Introduction
Acute hepatic failure (AHF) is a severe liver injury
accompanied by hepatic encephalopathy, which leads to multi-organ
failure with an extremely high mortality rate (1). Acute-on-chronic hepatic failure
(AOCHF) has been defined as an acute deterioration of liver
function in chronic liver disease that ultimately leads to
multi-organ failure within 4–6 weeks, with a mortality rate of 53%
(2). Liver transplantation has
long been recognized as the most effective therapy in the treatment
of AHF and AOCHF (3). However,
this therapeutic strategy is limited by the insufficient organ
resources and a significantly elevated demand for liver
transplantation. Therefore, extracorporeal liver support systems
(LSSs), as an alternative source for liver transplantation, have
attracted increased focus over the last four decades (4).
Artificial LSSs were originally developed in Germany
and were designed to remove toxic substances from the blood that
would normally be filtered out by a functioning liver (5). Artificial LSSs transport a patient’s
blood through a filter where it is mixed with albumin. The toxins
and metabolic waste from the blood that are mixed with the albumin
molecules are then carried out of the blood (6). Bioartificial LSSs, which are
essentially bioreactors, utilize either human hepatocytes or
porcine liver cells to process oxygenated blood plasma, which is
subsequently separated from the other blood constituents (7). The aim of artificial and
bioartificial LSSs is to temporarily replace liver functions until
a transplant is available (8). It
has been demonstrated that artificial and bioartificial LSSs are
important in the improvement of jaundice, the amelioration of
hemodynamic instability, the reduction of portal hypertension, the
lowering of intracranial pressure and the reduction in short-term
mortality in patients with AHF and AOCHF (9). Moreover, in cases of hepatic
encephalopathy, patients have shown marked reductions in ammonia
levels, clearance of aromatic amino acids and improvements in
systemic hemodynamics, which may partially explain the potential
benefits of artificial and bioartificial LSSs in improving hepatic
encephalopathy in patients with hepatic failure (10). Previous meta-analyses have
demonstrated that artificial and bioartificial LSSs may lead to
significant improvements in total bilirubin levels, hepatic
encephalopathy, the incidence of bleeding and bridging to
transplantation (11–13). However, the results remain
debatable with regard to whether artificial and bioartificial LSSs
are able to improve survival in patients with AHF or AOCHF. These
inconsistent results may be due to the limited number of studies
and relatively small number of patients suitable for study in the
previous meta-analyses. Therefore, in the present study, an updated
meta-analysis was performed on all the eligible literature to
evaluate the benefits and harmful effects of artificial and
bioartificial LSSs in patients with AHF and AOCHF.
Materials and methods
Literary search
Relevant papers published prior to March 1, 2013
were identified through a search of the PubMed, Embase, Web of
Science and Chinese Biomedical (CBM) databases using the following
terms: (‘liver support system’ or ‘liver, artificial’ or
‘artificial liver’ or ‘bioartificial liver’ or ‘extracorporeal
liver’) and (‘hepatic failure’ or ‘liver failure’ or ‘liver
failure, acute’ or ‘liver failure, acute’ or ‘end stage liver
disease’ or ‘liver failure, chronic’). The references used in
eligible articles or textbooks were also reviewed to examine other
potential sources. Disagreements were resolved through discussions
between the authors.
Inclusion and exclusion criteria
Studies included in the meta-analysis had to meet
the following criteria: i) randomized controlled trials (RCTs)
focused on the effects of artificial and bioartificial LSSs in
patients with AHF and AOCHF; ii) study populations included
patients with AHF and AOCHF; iii) interventions (treatment groups)
included artificial and bioartificial LSSs, while the comparison
intervention (control group) used standard medical therapy,
including electrolyte substitution, fluid substitution, antacid
therapy, coagulation therapy and N-acetylcysteine; and iv)
published data on the clinical outcomes must be sufficient. The
exclusion criteria were as follows: i) not an RCT on the effects of
artificial and bioartificial LSSs in patients with AHF and AOCHF;
ii) duplicates of previous publications; iii) based on incomplete
data; and iv) meta-analyses, letters, reviews and editorial
articles. If more than one study by the same author using the same
case series was published, either the study with the largest sample
size or the most recently published study was included.
Data extraction
Data from the published studies were extracted
independently by two authors into a standardized form. For each
study, the following characteristics and numbers were collected:
first author, year of publication, country, language, study design,
numbers of subjects, subtype of hepatic failure, inclusion criteria
for subjects, type of liver support system, duration of follow-up,
outcomes and methodological quality. In cases of conflicting
evaluations, disagreements were resolved through discussions
between the authors.
Study outcome
All outcomes were assessed subsequent to the maximum
follow-up. The following outcome data were extracted from the
studies: i) mortality; ii) bridging to liver transplantation; iii)
total bilirubin levels; iv) hepatic encephalopathy; and v)
incidence of bleeding.
Methodological quality assessment
This meta-analysis was performed according to
recommendations from the Preferred Reporting Items for Systematic
Reviews and Meta-analyses (PRISMA) statement (14). Two authors independently assessed
the quality of the papers according to the Consolidated Standards
of Reporting Trials (CONSORT) criteria (15). A point was awarded for each
criterion met. The mean CONSORT score was calculated for each
trial. Disagreements were resolved through discussions between the
authors.
Statistical analysis
Crude relative risks (RRs) or standardized mean
differences (SMDs) with 95% confidence intervals (95% CI) were
calculated for dichotomous outcomes and continuous outcomes,
respectively. The statistical significance of the pooled value was
examined using the Z-test. Interstudy variations and
heterogeneities were estimated using Cochran’s Q-statistic with
P<0.05 as a cutoff for statistically significant heterogeneity
(16). The effects of
heterogeneity were also quantified using the I2 test,
which ranges from 0 to 100% and represents the proportion of
interstudy variability that may be contributed to heterogeneity
rather than by chance (17). When
a significant Q-test (P<0.05) or I2 >50% indicated
that heterogeneity existed among the studies, the random effects
model (DerSimonian-Laird method) was conducted for meta-analysis;
otherwise, the fixed effects model (Mantel-Haenszel method) was
used. To explore sources of heterogeneity, univariate and
multivariate regression analyses were also performed (18). A sensitivity test was performed by
omitting each study randomly and assessing the stability of the
results. Begg’s funnel plot and Egger’s linear regression test were
used to evaluate the publication bias (19). All P-values were two-sided and
P<0.05 was considered to indicate a statistically significant
difference. All analyses were calculated using STATA statistical
software version 12.0 (Stata Corp., College Station, TX, USA).
