disease and decrease cardiac ischemia; however, PCI
has not been shown to reduce mortality rates in large clinical
trials (3). The implantable
vascular stents used during PCI procedures appear to increase the
risk of coronary artery intimal injury and platelet activation, and
may thereby increase the risk of thrombosis (4). This is significant, as the 1-year
mortality rate of patients with myocardial infarction (MI) induced
by thrombotic diseases is ∼15.8% (5). Therefore, antiplatelet therapy has
become the focus of basic interventional cardiology studies and has
received increased clinical attention in the last decade (6).
Dual antiplatelet therapy (DAT; aspirin and
clopidogrel) is a mainstay of medical treatment following PCI,
which reduces the risk of occurrence of stent thrombosis. However,
studies have demonstrated that aspirin and clopidogrel resistance
are occurring clinically with potentially severe consequences,
including recurrent MI, stroke or mortality (7). Therefore, triple antiplatelet therapy
(TAT; addition of cilostazol to DAT) has been increasingly studied
(8). Cilostazol is a selective
phosphodiesterase-3 inhibitor that is commonly used as a
vasodilator with antiplatelet activity in patients with peripheral
arterial disease (9). Adding
cilostazol to aspirin and clopidogrel regimens may provide a more
effective suppression of platelet P-selectin expression in patients
with relatively high platelet activity (10). In addition, TAT has also been
demonstrated to have anti-inflammatory, -apoptotic and
-proliferative effects, as demonstrated by its reduction of intimal
hyperplasia and restenosis following balloon angioplasty and stent
implantation (11). Registry data
have further identified that TAT reduces the rate of restenosis,
incidence of clinical events and stent thrombosis, compared with
DAT (12). However, controlled
clinical studies that have examined the benefits of adding
cilostazol to DAT in patients with CHD undergoing PCI with coronary
stent implantation have obtained conflicted or inconclusive
results. Therefore, the present meta-analysis aimed to compare
differences in the clinical outcomes between DAT and TAT in
patients with CHD undergoing PCI.
Methods
Literature search strategy
Relevant studies published prior to March 1, 2013,
were identified by searches in Pubmed, Embase, Web of Science and
Chinese BioMedical databases using the following terms: (‘coronary
disease’ or ‘coronary diseases’ or ‘disease, coronary’ or ‘coronary
heart disease’ or ‘heart disease, coronary’ or ‘stents’ or ‘stent’
or ‘drug-eluting stents’ or ‘bare metal stent’ or ‘percutaneous
coronary intervention’, and ‘triple antiplatelet therapy’ or ‘dual
antiplatelet therapy’ or ‘TAT’ or ‘DAT’ or ‘cilostazol’ or
‘clopidogrel’ or ‘aspirin’). References from eligible articles or
textbooks were also reviewed to find further potential sources.
Disagreements were resolved through discussion between authors.
Inclusion and exclusion criteria
Studies included in the meta-analysis were required
to meet the following criteria: i) controlled clinical study
focusing on the differences in the clinical outcomes between TAT
and DAT in patients with CHD undergoing PCI with coronary stent
implantation; ii) patients in the DAT group were treated with
aspirin and clopidogrel and patients in the TAT group were treated
with cilostazol in addition to aspirin and clopidogrel; iii) the
follow-up period was >1 month and iv) published data of the
clinical outcomes were sufficient. Studies were excluded from the
meta-analysis when they were: i) not controlled clinical studies
relevant to TAT and DAT in patients with CHD undergoing PCI with
coronary stent implantation; ii) duplicates of previous
publications; iii) based on incomplete data; or iv) meta-analyses,
letters, reviews or editorial articles. When more than one study by
the same author and using the same case series was published,
either the study with the largest sample size or the most recently
published study was included.
Data extraction
Using a standardized form, data from published
studies were extracted independently by two authors. For each
study, the following characteristics and numbers were collected:
the authors, year of publication, country, ethnicity, study design,
number of subjects, follow-up period, antiplatelet drug and dose,
rate of restenosis and clinical events [major adverse cardiac
events (MACE) and stent thrombosis]. MACE included mortality rate,
MI, target lesion revascularization (TLR) and target vessel
revascularization (TVR). In cases of conflicting evaluations, two
authors discussed the issue in order to meet a consensus; if no
agreement was reached, a third author decided.
Statistical analysis
The crude odds ratio (OR) with 95% confidence
interval (CI) was calculated. The statistical significance of the
pooled OR was examined by the Z-test. Interstudy variations and
heterogeneities were estimated using Cochran’s Q-statistic with
P<0.05 indicating a statistically significant heterogeneity
(13,14). The present meta-analysis also
quantified the effect of heterogeneity by using the I2
index (range, 0–100%), which represents the proportion of
interstudy variability attributed to heterogeneity, rather than to
chance. When a significant Q-test (P<0.05) or an I2
index of >50% was obtained, indicating that heterogeneity
existed among studies, the random effects model using the
DerSimonian and Laird method (15)
was conducted. However, when the Q-test was not significant
(P>0.05) or the I2 index was <50%, the fixed
effects model using the Mantel-Haenszel method (16) was used. To explore sources of
heterogeneity, subgroup analysis was performed by follow-up
periods. A sensitivity analysis was performed by omitting each
study in turn, to assess the quality and consistency of the
results. Begg’s funnel plot was used to detect publication bias.
Egger’s linear regression test, which measures funnel plot
asymmetry using a natural logarithm scale of OR, was also used to
evaluate publication bias (17).
