Introduction
Cerebrovascular disease, due to its high incidence,
morbidity and mortality, is one of the three most serious human
diseases (1–3). Ischemic stroke accounted for 70–90%
of cerebral stroke cases and middle cerebral artery thromboembolism
is the main cause of ischemic stroke (4,5). An
in-depth study of ischemic cerebrovascular disease may useful for
the prevention, treatment and prognosis of the disease. The aim of
experimental studies of ischemic stroke is to establish animal
models that are similar to human cerebral ischemia. Currently,
methods of physically blocking blood flow by craniotomy,
suture-occlusion and microembolism are used to establish animal
models of cerebral ischemia (6–8). As
animal models of focal cerebral ischemia are usually prepared
without direct visualization, it is often not possible to
accurately determine whether vascular occlusion is affected by the
experimental strain, batch or weight of the animal, the proficiency
of the operator or other factors. These methods have certain
limitations, including poor experimental controllability and high
animal mortality, which render them unsuitable for studies of
thrombolytic therapy. Therefore, the establishment of an easily
controllable, stable, reliable and reproducible focal cerebral
ischemia model with minimal trauma to the animals, which is similar
to human brain ischemia, is essential for the study of the
pathophysiology, pathogenesis, prevention and control of cerebral
ischemia. In the present study, an animal model of cerebral
infarction was established using neurovascular interventional
techniques. The technical feasibility and stability of the model
have been summarized to assess the value of its clinical
application.
Material and methods
Animals
Ten healthy male New Zealand rabbits, weighing
2.5–3.0 kg, were used in the study. This study was carried out in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (9). The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of Jining First People’s Hospital,
Jining, China.
Thrombus preparation
The New Zealand rabbits were anesthetized with 3%
sodium pentobarbital (1 ml/kg, i.v.). Following iodinated
disinfection of the back of the rabbit ear, rabbit auricular
endarteria were punctured and scraped with a modified lumbar
puncture needle to form abrasions of ∼2 cm in the endarterium. A
silk suture was tied loosely around the blood vessel to reduce
blood flow, retard blood flow velocity and increase the likelihood
of embolus formation. The rabbits were anesthetized again 24 h
later and the scraped auricular artery was removed. The
intravascular thrombus was stripped under a magnifying glass and
was cut with an ophthalmic scalpel into 0.5×0.4 mm samples. The
samples were placed in sterile normal saline ready for use.
Establishing the experimental embolism
model
The rabbit from which the intravascular thrombosis
was stripped was anesthetized and placed supine on the operating
table of the bed of the subtraction angiography machine (OEC 9800;
GE Healthcare, Salt Lake City, UT, USA). The limbs were fixed,
routine disinfection was performed, the right vastus medialis skin
was incised and the right femoral artery was separated for
preparation of intubation and thrombolysis. After the distal end of
the right femoral artery was ligated and the proximal end of the
artery was clipped with a temporary occlusion clip, half of the
right femoral artery diameter was removed using ophthalmic scissors
and a 4F arterial sheath (Terumo, Tokyo, Japan) was placed via
right femoral artery puncture. Under the guidance of a TV monitor
and ancillary micro-guide wire, the Echelon-10 microcatheter system
(eV3, Micro Therapeutics, Inc., Irvine, CA, USA) was inserted via
the right femoral artery into the right or left common carotid
artery. Iodixanol (Jiangsu Hengrui Medicine Co., Ltd., Lianyungong,
China) was used as the contrast agent in a ratio of 2:1 to normal
saline. The catheter tip was placed flush with the lower edge of
the second cervical vertebra and the lateral roadmap was made. The
internal carotid artery was considered as a backward-running branch
of carotid artery, with an obvious sign of ampulla-like
enlargement. The micro-catheter crossed the occipital artery
opening at the proximal end of the carotid artery and lateral
angiography was conducted to assess the blood vessels. After the
contrast agent was administered by hand to demonstrate the absence
of evident reflux, three blood clot samples were injected with a 1
ml syringe through the micro-catheter. After occlusion of the
middle cerebral artery was demonstrated by angiography, the
micro-catheter was withdrawn. The temperature was maintained at
∼37°C after embolization and the vessel recanalization was reviewed
using angiography at 2 and 5 h, respectively, after embolization.
The catheter was flushed with heparin saline throughout the entire
process.