Results
Characteristics of included studies
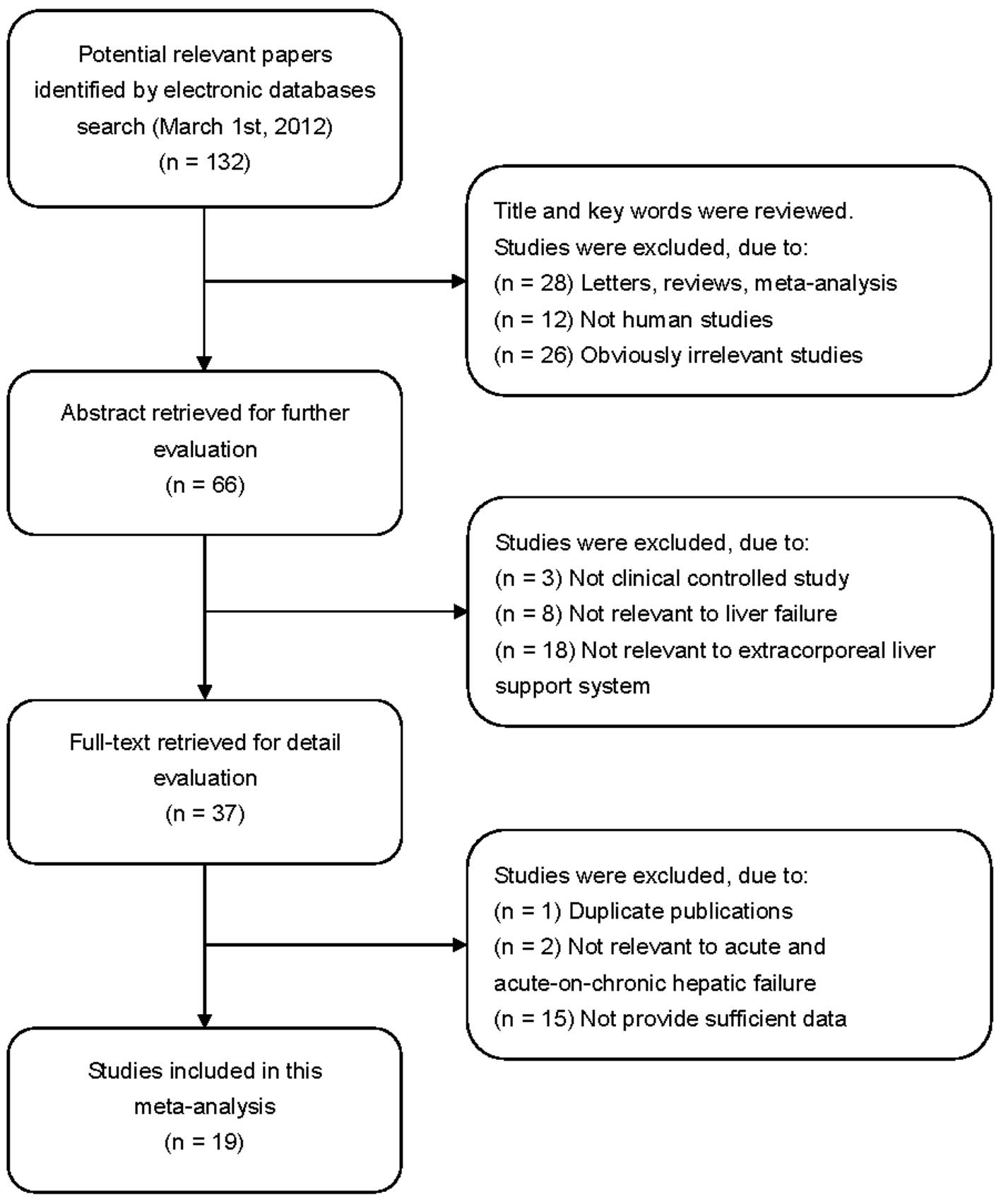
In accordance with the inclusion criteria, 19 RCTs
(20–38) were assessed in this meta-analysis
and 113 studies were excluded. The publication years of the
included studies ranged from 1973 to 2012. A flow chart of the
study selection process is shown in Fig. 1. A total of 937 patients with
hepatic failure were involved in this meta-analysis, including 566
patients with AHF and 371 patients with AOCHF. With regard to the
therapeutic strategy in the treatment group, 16 studies adopted
artificial LSSs while the remaining studies adopted bioartificial
LSSs. The control groups received standard medical therapy aimed at
preventing the complications associated with severe liver failure.
The characteristics and methodological quality of the included
studies are summarized in Table
I.
 | Table I.Characteristics of included studies in
this meta-analysis. |
Table I.
Characteristics of included studies in
this meta-analysis.