To ensure the reliability and accuracy of the results, two authors
examined the data independently using statistical software programs
and obtained identical results. The P-values were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. All analyses were calculated using Stata software,
version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the included
studies
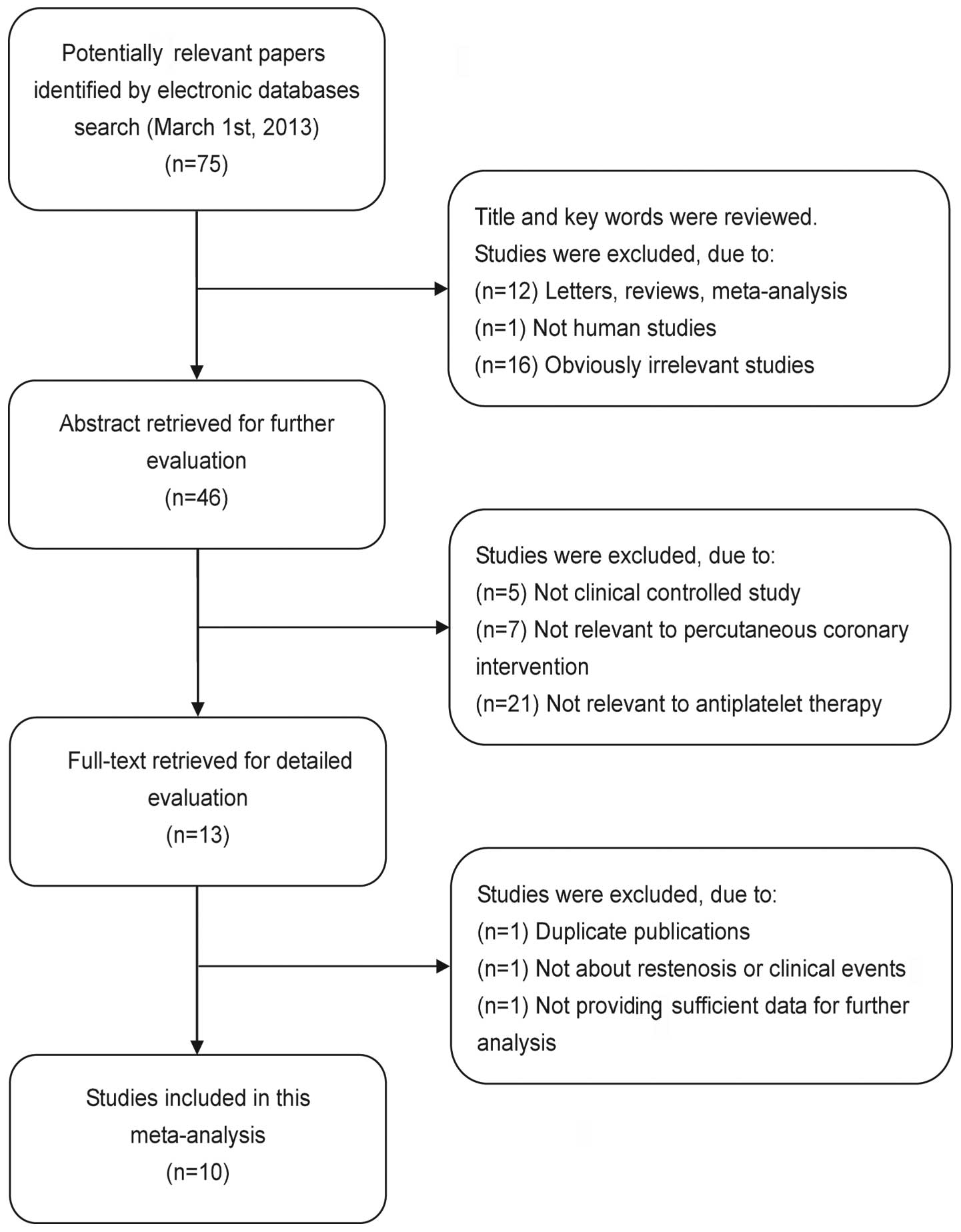
In the present meta-analysis, according to the
inclusion criteria, 10 controlled clinical studies were included
(8,12,18–25)
and 65 were excluded. The publication year of the included studies
ranged from 2005 to 2011. The flow chart of study selection is
shown in Fig. 1. A total of 7,670
patients with CHD undergoing PCI were involved in the present
meta-analysis, including 3,925 patients treated with DAT (aspirin
and clopidogrel) and 3,745 patients treated with TAT (addition of
cilostazol to DAT). The doses of aspirin ranged from 100 to 200
mg/day, those of cilostazol ranged from 100 to 200 mg/day and the
dose of clopidogrel was 75 mg/day. The main characteristics of the
eligible studies are listed in Table
I.
 | Table I.Characteristics of studies included in
this meta-analysis. |
Table I.
Characteristics of studies included in
this meta-analysis.
| Author | Year | Country | Ethnicity | | Follow-up
(months) | Drug doses | Clinical
outcomes |
|---|
|
|---|
| Number | TAT | DAT |
|---|
|
|
|
|---|
| TAT | DAT | Aspirin | Clopidogrel | Cilostazol | Aspirin | Clopidogrel |
|---|
| Douglas et
al | 2005 | USA | Caucasian | 354 | 351 | 6 | NR | 75 mg/day | 100 mg/bid | NR | 75 mg/day | Clinical events
Restenosis |