Cerebral angiography
The contrast agent iodixanol (100 mg/ml) was
injected into the vessel with a high-pressure syringe bolus through
the Echelon-10 micro-catheter in the internal carotid artery prior
to and at 0, 2 and 5 h after embolization in each rabbit. Digital
subtraction angiography (DSA) (pressure, 50 psi; speed, 0.5 ml/sec)
was conducted to observe vascular thrombosis.
Magnetic resonance imaging (MRI)
examination
Diffusion weighted imaging (DWI), T1WI
and T2WI scans were performed using a GE Signa HDe 1.5 T
superconducting MRI scanning machine (GE Healthcare) at 2 and 5 h
after successful establishment of the animal models. A small knee
coil was used. The DWI parameters were as follows: echo planner
imaging (EPI) list, repetition time (TR) 5,000 msec, echo time (TE)
10 msec, thickness 3 mm, interval 1 mm, field of vision (FOV) 16×16
cm, matrix 128×128 and scanning time 40 sec. The T2WI
parameters were as follows: TR 2,500 msec, TE 99 msec, thickness 3
mm, interval 1 mm, FOV 24×24 cm and matrix 128×128. The
T1WI parameters were as follows: TR 564 msec, TE 15
msec, thickness 3 mm, interval 1 mm, FOV 24×24 cm and matrix
128×128.
Scoring physical signs of neurological
deficits
The modified Bederson scoring (10)was used to evaluate the neurological
deficits 24 h after the anesthetized animals regained
consciousness. The scores were: 0, no neurologic deficit symptoms;
1, buckling of the contralateral forelimb; 2, grip of the
contralateral fore-limb decreased when the tail was pulled; 3,
movement without clear direction and turning in circles towards the
contralateral side when the tail was grasped; 4, spontaneously
turning in circles to the contralateral side. The higher the score,
the more serious the neurological disorder was.
Pathological examination
The animals were sacrificed by carotid phlebotomy 24
h after embolization. The brain tissue was completely separated
from the medulla oblongata. The brain was quickly removed and
placed in the freezer. When frozen for ∼20 min, the brain was cut
into six coronal brain slices of ∼5 mm in thickness. The slices
were checked for bleeding and incubated in 2%
2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO,
USA) at 37°C for 30 min. The slices were then transferred into 10%
formalin solution for fixation. The ischemic range of brain tissue
was observed after one week. The percentage of infarction area of
each rabbit was calculated using the following formula: Total
infarction area/hemispheric infarction zone ×100. After the sample
was hematoxylin and eosin (H&E)-stained and paraffin-embedded,
histological evaluation of the sample was conducted under a light
microscope.
Results
Signs of neurological deficits
All animals survived for 24 h after embolization.
Different degrees of paralysis were observed in the limb
contralateral to the embolization, including collapsing to the
contralateral side, hindlimb abduction, weakness, muscle tension
reduction and retractile reaction weakening. There were eight cases
of hemiplegia (collapsing to the hemiplegic contralateral side or
circling) and two cases of weakness of the contralateral forelimb
to embolism.