| First author | Year | Country | n
| Subtype of HF | Etiology | Interventions | CONSORT score |
|---|
| Treatment | Control |
|---|
| Redeker et al
(20) | 1973 | USA | 8 | 20 | AHF | Viral hepatitis | Transfusion
(artificial)a | 13 |
| O’Grady et al
(21) | 1988 | UK | 29 | 33 | AHF | Multi-etiology | Hemoperfusion
(artificial)a | 16 |
| Davenport et
al (22) | 1993 | UK | 12 | 18 | AHF | Multi-etiology | Hemofiltration
(artificial)a | 16 |
| Hughes et al
(23) | 1994 | UK | 5 | 5 | AHF | Drug induced liver
disease/viral hepatitis | BioLogic-DT
(artificial)acd | 17 |
| Ellis et al
(24) | 1996 | UK | 12 | 12 | AHF | Multi-etiology | ELAD
(bioartificial)acd | 14 |
| Mazariegos et
al (25) | 1997 | USA | 5 | 5 | AHF | NR | BioLogic-DT
(artificial)a–c | 16 |
| Kramer et al
(26) | 1998 | Austria | 10 | 10 | AOCHF | Multi-etiology | BioLogic-DT
(artificial)a–d | 17 |
| Wilkinson et
al (27) | 1998 | USA | 6 | 5 | AHF | Multi-etiology | BioLogic-DT
(artificial)a–c | 17 |
| Ellis et al
(28) | 1999 | UK | 5 | 5 | AOCHF | Alcoholic liver
disease | BioLogic-DT
(artificial)abd | 11 |
| He et al-a
(29) | 2000 | China | 37 | 33 | AHF | Viral
hepatitis |
Hemoperfusion/hemofiltration
(artificial)abd | 11 |
| He et al-b
(29) | 2000 | China | 27 | 27 | AOCHF | Viral
hepatitis |
Hemoperfusion/hemofiltration
(artificial)ad | 11 |
| Mitzner et
al (30) | 2000 | Germany | 8 | 5 | AOCHF | Multi-etiology | MARS
(artificial)ade | 16 |
| Stevens et
al (31) | 2001 | USA/Europe | 73 | 74 | AHF | Multi-etiology | HepatAssist
(bioartificial)acd | 12 |
| Heemann et
al (32) | 2002 | Germany | 12 | 11 | AOCHF | Multi-etiology | MARS
(artificial)acd | 17 |
| Demetriou et
al (33) | 2004 | Denmark | 73 | 74 | AHF | Multi-etiology | HepatAssist
(bioartificial)a | 18 |
| El Banayosy et
al (34) | 2004 | Germany | 14 | 13 | AHF | Cardiogenic
shock | MARS
(artificial)ade | 11 |
| Sen et al
(35) | 2004 | UK | 9 | 9 | AOCHF | Alcoholic liver
disease | MARS
(artificial)acd | 15 |
| Laleman et
al (36) | 2006 | Belgium | 12 | 6 | AOCHF | Alcoholic liver
disease | MARS/Prometheus
(artificial)e | 13 |
| Hassanein et
al (37) | 2007 | Germany | 39 | 31 | AOCHF | Liver
cirrhosis | MARS
(artificial)acd | 20 |
| Kribben et
al (38) | 2012 | Europe | 77 | 68 | AOCHF | Multi-etiology | Prometheus
(artificial) ade | 21 |
Mortality
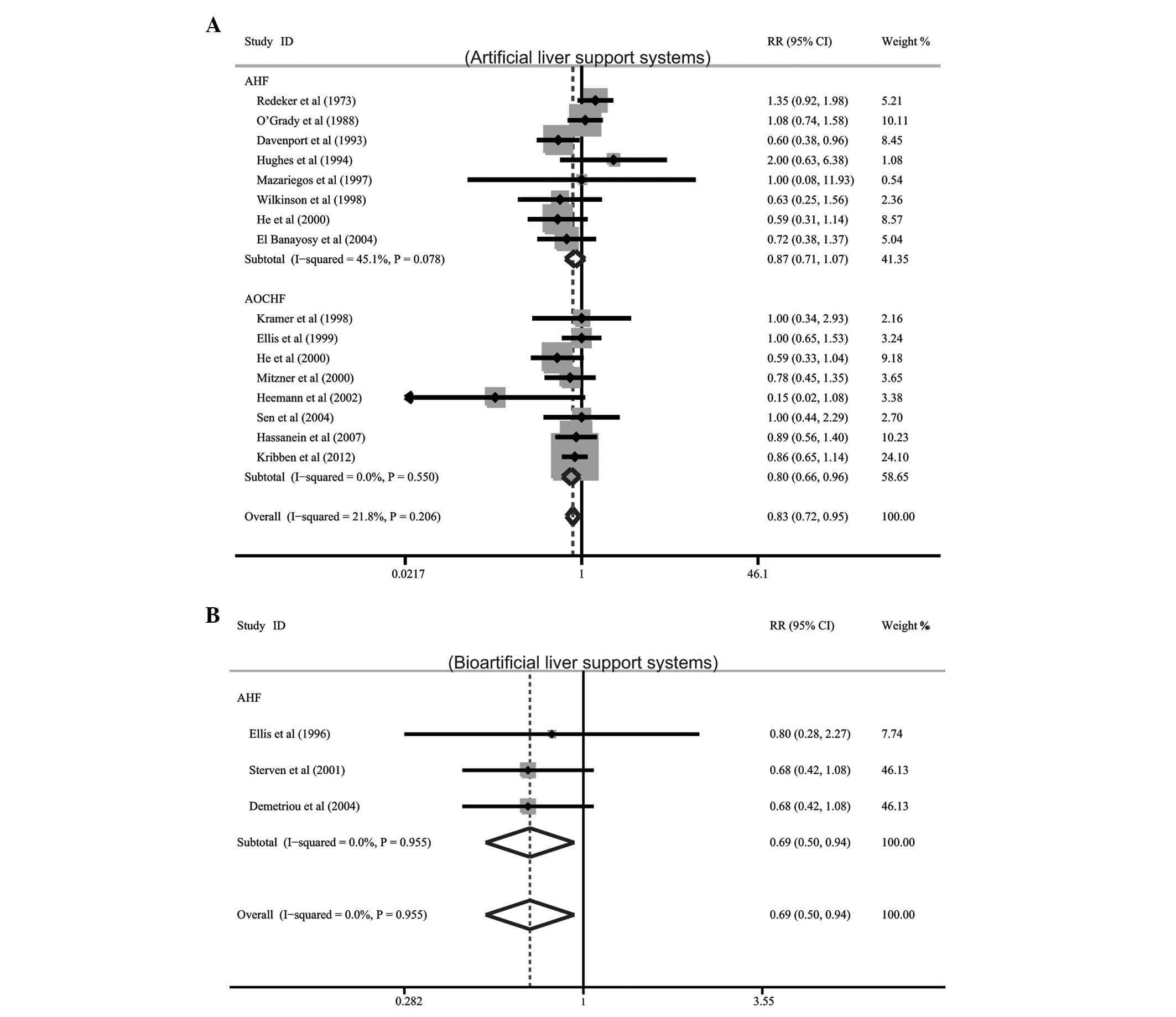
Among the 19 included studies, 16 described data on
the effects of artificial LSSs on mortality in patients with AHF
and AOCHF, while only three studies referred to the effects of
bioartificial LSSs on mortality. Meta-analysis showed that
artificial LSS therapy significantly reduced mortality in patients
with AOCHF (RR= 0.80, 95% CI= 0.66–0.96, P= 0.018). The results
also showed that the use of bioartificial LSSs was correlated with
decreased mortality in patients with AHF (RR=0.69, 95%
CI=0.50–0.94, P=0.018). However, it was observed that artificial
LSSs had no apparent effect on total mortality in patients with AHF
(RR=0.87, 95% CI=0.71–1.07, P=0.187; Fig. 2).