| Lee et al | 2005 | Korea | Asian | 1415 | 1597 | 1 | 200 mg/day | 75/300 mg/day | 100/200 mg/bid | 200 mg/day | 75/300 mg/day | Clinical events |
| Chen et
al | 2006 | China | Asian | 60 | 60 | 6 | 100 mg/day | 75 mg/day | 200 mg/day | 100 mg/day | 75 mg/day | Restenosis |
| Lu et al | 2006 | China | Asian | 60 | 58 | 6 | 100 mg/day | 75 mg/day | 100 mg/bid | 100 mg/day | 75 mg/day | Restenosis |
| Kim et al | 2007 | Korea | Asian | 31 | 28 | 6 | 100 mg/day | 75 mg/day | 100 mg/bid | 100 mg/day | 75 mg/day | Restenosis |
| Han et al | 2009 | Korea | Asian | 604 | 608 | 12 | 300 mg/day; 100
mg/day after 1 month | 75 mg/day | 100 mg/bid | 300 mg/day; 100
mg/day after 1 month | 75 mg/day | Clinical events |
| Lee et al | 2010 | Korea | Asian | 450 | 450 | 24 | 200 mg/day | 75 mg/day | 200 mg/day | 200 mg/day | 75 mg/day | Clinical events |
| Ahn et al | 2011 | Korea | Asian | 64 | 66 | 8 | 100 mg/day | 75 mg/day | 200 mg/day | 100 mg/day | 75 mg/day | Restenosis |
| Lee et
al | 2011 | Korea | Asian | 250 | 249 | 8 | 200 mg/day | 75 mg/day | 200 mg/day | 200 mg/day | 75 mg/day | Clinical events
Restenosis |
| Suh et
al | 2011 | Korea | Asian | 457 | 458 | 6 | 100 mg/day | 75 mg/day | 200 mg/day | 100 mg/day | 75 mg/day | Clinical
events |
Quantitative data synthesis
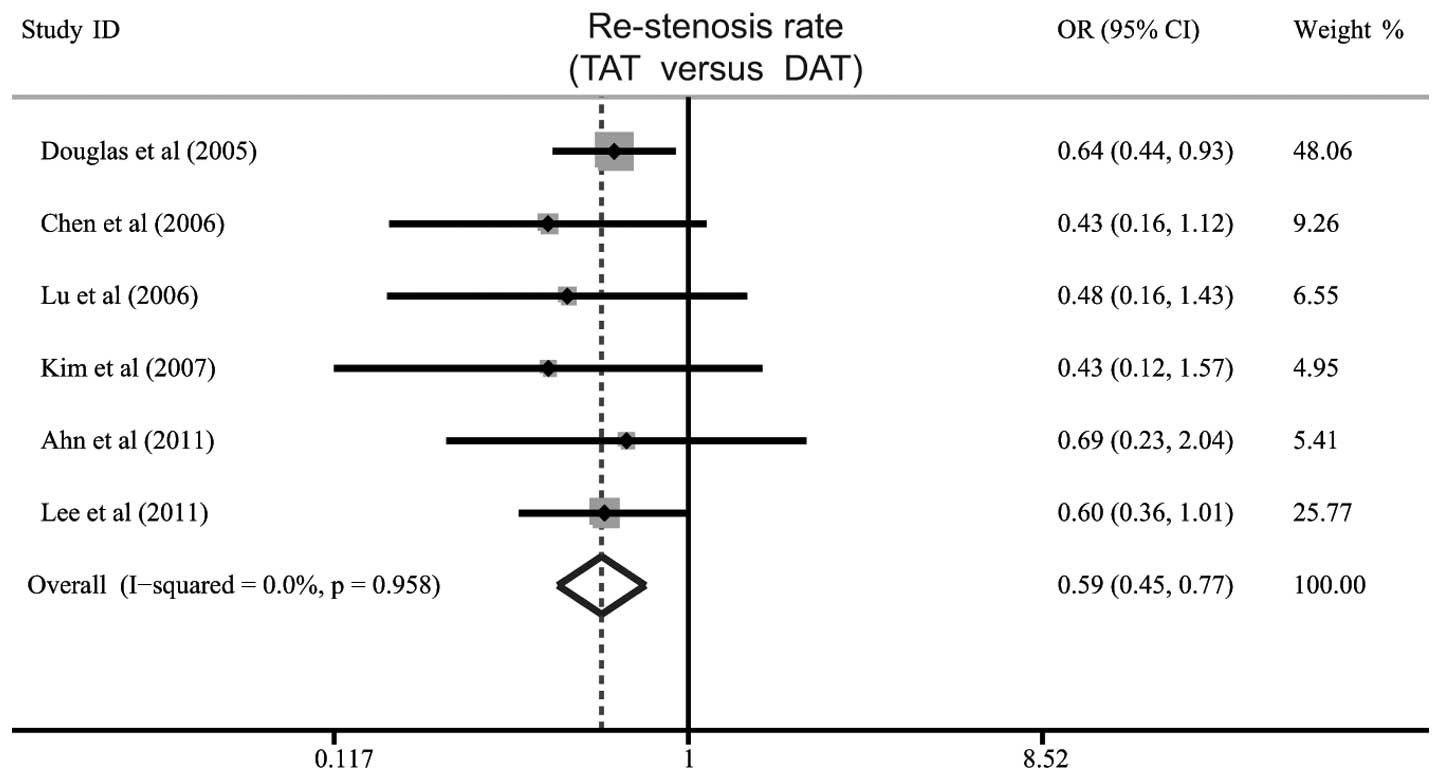
Six studies identified a difference in the rate of
restenosis between TAT and DAT for patients with CHD undergoing
PCI. The heterogeneity was not significant (P=0.958,
I2=0%), therefore, the fixed effects model was used. The
meta-analysis results showed that patients in the TAT group had a
significantly lower rate of restenosis than those in the DAT group
(OR=0.59, 95% CI: 0.45–0.77, P<0.001; Fig. 2).