TTC staining
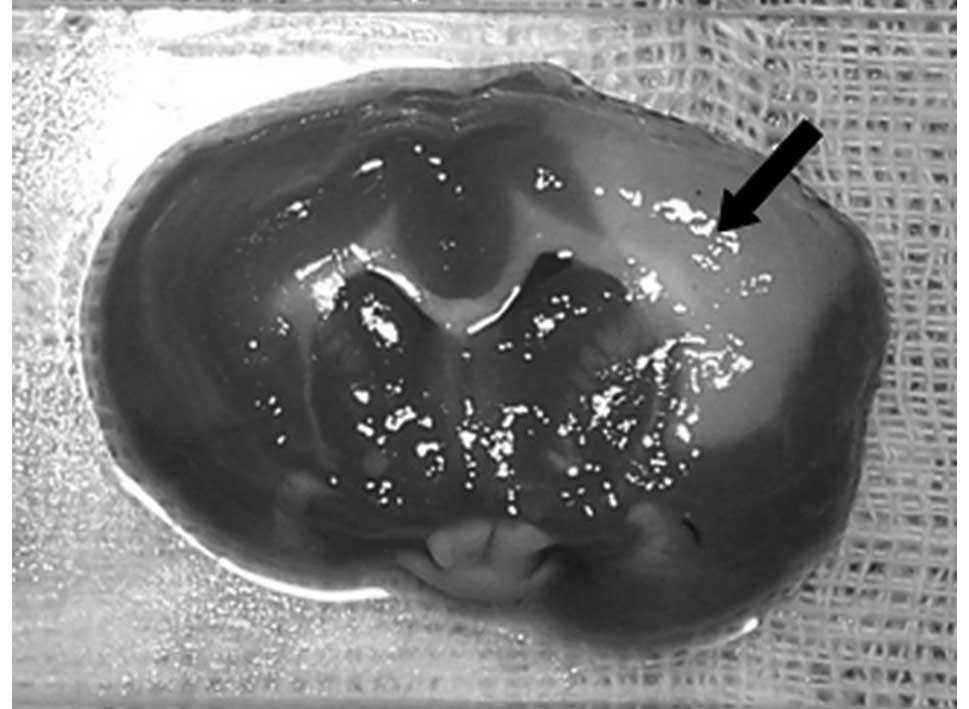
The infarction was clearly visible in the
distribution of the ipsilateral middle cerebral artery 24 h after
embolism. It was also possible to observe an irregular pattern of
infarction with the naked eye. This ranged in size from 0.5 to 2.0
cm with a gray central region and clear boundary (Fig. 1). The percentage of infarction area
was 38.67±2.23%.
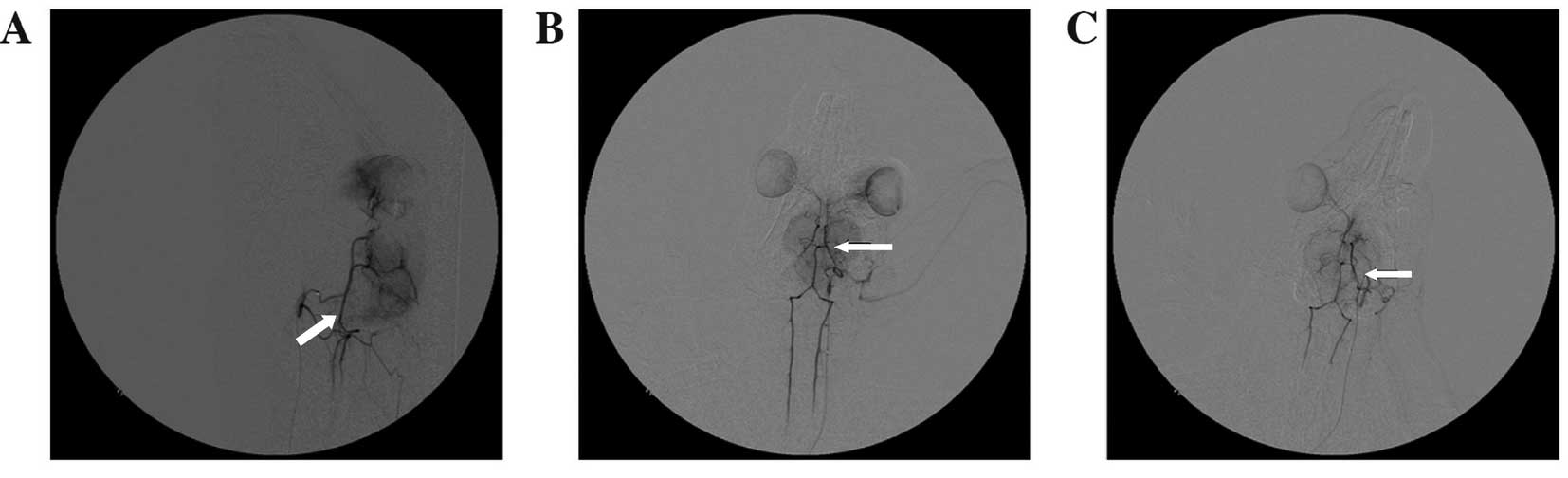
Cerebral angiography
Part of middle cerebral artery branches did not
develop after the injection of autologous blood clots in the
internal carotid artery (Fig. 2).
The results of angiography showed that no recanalization occurred
in the clogged vessels at 2 or 5 h after embolization.