Bridging to liver transplantation
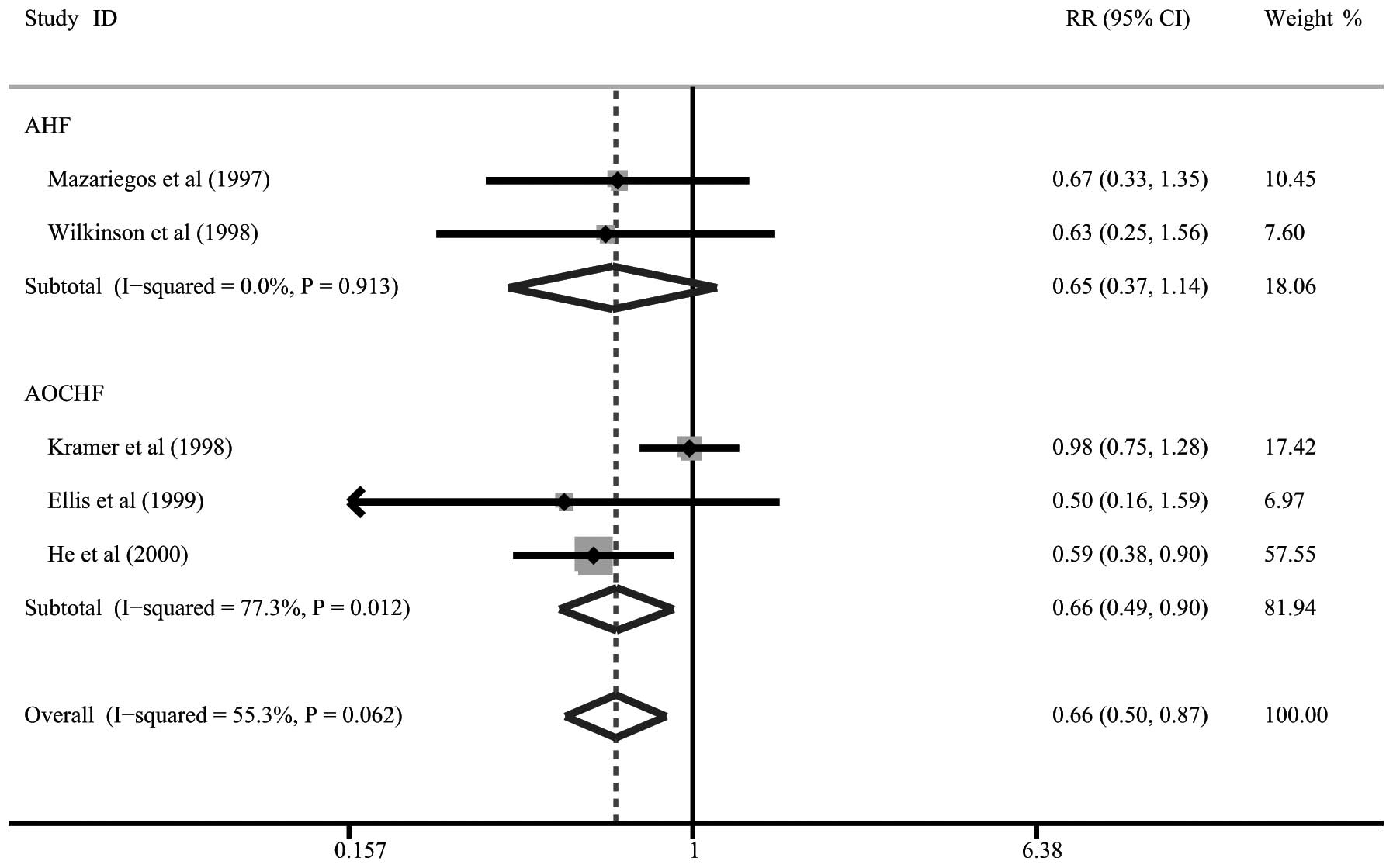
Five of the 19 studies described data on the
bridging to liver transplantation with artificial LSS therapy. Our
meta-analysis demonstrated significant reductions in the bridging
to liver transplantation in patients with AOCHF following
artificial LSS therapy (RR= 0.66, 95% CI= 0.49–0.90, P= 0.009).
However, artificial LSSs had no significant effect on the bridging
to liver transplantation in patients with AHF (RR= 0.65, 95% CI=
0.37–1.14, P=0.131; Fig. 3).
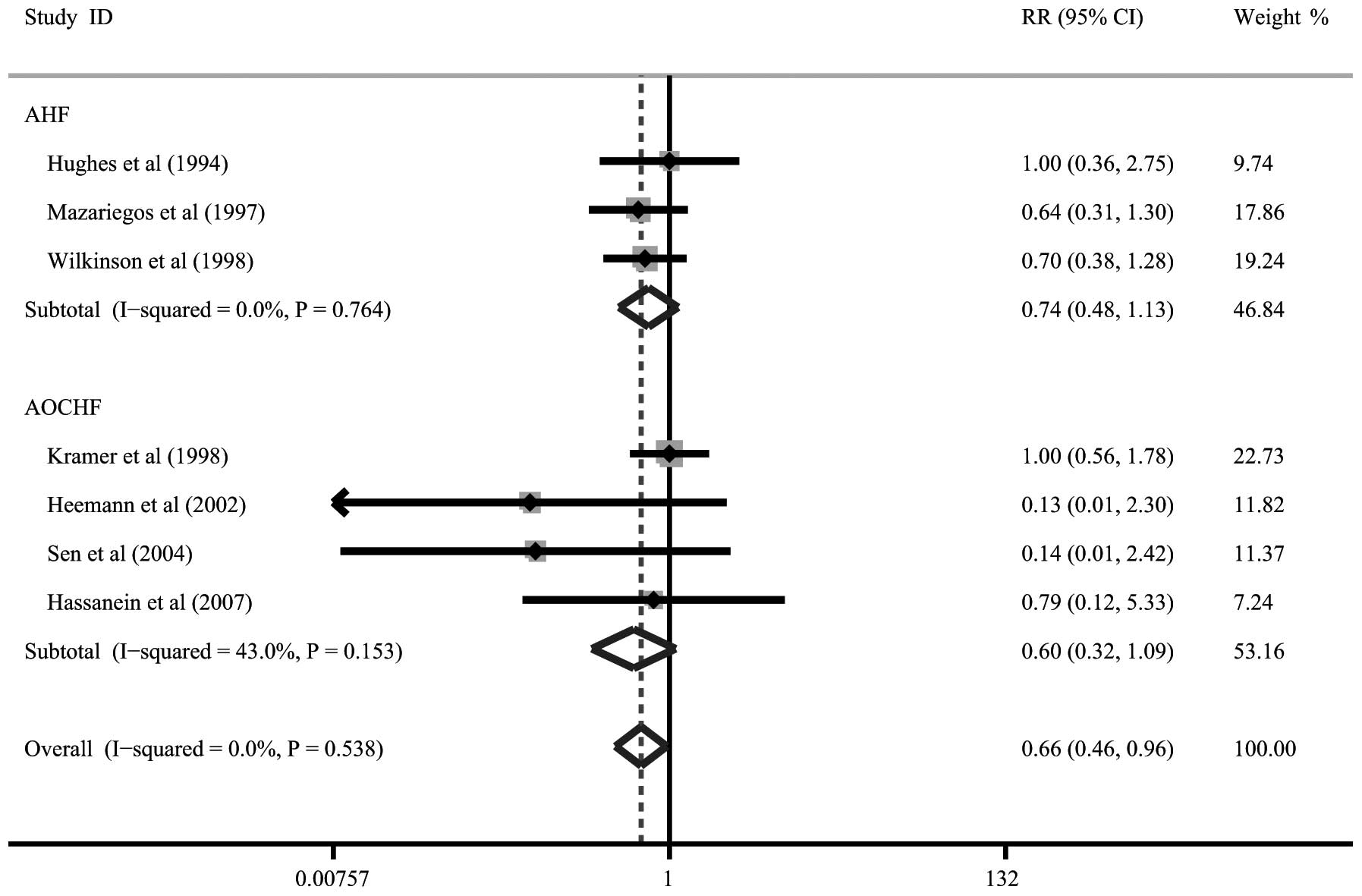
Total bilirubin levels
Seven trials presented data on total bilirubin
levels following artificial LSS therapy. Patients with AHF and
those with AOCHF were revealed to have significant reductions in
total bilirubin levels following artificial LSS therapy (RR= 0.74,
95% CI= 0.48–1.13, P= 0.357; RR=0.60, 95% CI=0.32–1.09, P=0.357,
respectively; Fig. 4).