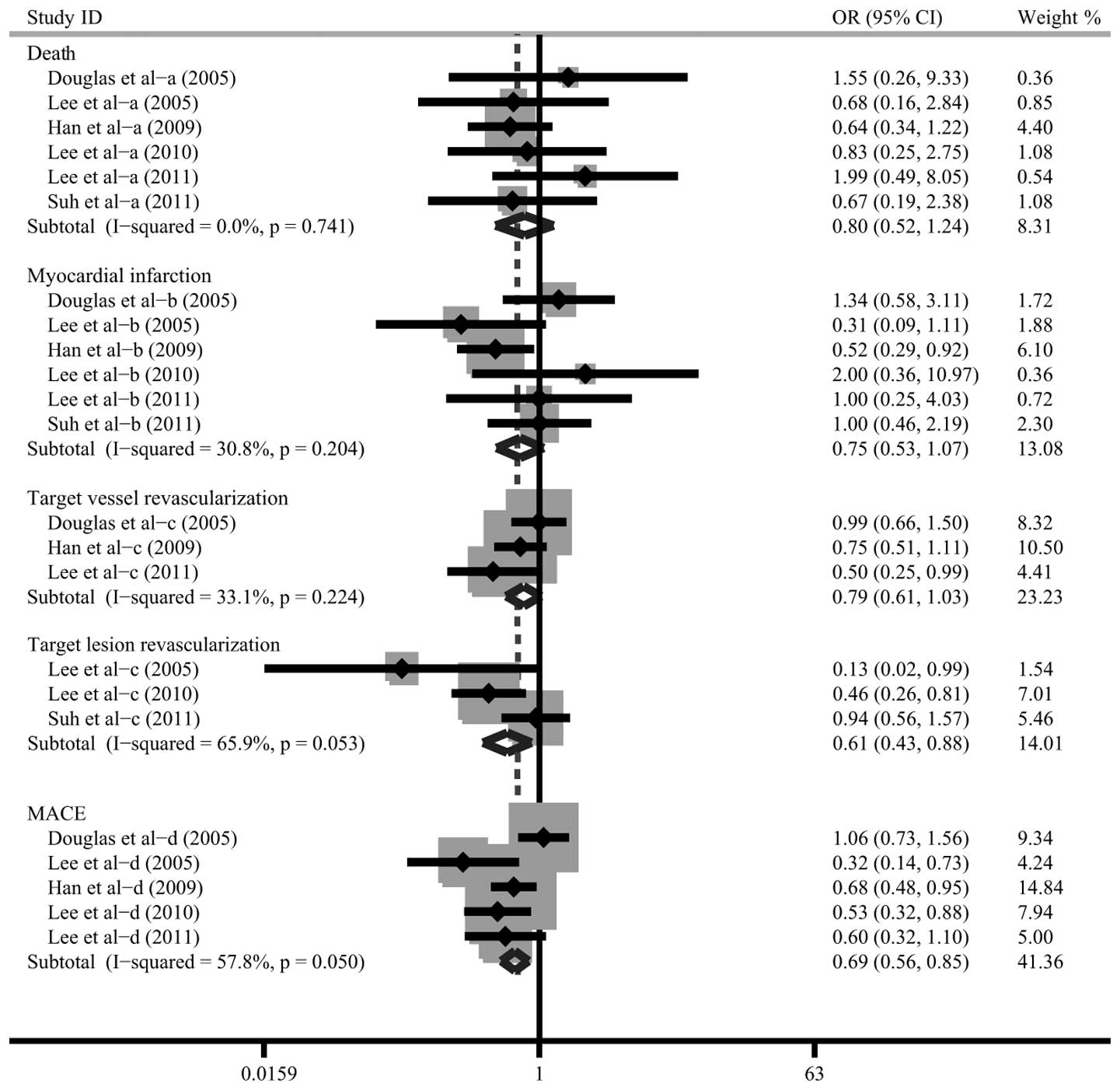
The differences in the clinical events between TAT
and DAT were compared in six studies. As no heterogeneity was
identified, the fixed effects model was used. The meta-analysis
results indicated that the rates of MACE and TLR in the TAT group
were significantly lower compared with those in the DAT group
(MACE: OR=0.69, 95% CI: 0.56–0.85, P<0.001; TLR: OR=0.61, 95%
CI: 0.43–0.88, P=0.008). However, no significant differences were
indicated in mortality rates (OR=0.80, 95% CI: 0.52–1.24, P=0.319),
MI (OR=0.75, 95% CI: 0.53–1.07, P=0.109) and TVR (OR=0.79, 95% CI:
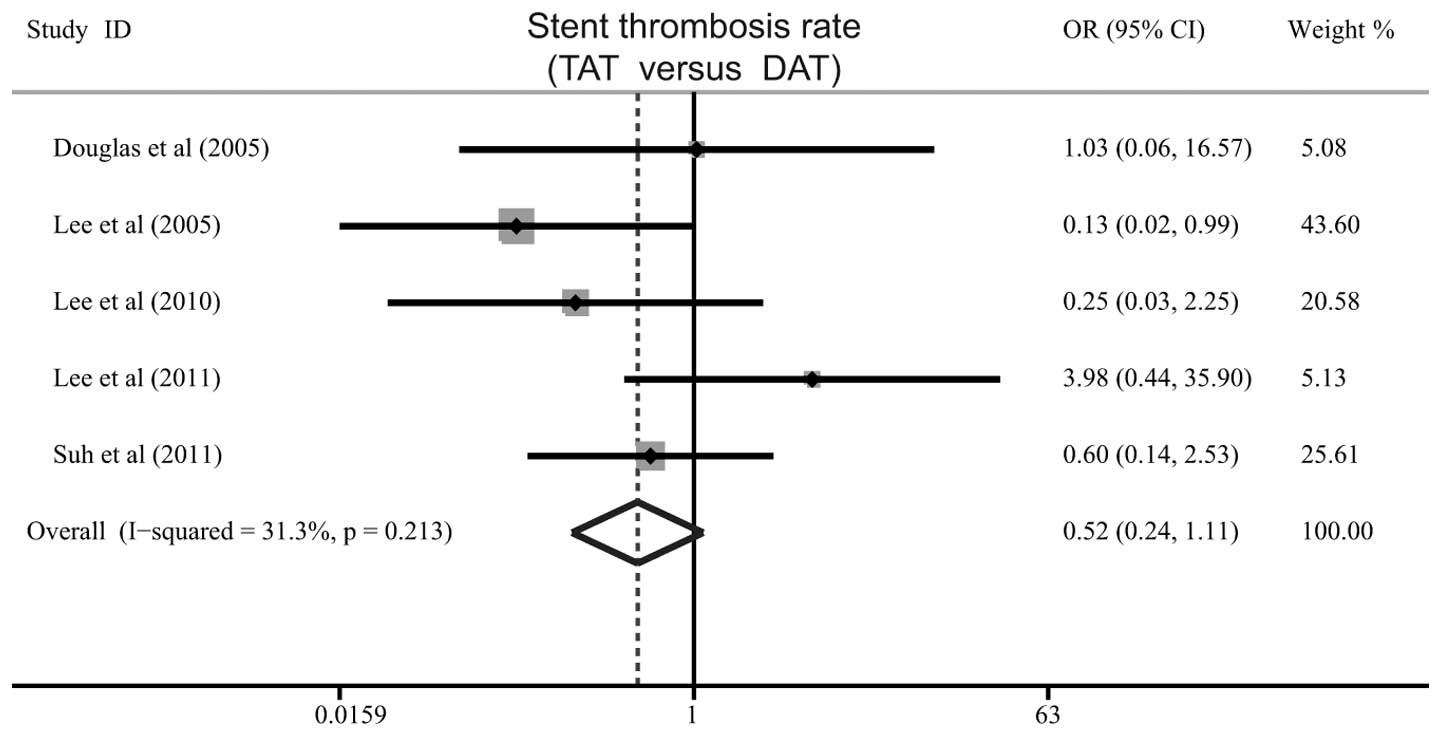
0.61–1.03, P=0.077) between the TAT and DAT groups (Fig. 3). Additionally, no significant
difference was identified in the rate of stent thrombosis between
the TAT and DAT groups (OR=0.52, 95% CI: 0.24–1.11, P=0.091;
Fig. 4).