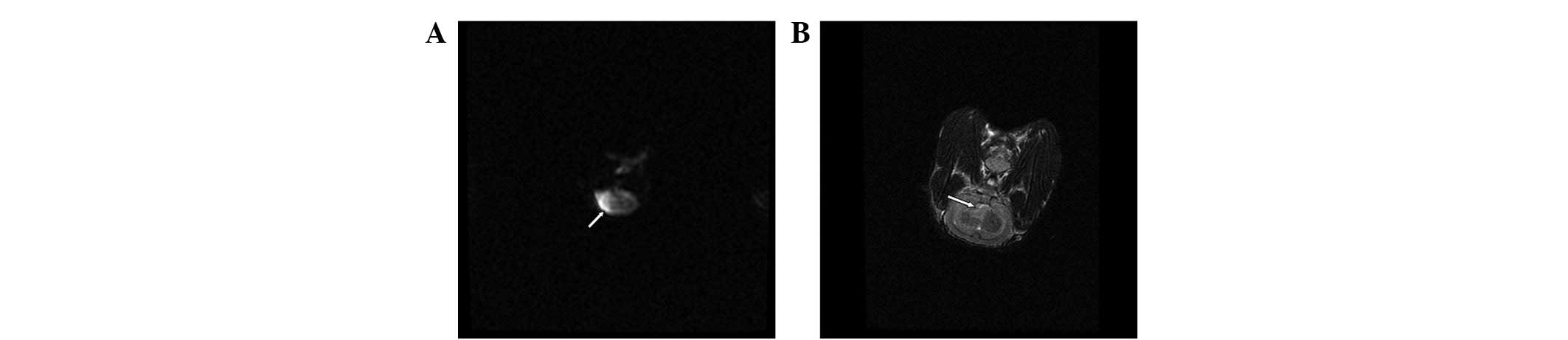
MRI examination
T2WI was negative when ischemia had been
induced for 2 h in the ten rabbits. After 5 h of induced ischemia,
there was a higher sign or greater signal change in all ten rabbits
(Fig. 3).
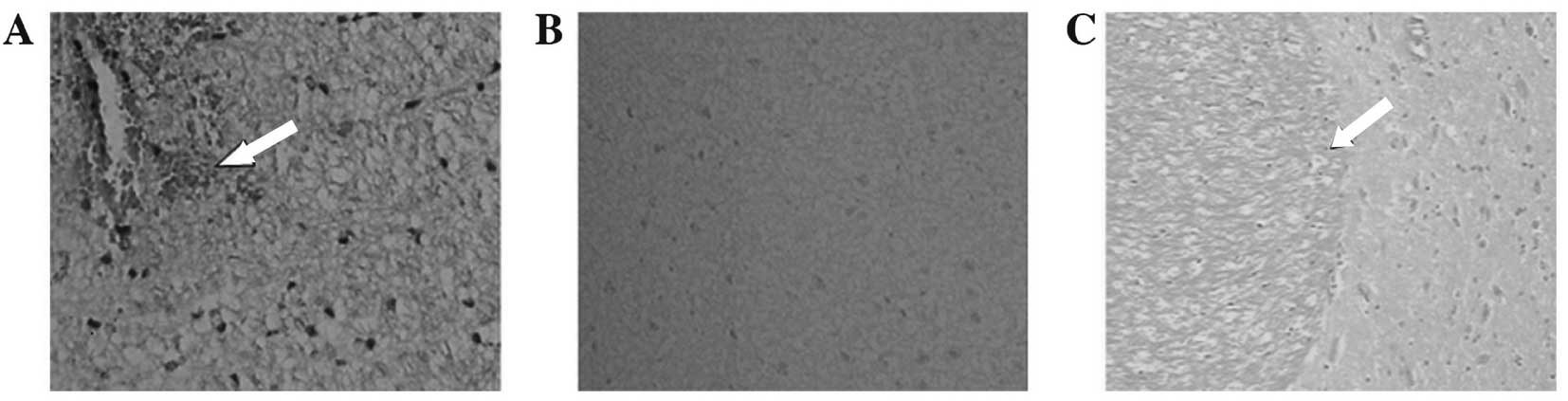
Light microscopic examination
The results of examination by light microscopy
showed that the brain tissues contralateral to the embolization
stained by H&E were normal. Observation of the embolization
under a light microscope indicated that edema was present in the
brain tissue of the infarct area and coagulative necrosis occurred
widely in the nerve cells. The nuclei became pyknotic or
disappeared and the cell outline was vague. The nerve and glial
cells were significantly reduced or disappeared. The neutrophil
infiltration occurred in a discrete area. Microglia hyperplasia and
neurotropic phenomena were also observed. The boundary between the
infarction and the normal area was clear and an infarcted area,
zone of edema and normal area were visible from the infarction
outwards (Fig. 4).