Hepatic encephalopathy
Nine trials described improvements in hepatic
encephalopathy. Patients with AHF and those with AOCHF had no
significantly increased risk of hepatic encephalopathy following
artificial LSS therapy (RR= 0.76, 95% CI= 0.49–1.16, P= 0.202; RR=
0.64, 95% CI= 0.36–1.15, P= 0.137, respectively). There was also no
increased risk of hepatic encephalopathy in patients with AHF
following bioartificial LSS therapy (RR= 0.43, 95% CI= 0.14–1.28,
P= 0.128).
Incidence of bleeding
Thirteen trials described the incidence of bleeding.
No statistically significant increase in the incidence of bleeding
was observed in patients with AHF or AOCHF following artificial LSS
therapy (RR=0.97, 95% CI= 0.15–6.19, P= 0.973; RR=1.20, 95% CI=
0.87–1.64, P=0.270, respectively).
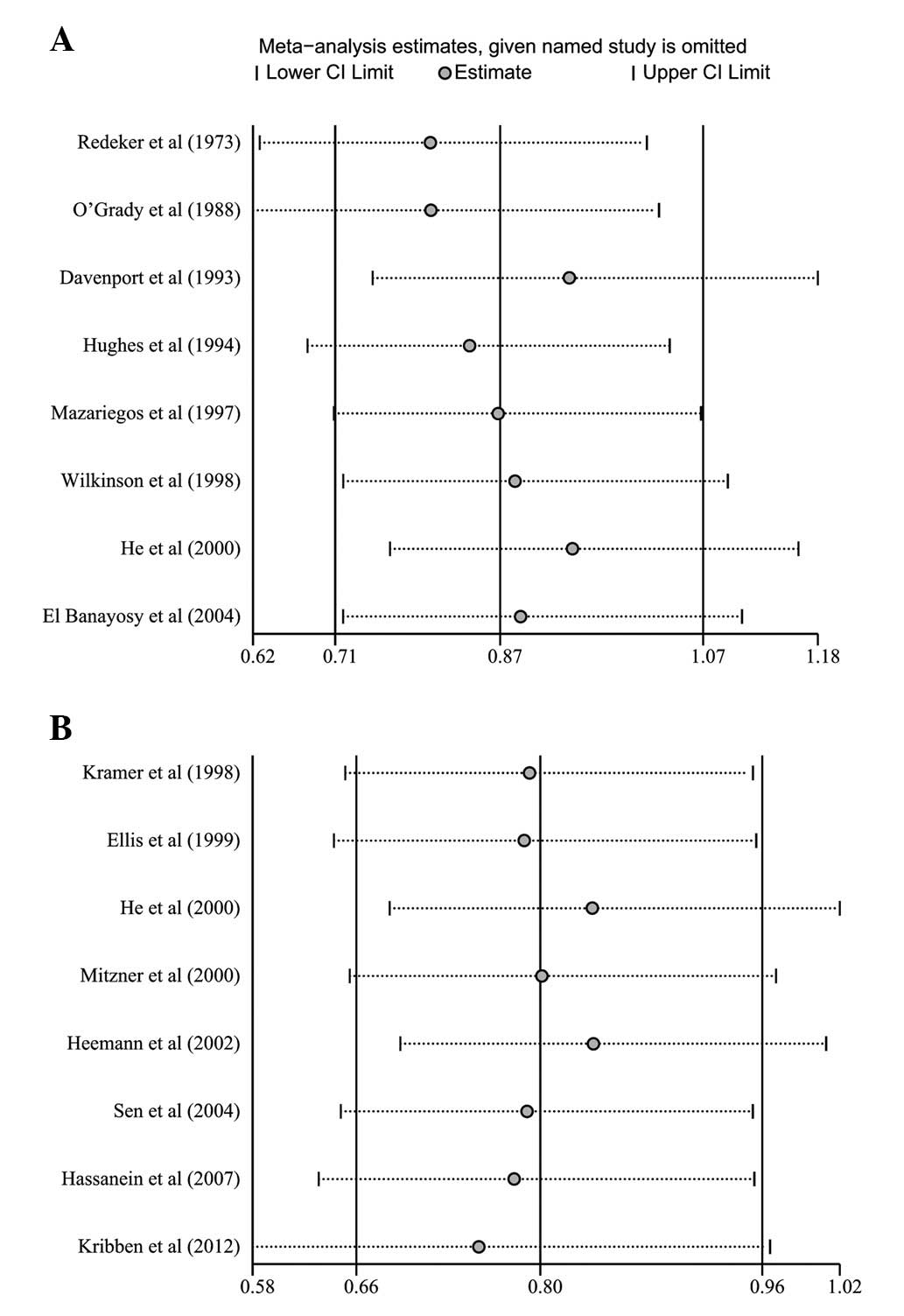
Meta-regression and sensitivity
analyses
Univariate and multivariate meta-regression analyses
were used to explore possible sources of heterogeneity among the
studies (Table II). The results
showed that none of the factors explained the heterogeneity (all
P>0.05). Sensitivity analysis was performed to assess the
stability of the conclusions on the pooled RR of mortality by
omitting individual studies. The sensitivity analysis results
suggested that no individual study significantly affected the
pooled values of the clinical events (Fig. 5), indicating that the results of
the meta-analysis were statistically robust.
 | Table II.Univariate and multivariate
meta-regression analyses of potential sources of heterogeneity. |
Table II.