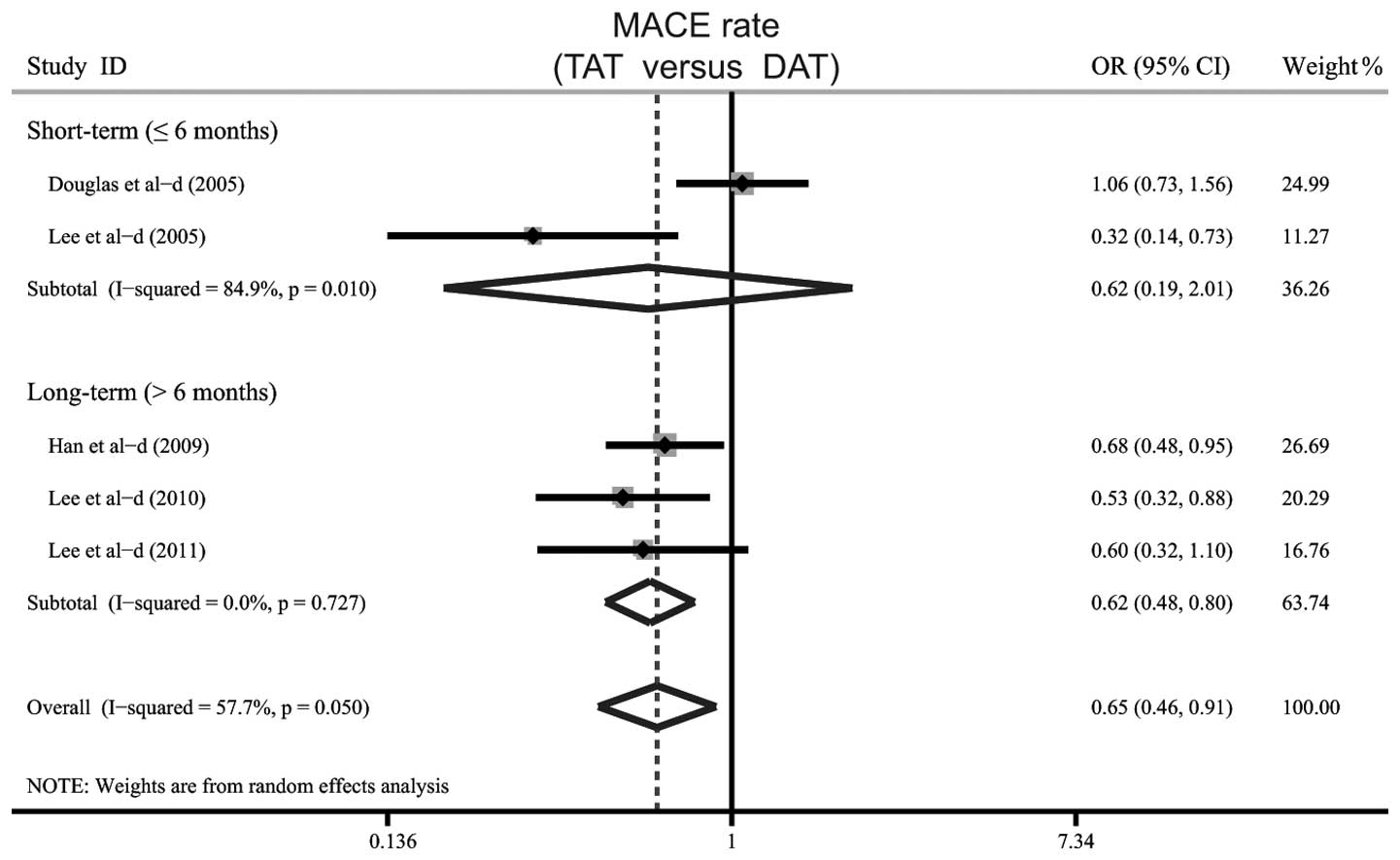
When examining the potential factors that may have
impacted the results, a further subgroup analysis was performed
based on the follow-up periods. The results indicated a significant
difference in the rate of MACE between TAT and DAT in the long-term
(>6 months) follow-up subgroups (OR=0.62, 95% CI: 0.48–0.80,
P<0.001). However, no significant difference in the rate of MACE
was indicated in the short-term (≤6 months) follow-up subgroups
(OR=0.62, 95% CI: 0.19–2.01, P=0.421; Fig. 5).