Discussion
Prior to an experimental study of the thrombolytic
treatment of acute cerebral infarction, an ideal animal model of
cerebral ischemia should first be established. Focal ischemic
cerebrovascular diseases are common in the clinic, particularly
middle cerebral artery occlusion. Due to poor collateral
circulation, the middle cerebral artery occlusion model has been
recognized as the standard animal model of focal cerebral
infarction (11). The experimental
results in the current study indicated that the method used for
establishing the animal model is reliable and repeatable. The
process used to generate the model is similar to that by which
human ischemic stroke occurs and the cerebral blood flow is easily
observed. The model established by this method is conducive to the
observation of the subsequent effect of thrombolysis and is
applicable to neuroimaging research on cerebral ischemia.
Common methods for creating models of focal cerebral
ischemia include opening the skull to physically block the middle
cerebral artery with electric coagulation (12), suture-occlusion (13,14)
and microembolism (6,7). As these animal models are different
from human stroke, they are not suitable for use in studies of
thrombolysis and anticoagulation therapy. An animal model of focal
cerebral infarction caused by autologous thromboembolism is similar
to the pathological process of human ischemic stroke and therefore
has a certain value for the evaluation of thrombolytic or
anticoagulant efficacy (15–19).
The ideal animal model of acute brain artery occlusion used for
superselective intra-arterial thrombolysis should meet the
following requirements: i) The animal vascular anatomy should be
close to that of human brain blood vessels, and cerebral
angiography and superselective intra-arterial thrombolysis should
be relatively easy to perform. ii) Cerebrovascular variation of the
animal should be minimal, with the location and extent of the
embolism being fixed readily and with good reproducibility. iii)
Success and animal survival rates should be high, with minimal
surgical injury. iv) Observation of vascular occlusion should be
possible from the changes in the blood flow. v) The animal blood
should be sufficient to be drawn repeatedly and be suitable for
dynamic observation of certain biochemical changes. Embolization in
the rabbit middle cerebral artery infarction under DSA may make the
control of the infarction conditions relatively consistent. Rabbit
blood is sufficient in quantity for repeated drawing and is also
suitable for dynamic observation of certain biochemical changes.
The rabbit vascular anatomy is close to that of human brain blood
vessels and cerebral angiography may be easily completed.
Therefore, rabbits are suitable for use as a thrombolytic animal
model.
The preparation of thromboemboli is critical for the
successful establishment of a model. In humans, 75% of cerebral
emboli comprise ‘white thrombus’, i.e., are rich in platelets and
fibrin (20). An ideal thrombosis
should be rich in cellulose, with no deformation, be difficult to
autolyze and of sufficient size to block the middle cerebral artery
initial segment. Thrombi formed within arterial or venous blood
vessels are red and the main constituents are red blood cells and
platelets. The thrombi that readily undergo autolysis are subject
to further processing (15,16,21–23).
In the present study, autologous arterial thrombi were prepared by
puncturing and scraping the rabbit auricular endarterium to form
abrasions with a modified lumbar puncture needle. The thrombus
formed in this way is rich in fibrin and platelets, and similar to
those formed in human atherosclerosis, and so is more suitable for
the study of thrombolytic therapy.
In a previous study, the embolus was injected by
intubation through the internal carotid artery using the Hamilton
method (24). This model may often
result in injury to the internal carotid artery and readily cause
the formation of thrombosis that may affect the normal flow of the
internal carotid artery. In the Benes method (22), external and internal carotid
arteries near the skull base are anatomized. Following the ligation
of each branch of the external carotid artery, intubation was
inserted retrogradely into the opening of the internal carotid
artery. As it is difficult to surgically expose the bifurcation,
microsurgery is often required to anatomize the carotid
bifurcation. Therefore, the high technical requirement and
complexity of the modeling process may result in serious animal
trauma and high mortality. In addition, 25.7% of the rabbit
occipital artery originates from the internal carotid artery, and
13.3% of occipital arteries with a large diameter originate from
the distal end of the internal carotid artery near the skull base
(25). Since the previous method
may fail to ligate the occipital artery separated from the internal
carotid artery, resulting in some emboli entering into the
occipital artery, the success rate was only 50–85% (24). To ensure the probability of
success, superselective intubation of the internal carotid artery
was used for embolization of the middle cerebral artery after
crossing the opening of occipital artery near the proximal end of
the internal carotid artery or embolization of the occipital
artery.