Univariate and multivariate
meta-regression analyses of potential sources of heterogeneity.
| Heterogeneity
factor | Coefficient | SE | z | P-value | 95% CI
|
|---|
| UL | LL |
|---|
| Publication
year | | | | | | |
| Univariate | −0.005 | 0.015 | −0.34 | 0.736 | −0.034 | 0.024 |
| Multivariate | 0.032 | 0.063 | 0.51 | 0.613 | −0.092 | 0.155 |
| Country | | | | | | |
| Univariate | 0.077 | 0.073 | 1.05 | 0.293 | −0.066 | 0.220 |
| Multivariate | −0.059 | 0.256 | −0.23 | 0.817 | −0.562 | 0.443 |
| Subtype of hepatic
failure | | | | | | |
| Univariate | −0.019 | 0.036 | −0.52 | 0.603 | −0.088 | 0.051 |
| Multivariate | −0.024 | 0.065 | −0.37 | 0.713 | −0.150 | 0.103 |
| Etiology | | | | | | |
| Univariate | 0.010 | 0.036 | 0.28 | 0.777 | −0.059 | 0.080 |
| Multivariate | −0.040 | 0.105 | −0.38 | 0.704 | −0.246 | 0.166 |
| Interventions | | | | | | |
| Univariate | −0.004 | 0.030 | −0.13 | 0.893 | −0.063 | 0.054 |
| Multivariate | 0.004 | 0.078 | 0.05 | 0.957 | −0.148 | 0.156 |
| CONSORT score | | | | | | |
| Univariate | −0.044 | 0.033 | −1.34 | 0.179 | −0.108 | 0.020 |
| Multivariate | −0.112 | 0.139 | −0.81 | 0.419 | −0.384 | 0.160 |
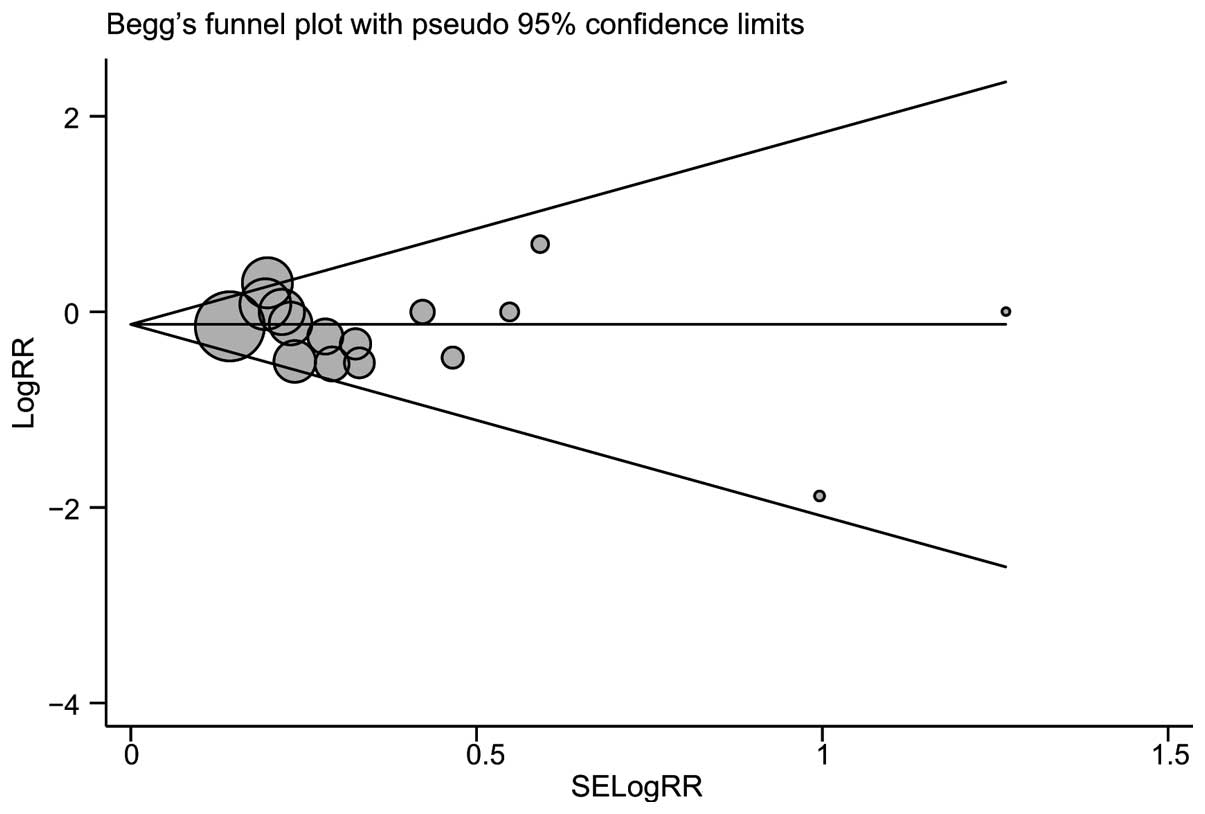
Publication bias evaluation
Begg’s funnel plot and Egger’s linear regression
test were performed to assess the publication bias of the included
studies. The shapes of the funnel plots of mortality did not reveal
any evidence of obvious asymmetry (Fig. 6). Egger’s test also displayed no
significant statistical evidence of publication bias with regard to
mortality (t=−1.02, P= 0.327).