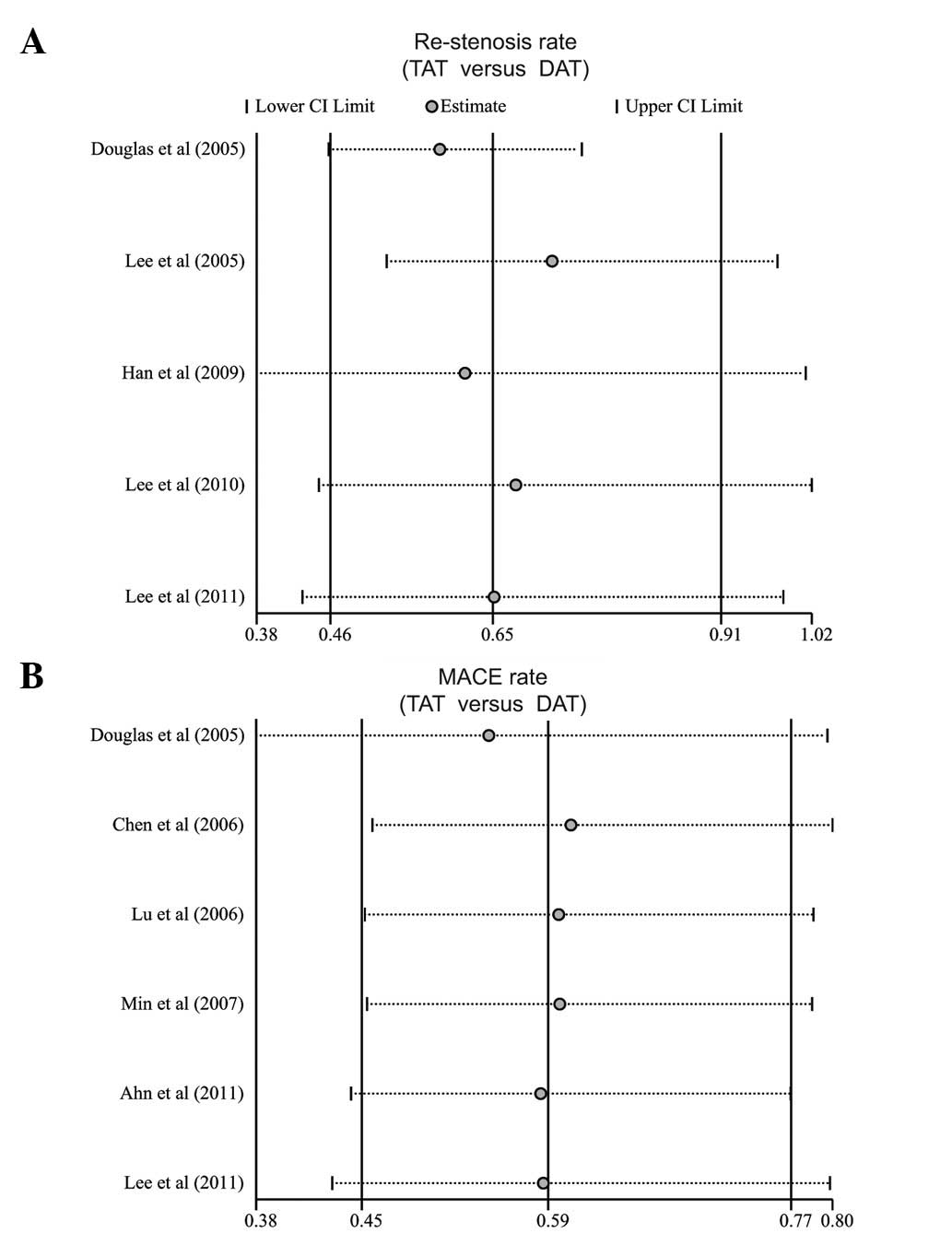
Sensitivity analysis and publication
bias
Sensitivity analysis was performed to assess the
influence of each individual study on the pooled ORs by omitting
individual studies. Analysis of the results suggested that no
individual study significantly affected the pooled ORs of the rate
of restenosis and MACE (Fig. 6),
indicating a statistically robust result.
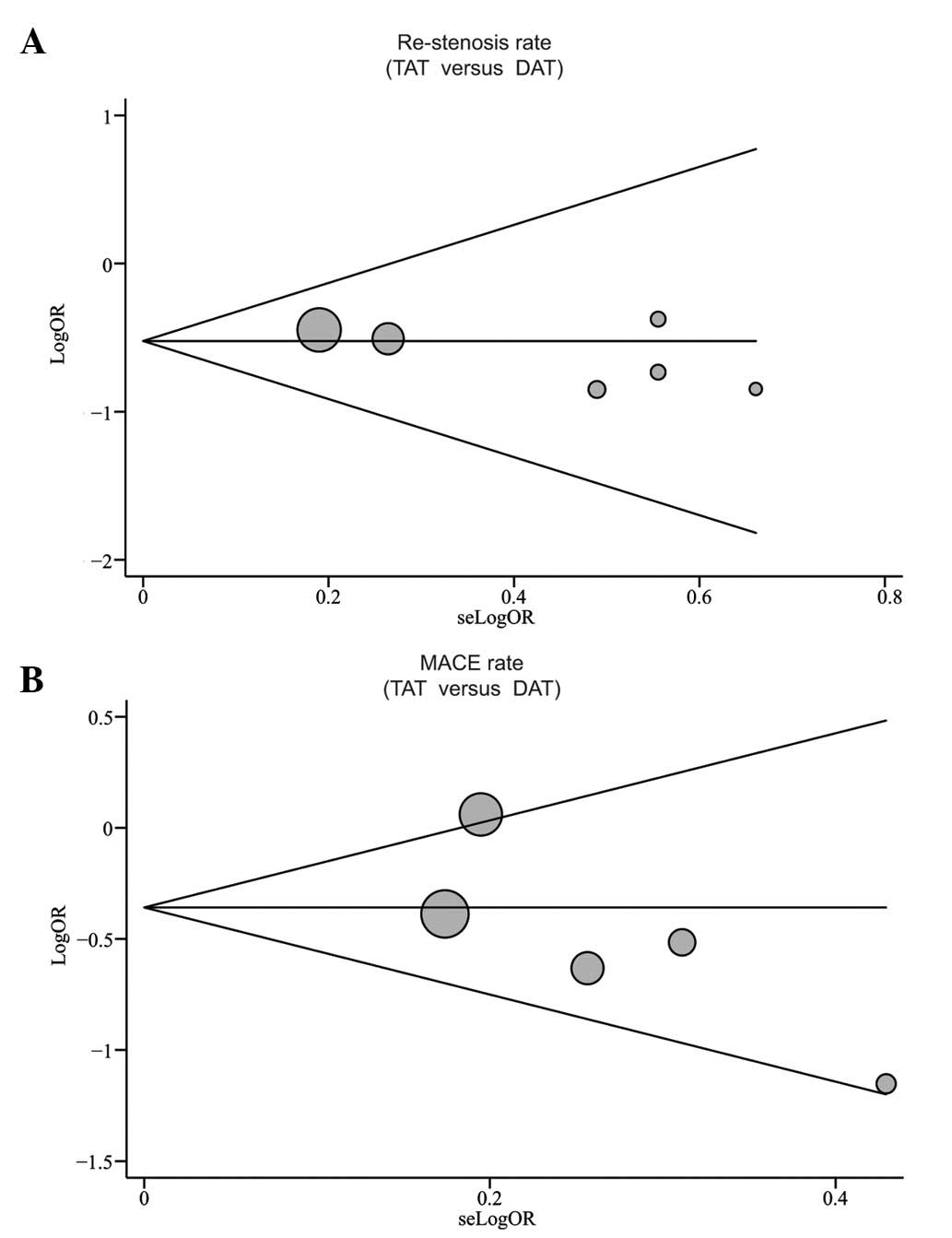
Publication bias exists to the extent that the
available research results are not representative of all research
results. Begg’s funnel plot and Egger’s linear regression test were
performed to assess the publication bias of the included studies.