The results of angiography demonstrated that the
internal carotid artery of the rabbit is the main blood supply of
the intracranial artery and there is no anastomosis network between
the intracranial and extracranial vessels. End-to-end anastomosis
among branches of the brain blood vessels is similar to that of the
human brain. The occipital artery originates from the internal
carotid artery and its distal end may diminish in size to half that
of the initial part. The distal diameter of the internal carotid
artery, other than the separated occipital artery branch, was not
observed to change significantly. The extracranial segment of the
rabbit carotid artery is tortuous and slender to form a loop during
ascent, which is similar to the human carotid siphon. Two branches
are separated from its ends; following the separation of the middle
and anterior cerebral arteries, some of the blood vessels
originating from the former branch supply blood to the eye, head
and facial organs while the latter branch, which is rearward and
downward in direction, is the posterior communicating artery. The
two bilateral branches are able to form the circle of Willis with
the basilar artery and have no anastomosis with the external
carotid artery. As the rabbit circle of Willis has good
compensation, it is difficult for a large thrombus to form a
cerebral infarction due to embolization in the internal carotid
artery. The size of the autologous thrombus in the current study
was 0.5×0.4 mm, which is not sufficient to directly block the
internal carotid artery. Following the insertion of a
micro-catheter into the internal carotid artery by ∼1 cm, a
contrast agent may be injected and, if no regurgitation is evident,
a thrombus may subsequently be injected.
Since the middle cerebral artery with its relatively
large blood flow is the largest branch of the internal carotid
artery, the probability of occlusion in the middle cerebral artery
is the highest. The incidence of embolization in posterior
communicating artery running backward and downward was the lowest,
which was basically in line with the morbidity in humans.
Embolization to the proximal end in M1 infarction occurs in the
cerebral hemispheres and basal ganglia. Embolization to the distal
end of M1 mainly manifests in the lobular cortex. Embolization to
the distal end of M2 generally results in focal cerebral
infarction. Since the current interventional devices are not yet
able to selectively insert emboli into the rabbit middle cerebral
artery, shortcomings remain. These include instability in the
blocking of the vessels and the occasional entry of emboli into the
anterior cerebral or contralateral intracranial artery.
DSA cerebral angiography may accurately display
thromboembolic site thrombolysis and artery recanalization, but not
abnormal brain parenchyma and infarct range. Functional magnetic
resonance DWI may be used to determine the ischemic range. It also
may be used to evaluate the efficacy of thrombolytic therapy and
for the study of ischemic penumbra. In the current study, all
animals were sacrificed 24 h after embolization and the results of
TTC staining also demonstrated the infarction area, location and
the scope in the middle cerebral artery, which were consistent with
the results of examination by functional magnetic resonance
DWI.
In the current study, we noted the following: i) In
order to achieve good controllability, reduce surgery time and
increase the success rate, the roadmap function may be used to make
the operation of the catheter and guide wire visible. ii) Operation
of the micro-guide wire should be performed gently. The micro-guide
wire tension should be released, particularly when entering into
the middle cerebral artery, so as not to pierce the blood vessel,
and thereby cause cerebral hemorrhage and model failure. iii)
Continuously washing with heparin saline during surgery may
effectively prevent thrombosis from the cerebral infarction
occurring outside the target area. This model may avoid the large
trauma caused by surgery at the neck and is conducive to the
animal’s rapid recovery and the long-term observation of the model,
as well as the treatment effect. The disadvantage is the
requirement for a subtraction angiography machine and professional
personnel who are capable of carrying out the endovascular
interventional technique.