Discussion
Previous meta-analyses (11–13)
have attempted to evaluate the effects of artificial and
bioartificial LSSs on the clinical outcomes in patients with AHF
and AOCHF; however, the results were inconclusive due to small
sample sizes, different study designs, methodological limitations
and a wide variety of observed outcome measures. In the
meta-analysis by Kjaergard et al in 2003 (12), it was revealed that artificial LSSs
reduced mortality in patients with AOCHF, while neither artificial
nor bioartificial LSSs appeared to affect mortality in patients
with AHF. Results from another meta-analysis by Liu et al
(11) also indicated that
artificial LSSs reduced mortality in patients with AOCHF; however,
there appeared to be no correlation between the use of artificial
or bioartificial LSSs and reductions in mortality in patients with
AHF (11). By contrast, in the
meta-analysis performed by Stutchfield et al (13) in 2011, it was demonstrated that
artificial and bioartificial LSSs appeared to improve survival in
patients with AHF, although not in patients with AOCHF (13). Therefore, it was imperative to
conduct a more systematic and comprehensive meta-analysis to
reassess the effects of artificial and bioartificial LSSs on the
clinical outcomes of patients with different types of hepatic
failure.
In the present study, compared with previous
meta-analyses, more stringent inclusion criteria were used (only
RCTs were evaluated), more studies were included (19 versus 12 in
the analyses by Kjaergard et al and Liu et al,
respectively, and eight in the analysis by Stutchfield et
al), more patients were assessed (566 with AHF and 371 with
AOCHF) and a wider range of eligible articles were analyzed (from
1973 to 2012 compared with from 1973 to 2002 in the study by
Kjaergard et al, from 1973 to 2001 in the study by Liu et
al and from 1996 to 2007 in the study by Stutchfield et
al). The present meta-analysis also evaluated more clinically
relevant endpoints (mortality, bridging to transplantation, total
bilirubin level, hepatic encephalopathy and bleeding) with greater
inferential power. When all available studies were pooled into the
present meta-analysis, the results showed that artificial LSS
therapy significantly reduced mortality in patients with AOCHF;
however, it had no apparent effect on total mortality in patients
with AHF. The findings from this meta-analysis were consistent with
the previous studies conducted by Kjaergard et al (12) and Liu et al (11), suggesting that no survival benefits
may be derived from artificial LSS therapy for patients with AHF.
However, in contrast to the previous meta-analyses, it was observed
in the present meta-analysis that the use of bioartificial LSS
therapy was correlated with decreased mortality in patients with
AHF. Moreover, the current meta-analysis results revealed that
there was a significant reduction in the bridging to liver
transplantation in patients with AOCHF following artificial LSS
therapy, although similar results were not observed in patients
with AHF. Significant reductions were observed in total bilirubin
levels in the patients with AHF and with AOCHF following artificial
LSS therapy, which was consistent with the previous study by Liu
et al (11). This indicated
that an effective clearance of albumin-bound substances was
performed in the liver support device (11). There was no significantly increased
risk of hepatic encephalopathy in either the patients with AHF or
AOCHF following artificial and bioartificial LSS therapies.
Over the past decade, a number of studies have
indicated that the use of LSSs may be correlated with several
potentially life-threatening adverse effects, including bleeding,
infection, coagulopathy and an increase in intracranial pressure,
with the most frequently observed adverse effect being bleeding
(39,40). However, the result of the present
analysis did not reveal any significant increase in the incidence
of bleeding in either the patients with AHF or AOCHF following
artificial or bioartificial LSS therapy, which was inconsistent
with the previous study by Liu et al (11). However, in the largest trial of
LSSs in patients with AOCHF, published in 2012, there was no
difference in the incidence of bleeding between the use of LSSs and
standard medical therapy. Despite this, all of the patients in the
studies suffered from severe liver disease, so it may be difficult
for physicians to conclude whether the LSS therapies or the
underlying severe liver disease caused the bleeding. Therefore,
additional adequately powered studies addressing these issues in
larger populations are required to provide more definitive
conclusions.
Similar to other meta-analyses, the present study
showed certain limitations, such as a lack of adequate
double-blinding procedures. Adequate double-blinding procedures for
patients and caregivers were impossible due to the nature of the
support systems; this may have increased the risk of false-positive
conclusions from the outcomes. In addition, the heterogeneity of
the trials included follow-up periods of variable durations and a
diverse patient population, with regard to the severity and
etiology of the liver failure, which precluded definitive
conclusions. Despite these limitations, however, the present
meta-analysis also demonstrated several strengths, such as
including the largest number of patients with hepatic failure
treated with LSSs to date. Moreover, the results were relatively
consistent with those observed in the largest study to date and the
consistency of the results was maintained in almost each subgroup
analysis.
In conclusion, the present updated meta-analysis
demonstrated that artificial LSS therapy appeared to reduce
mortality in patients with AOCHF, while the use of bioartificial
LSS therapy was correlated with decreased mortality in patients
with AHF. Artificial LSS therapy also appeared to reduce the
bridging to transplantation and levels of total bilirubin; however
it did not appear to increase the risks of hepatic encephalopathy
and bleeding. Considering the limitations mentioned previously,
further adequately powered studies are essential to extend this
investigation before any support systems are able to be recommended
for routine use.
Acknowledgements
This study was funded by the Science
Foundation of Science and Technology Bureau of Liaoning Province of
China (grant no. 2008225008-8).
References
|
1.
|
Tuñón MJ, Alvarez M, Culebras JM and
González-Gallego J: An overview of animal models for investigating
the pathogenesis and therapeutic strategies in acute hepatic
failure. World J Gastroenterol. 15:3086–3098. 2009.PubMed/NCBI
|
|
2.
|
Stadlbauer V, Krisper P, Aigner R, et al:
Effect of extracorporeal liver support by MARS and Prometheus on
serum cytokines in acute-on-chronic liver failure. Crit Care.
10:R1692006. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bernal W, Auzinger G, Dhawan A and Wendon
J: Acute liver failure. Lancet. 376:190–201. 2010. View Article : Google Scholar
|
|
4.
|
van de Kerkhove MP, Hoekstra R, Chamuleau
RA and van Gulik TM: Clinical application of bioartificial liver
support systems. Ann Surg. 240:216–230. 2004.PubMed/NCBI
|
|
5.
|
Carpentier B, Gautier A and Legallais C:
Artificial and bioartificial liver devices: present and future.
Gut. 58:1690–1702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang Y, Susando T, Lei X, et al: Current
development of bioreactors for extracorporeal bioartificial liver
(Review). Biointerphases. 5:FA116–FA131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Pless G: Bioartificial liver support
systems. Methods Mol Biol. 640:511–523. 2010. View Article : Google Scholar
|
|
8.
|
Millis JM and Losanoff JE: Technology
insight: liver support systems. Nat Clin Pract Gastroenterol
Hepatol. 2:398–405; quiz 434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nevens F and Laleman W: Artificial liver
support devices as treatment option for liver failure. Best Pract
Res Clin Gastroenterol. 26:17–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Schilsky ML: Acute liver failure and liver
assist devices. Transplant Proc. 43:879–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu JP, Gluud LL, Als-Nielsen B and Gluud
C: Artificial and bioartificial support systems for liver failure.