The shapes of the funnel plots did not demonstrate any obvious
asymmetry (Fig. 7). Egger’s test
also showed that there was no statistical indication of publication
bias for the rates of restenosis and MACE (restenosis: t=−2.04,
P=0.111; MACE: t=−1.76, P=0.177).
Discussion
The clinical application of PCI with coronary stent
implantation has markedly increased, which has significantly
improved the outcomes in patients with CHD (26). However, serious complications
following implantation of coronary stents remain a significant
clinical problem and may result in failure of PCI to provide
long-term benefits. Over the past decade, numerous studies have
indicated that the use of adjunctive antiplatelet agents has
resulted in decreased stent-associated thrombosis and is important
in improving the final outcomes of PCI (27). Two meta-analyses by Tamhane et
al (11) and Singh et
al (28) also indicated that
patients treated with TAT exhibited a lower restenosis rate than
those treated with DAT (11,28).
However, these meta-analyses did not provide reliable results
demonstrating the differences in the clinical outcomes between TAT
and DAT for patients with CHD undergoing PCI with coronary stent
implantation. Therefore, in view of the conflicting results from
previous studies and the insufficient statistical power of the two
previous meta-analyses, the present meta-analysis was performed in
order to update previous meta-analyses and provide a comprehensive
conclusion.
In the present meta-analysis, 10 clinical studies
were included with a total of 7,670 patients with CHD undergoing
PCI with coronary stent implantation, including 3,745 patients in
the TAT group and 3,925 patients in the DAT group. The predominant
finding of this meta-analysis was that the rates of restenosis,
MACE and TLR in the TAT group were significantly lower compared
with those of the DAT group. Furthermore, when the follow-up
periods were stratified into short-term (≤6 months) and long-term
(>6 months) subgroups, it was noteworthy that a significant
difference in the rate of MACE between TAT and DAT was only
observed in the long-term follow-up subgroup. The substantial
improvement due to the addition of cilostazol to DAT identified by
the current meta-analysis is consistent with the findings of
several previous studies. This is due to cilostazol inhibiting the
progression of carotid intima-media thickness and thus producing an
additional anti-proliferative effect, regardless of the fact that
DAT itself is a potent therapy (23,29–31).
Notably, the significantly reduced rate of MACE in the long-term
follow-up subgroup is inconsistent with the findings of two
previous meta-analyses, which indicated that there was no
difference in the clinical outcomes of MACE between DAT and TAT
treatment groups (11,28). A potential reason for the
discrepancy may be the limited number of included studies. The
controversial results may also be caused by the high degree of
heterogeneity in the different follow-up periods. In the present
meta-analysis, no significant differences in the rates of
mortality, MI and TVR were observed between TAT and DAT for
patients with CHD undergoing PCI. Additionally, no significant
difference in the rate of stent thrombosis between TAT and DAT was
determined, which is in accordance with a previous meta-analysis
(32). These results suggest that
TAT may be no different from DAT in terms of its effectiveness as
an adjunctive therapy to coronary stents and as a preventative
measure against stent-associated thrombosis. This is regardless of
the different antiplatelet mechanisms of TAT, which may result in
suppression of platelet aggregation (32). The precise effect of adding
cilostazol to DAT on the incidence rates of various clinical events
requires further study before conclusions regarding these outcomes
may be made.
Similar to previous meta-analyses, the present
meta-analysis also has certain limitations. The sample size was
relatively small and may not have provided sufficient statistical
power to estimate the differences in the clinical outcomes between
TAT and DAT for patients with CHD undergoing PCI. A meta-analysis,
as a type of retrospective study, may encounter recall or selection
bias, which may influence the reliability of the results. A further
limitation, was that each pretreatment regimen was not consistent
and the doses of aspirin or cilostazol were variable. Moreover, the
type of stent used in each patient was not consistent and there may
have been differences with regard to the effectiveness of the
antiplatelet agents. Although all participants in each study were
required to meet similar inclusion criteria, there may have been
potential factors that were not taken into account that may have
influenced the results. Therefore, the results of this
meta-analysis should be interpreted with caution, owing to the
potential heterogeneity among trials.
In conclusion, the present meta-analysis suggests
that the efficacy and safety of cilostazol-based TAT may be greater
than that of conventional DAT for treating patients with CHD
undergoing PCI. However, additional well-designed and randomized
controlled trials are required to further investigate and clarify
the differences in the clinical outcomes between TAT and DAT for
patients with CHD.
References
|
1.
|
Aradi D, Komócsi A, Price MJ, et al:
Efficacy and safety of intensified antiplatelet therapy on the
basis of platelet reactivity testing in patients after percutaneous
coronary intervention: Systematic review and meta-analysis. Int J
Cardiol. Jun 15–2012.(Epub ahead of print).
|
|
2.
|
Meryon I, Patel N, Millane T and Varma C:
Normal coronary angiography and primary percutaneous coronary
intervention for ST elevation myocardial infarction: a literature
review and audit findings. Int J Clin Pract. 64:1245–1251. 2010.
View Article : Google Scholar
|
|
3.
|
Mercado N and Serruys PW: A
meta-analytical approach for the treatment of in-stent restenosis.
Eur Heart J. 24:217–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Meier B, Bachmann D and Lüscher T: 25
years of coronary angioplasty: almost a fairy tale. Lancet.
361:5272003.PubMed/NCBI
|
|
5.
|
Mrdovic I, Savic L, Lasica R, et al:
Usefulness of the RISK-PCI score to predict stent thrombosis in
patients treated with primary percutaneous coronary intervention
for ST-segment elevation myocardial infarction: a substudy of the
RISK-PCI trial. Heart Vessels. 28:424–433. 2013. View Article : Google Scholar
|
|
6.
|
Gurbel PA and Tantry US: Aspirin and
clopidogrel resistance: consideration and management. J Interv
Cardiol. 19:439–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Takahashi S, Kaneda H, Tanaka S, et al:
Late angiographic stent thrombosis after sirolimus-eluting stent
implantation. Circ J. 71:226–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Suh JW, Lee SP, Park KW, et al:
Multicenter randomized trial evaluating the efficacy of cilostazol
on ischemic vascular complications after drug-eluting stent
implantation for coronary heart disease: results of the CILON-T
(influence of CILostazol-based triple antiplatelet therapy ON
ischemic complication after drug-eluting stenT implantation) trial.
J Am Coll Cardiol. 57:280–289. 2011.
|
|
9.
|
Regensteiner JG and Stewart KJ:
Established and evolving medical therapies for claudication in
patients with peripheral arterial disease. Nat Clin Pract
Cardiovasc Med. 3:604–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jeong YH, Lee SW, Choi BR, et al:
Randomized comparison of adjunctive cilostazol versus high
maintenance dose clopidogrel in patients with high post-treatment
platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive
Cilostazol Versus High Maintenance Dose Clopidogrel in Patients
With Clopidogrel Resistance) randomized study. J Am Coll Cardiol.
53:1101–1109. 2009.
|
|
11.
|
Tamhane U, Meier P, Chetcuti S, et al:
Efficacy of cilostazol in reducing restenosis in patients
undergoing contemporary stent based PCI: a meta-analysis of
randomised controlled trials. EuroIntervention. 5:384–393. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lee SW, Park SW, Hong MK, et al: Triple
versus dual antiplatelet therapy after coronary stenting: impact on
stent thrombosis. J Am Coll Cardiol. 46:1833–1837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
DerSimonian R and Kacker R: Random-effects
model for meta-analysis of clinical trials: an update. Contemp Clin
Trials. 28:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
17.
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ahn CM, Hong SJ, Park JH, Kim JS and Lim
DS: Cilostazol reduces the progression of carotid intima-media
thickness without increasing the risk of bleeding in patients with
acute coronary syndrome during a 2-year follow-up. Heart Vessels.
26:502–510. 2011.PubMed/NCBI
|
|
19.
|
Chen YD, Lu YL, Jin ZN, Yuan F and Lu SZ:
A prospective randomized antiplatelet trial of cilostazol versus
clopidogrel in patients with bare metal stent. Chin Med J (Engl).
119:360–366. 2006.PubMed/NCBI
|
|
20.
|
Douglas JS Jr, Holmes DR Jr, Kereiakes DJ,
et al: Coronary stent restenosis in patients treated with
cilostazol. Circulation. 112:2826–2832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Han Y, Li Y, Wang S, et al: Cilostazol in
addition to aspirin and clopidogrel improves long-term outcomes
after percutaneous coronary intervention in patients with acute
coronary syndromes: a randomized, controlled study. Am Heart J.
157:733–739. 2009. View Article : Google Scholar
|
|
22.
|
Kim JY, Lee K, Shin M, et al: Cilostazol
could ameliorate platelet responsiveness to clopidogrel in patients
undergoing primary percutaneous coronary intervention. Circ J.
71:1867–1872. 2007. View Article : Google Scholar
|
|
23.
|
Lee SW, Chun KJ, Park SW, et al:
Comparison of triple anti-platelet therapy and dual antiplatelet
therapy in patients at high risk of restenosis after drug-eluting
stent implantation (from the DECLARE-DIABETES and -LONG Trials). Am
J Cardiol. 105:168–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lee SW, Park SW, Kim YH, et al: A
randomized, double-blind, multicenter comparison study of triple
antiplatelet therapy with dual antiplatelet therapy to reduce
restenosis after drug-eluting stent implantation in long coronary
lesions: results from the DECLARE-LONG II (Drug-Eluting Stenting
Followed by Cilostazol Treatment Reduces Late Restenosis in
Patients with Long Coronary Lesions) trial. J Am Coll Cardiol.
57:1264–1270. 2011.
|
|
25.
|
Lu YL, Chen YZ, Lv SZ, Pan WZ and Liu X:
Cilostazol prevention in elderly coronary heart disease patients
with coronary bare metal stent restenosis after effect. Chin J
Geriatr. 25:537–538. 2006.(In Chinese).
|
|
26.
|
Schleinitz MD, Olkin I and Heidenreich PA:
Cilostazol, clopidogrel or ticlopidine to prevent sub-acute stent
thrombosis: a meta-analysis of randomized trials. Am Heart J.
148:990–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Smith SC Jr, Dove JT, Jacobs AK, et al:
ACC/AHA guidelines for percutaneous coronary intervention (revision
of the 1993 PTCA guidelines)-executive summary: a report of the
American College of Cardiology/American Heart Association task
force on practice guidelines (Committee to revise the 1993
guidelines for percutaneous transluminal coronary angioplasty)
endorsed by the Society for Cardiac Angiography and Interventions.
Circulation. 103:3019–3041. 2001.
|
|
28.
|
Singh I, Shafiq N, Pandhi P, et al: Triple
antiplatelet therapy vs. dual antiplatelet therapy in patients
undergoing percutaneous coronary intervention: an evidence-based
approach to answering a clinical query. Br J Clin Pharmacol.
68:4–13. 2009. View Article : Google Scholar
|
|
29.
|
Biondi-Zoccai GG, Lotrionte M, Anselmino
M, et al: Systematic review and meta-analysis of randomized
clinical trials appraising the impact of cilostazol after
percutaneous coronary intervention. Am Heart J. 155:1081–1089.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Friedland SN, Eisenberg MJ and Shimony A:
Meta-analysis of randomized controlled trials on effect of
cilostazol on restenosis rates and outcomes after percutaneous
coronary intervention. Am J Cardiol. 109:1397–1404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hong SJ, Choi SC, Ahn CM, Park JH, Kim JS
and Lim DS: Telmisartan reduces neointima volume and pulse wave
velocity 8 months after zotarolimus-eluting stent implantation in
hyper-tensive type 2 diabetic patients. Heart. 97:1425–1432.
2011.
|
|
32.
|
Hashiguchi M, Ohno K, Nakazawa R, Kishino
S, Mochizuki M and Shiga T: Comparison of cilostazol and
ticlopidine for one-month effectiveness and safety after elective
coronary stenting. Cardiovasc Drugs Ther. 18:211–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|