Cochrane Database Syst Rev. CD0036282004.PubMed/NCBI
|
|
12.
|
Kjaergard LL, Liu J, Als-Nielsen B and
Gluud C: Artificial and bioartificial support systems for acute and
acute-on-chronic liver failure: a systematic review. JAMA.
289:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Stutchfield BM, Simpson K and Wigmore SJ:
Systematic review and meta-analysis of survival following
extracorporeal liver support. Br J Surg. 98:623–631. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Moher D, Schulz KF and Altman DG; CONSORT
GROUP (Consolidated Standards of Reporting Trials): The CONSORT
statement: revised recommendations for improving the quality of
reports of parallel-group randomized trials. Ann Intern Med.
134:657–662. 2001. View Article : Google Scholar
|
|
16.
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ioannidis JP, Patsopoulos NA and Rothstein
HR: Reasons or excuses for avoiding meta-analysis in forest plots.
BMJ. 336:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Peters JL, Sutton AJ, Jones DR, Abrams KR
and Rushton L: Comparison of two methods to detect publication bias
in meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Redeker AG and Yamahiro HS: Controlled
trial of exchange-transfusion therapy in fulminant hepatitis.
Lancet. 1:3–6. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
O’Grady JG, Gimson AE, O’Brien CJ,
Pucknell A, Hughes RD and Williams R: Controlled trials of charcoal
hemoper-fusion and prognostic factors in fulminant hepatic failure.
Gastroenterology. 94:1186–1192. 1988.PubMed/NCBI
|
|
22.
|
Davenport A, Will EJ and Davison AM:
Effect of renal replacement therapy on patients with combined acute
renal and fulminant hepatic failure. Kidney Int Suppl.
41:S245–S251. 1993.PubMed/NCBI
|
|
23.
|
Hughes RD, Pucknell A, Routley D, Langley
PG, Wendon JA and Williams R: Evaluation of the BioLogic-DT
sorbent-suspension dialyser in patients with fulminant hepatic
failure. Int J Artif Organs. 17:657–662. 1994.PubMed/NCBI
|
|
24.
|
Ellis AJ, Hughes RD, Wendon JA, et al:
Pilot-controlled trial of the extracorporeal liver assist device in
acute liver failure. Hepatology. 24:1446–1451. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Mazariegos GV, Linden F, Kramer D, Patzer
J and Fung JJ: Randomized clinical trial of the BioLogic-DT in
treatment of acute hepatic failure (AHF) with advanced
encephalopathy. Hepatology. 26(Suppl): 559A1997.
|
|
26.
|
Kramer L, Gendo A, Madl C, Mullen K,
Kaminski-Russ K and Zauner C: A controlled study of the BioLogic-DT
System in chronic hepatic encephalopathy. Hepatology. 28(Suppl):
401A1998.
|
|
27.
|
Wilkinson AH, Ash SR and Nissenson AR:
Hemodiabsorption in treatment of hepatic failure. J Transpl Coord.
8:43–50. 1998.
|
|
28.
|
Ellis AJ, Hughes RD, Nicholl D, et al:
Temporary extracorporeal liver support for severe acute alcoholic
hepatitis using the BioLogic-DT. Int J Artif Organs. 22:27–34.
1999.PubMed/NCBI
|
|
29.
|
He JQ, Chen CY, Deng JT, Qi HX, Zhang XQ
and Chen ZQ: Clinical study on the treatment of fatal hepatitis
with artificial liver support system. Chinese Critical Care
Medicine. 12(2): 105–108. 2000.(In Chinese).
|
|
30.
|
Mitzner SR, Stange J, Klammt S, et al:
Improvement of hepatorenal syndrome with extracorporeal albumin
dialysis MARS: results of a prospective, randomized, controlled
clinical trial. Liver Transpl. 6:277–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Stevens AC, Busuttil R, Hans S, et al: An
interim analysis of a phase I/II prospective randomized,
multicenter, controlled trial of the HepatAssist®
bioartificial liver support system for the treatment of fulminant
hepatic failure. Hepatology. 34(Suppl): 299A2001.
|
|
32.
|
Heemann U, Treichel U, Loock J, et al:
Albumin dialysis in cirrhosis with superimposed acute liver injury:
a prospective, controlled study. Hepatology. 36:949–958. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Demetriou AA, Brown RS Jr, Busuttil RW, et
al: Prospective, randomized, multicenter, controlled trial of a
bioartificial liver in treating acute liver failure. Ann Surg.
239:660–670. 2004. View Article : Google Scholar
|
|
34.
|
El Banayosy A, Kizner L, Schueler V,
Bergmeier S, Cobaugh D and Koerfer R: First use of the Molecular
Adsorbent Recirculating System technique on patients with hypoxic
liver failure after cardiogenic shock. ASAIO J. 50:332–337.
2004.PubMed/NCBI
|
|
35.
|
Sen S, Davies NA, Mookerjee RP, et al:
Pathophysiological effects of albumin dialysis in acute-on-chronic
liver failure: a randomized controlled study. Liver Transpl.
10:1109–1119. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Laleman W, Wilmer A, Evenepoel P, et al:
Effect of the molecular adsorbent recirculating system and
Prometheus devices on systemic haemodynamics and vasoactive agents
in patients with acute-on-chronic alcoholic liver failure. Crit
Care. 10:R1082006. View
Article : Google Scholar
|
|
37.
|
Hassanein TI, Tofteng F, Brown RS Jr, et
al: Randomized controlled study of extracorporeal albumin dialysis
for hepatic encephalopathy in advanced cirrhosis. Hepatology.
46:1853–1862. 2007. View Article : Google Scholar
|
|
38.
|
Kribben A, Gerken G, Haag S, et al:
Effects of fractionated plasma separation and adsorption on
survival in patients with acute-on-chronic liver failure.
Gastroenterology. 142:782–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Allen JW, Hassanein T and Bhatia SN:
Advances in bioartificial liver devices. Hepatology. 34:447–455.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Sorkine P, Ben Abraham R, Szold O, et al:
Role of the molecular adsorbent recycling system (MARS) in the
treatment of patients with acute exacerbation of chronic liver
failure. Crit Care Med. 29:1332–1336. 2001. View Article : Google Scholar : PubMed/NCBI
|