In conclusion, we performed technological
improvements to an experimental animal model of focal cerebral
ischemia and successfully established focal cerebral ischemia
animal models using endovascular interventional techniques. The
results suggest that the rabbit animal model of acute cerebral
embolism was successfully established using embolization techniques
through superselective catheterization to the rabbit carotid artery
beyond the separated occipital artery branch. This method has
numerous advantages, including having no requirement for
craniotomy, minimal trauma, a reliable ischemic effect and a high
animal survival rate, and is particularly suitable for the study of
selective intra-arterial thrombolytic therapy. The model has the
following notable features: the uniformity of the embolus size,
stability, similarity to clinical cerebral thrombosis and
suitability for the study of thrombolytic therapy. In addition, the
probability of ectopic embolism is greatly reduced using
superselective embolization; small amounts of emboli may create
marked symptoms of cerebral ischemia; there is minimal injury to
the animal with a high success rate and low mortality; and the
model embolism is similar to human cerebral embolism. The present
model also has a wide range of applications with scalability and
may be commonly used in other experimental animals, including dogs
and monkeys. Further applications of the model are studies
concerning the diagnostic imaging of cerebral infarction and
pathophysiological changes. Angiography is able to accurately
display the thromboembolic site thrombolysis and artery
recanalization.
The embolism model established in this study is
similar to human cerebral embolism, with little injury, high
success rate and low mortality rate. This model may suitably
expanded to application in commonly used experimental animals,
including rabbits, dogs and monkeys. The establishment of this
model provides a broad prospect for further study of the
morphology, functional metabolomics, pharmacology, clinical
therapeutics, neurological interventional radiology and
neurosurgery of cerebral ischemia.
References
|
1.
|
Rothwell PM: The high cost of not funding
stroke research: a comparison with heart disease and cancer.
Lancet. 357:1612–1616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar
|
|
3.
|
Carro JJ, Huybrechts KF and Duchesne I:
Management patterns and costs of acute ischemic stroke: an
international study. Stroke. 3:582–590. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Roger VL, Go AS, Lloyd-Jones DM, et al:
Executive summary: heart disease and stroke statistics - 2012
update: a report from the American Heart Association. Circulation.
125:188–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mellado TP, Court LJ, Godoy FJ, et al:
Cerebrovascular disease in a Neurologic Intermediate Care Unit in
Chile. Analysis of 459 consecutive patients. Rev Med Chil.
133:1274–1284. 2005.(In Spanish).
|
|
6.
|
Jahan R, Stewart D, Vinters HV, et al:
Middle cerebral artery occlusion in the rabbit using selective
angiography: application for assessment of thrombolysis. Stroke.
39:1613–1615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Amiridze N, Gullapalli R, Hoffman G and
Darwish R: Experimental model of brainstem stroke in rabbits via
endovascular occlusion of the basilar artery. J Stroke Cerebrovasc
Dis. 18:281–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington: 2011
|
|
10.
|
Zhang Z, Zhang RL, Jiang Q, Raman SB,
Cantwell L and Chopp M: A new rat model of the thrombotic focal
cerebral ischemia. J Cereb Blood Flow Metab. 17:123–135. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Derouesné C, Cambon H, Yelnik A,
Duyckaerts C and Hauw JJ: Infarcts in the middle cerebral artery
territory. Pathological study of the mechanisms of death. Acta
Neurol Scand. 87:361–366. 1993.PubMed/NCBI
|
|
12.
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemia brain edema: a new
experimental model of cerebral embolism in rats in which
recirculation can be introduced in the ischemia area. Jpn J Stroke.
8:1–8. 1986.(In Japanese).
|
|
14.
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Revesible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zheng YL and Hu CL: Establishment of a
middle carotid arterial embolic stroke model in rats. Neural Injury
and Functional Reconstruction. 4:25–27. 2009.(In Chinese).
|
|
16.
|
Liu ZP and Liu HJ: Establishment of
embolic stroke model and study of the efficacy of intra-arterial
urokinase. Journal of Hebei Medical University. 28:105–110.
2007.(In Chinese).
|
|
17.
|
Zhang Z, Zhang RL, Jiang Q, Raman SB,
Cantwell L and Chopp M: A new rat model of the thrombotic focal
cerebral ischemia. J Cereb Blood Flow Metab. 17:123–135. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Kashiwabara S, Kurokawa Y, Uede T and
Hashi K: Direct continuous observation of in situ thrombolysis in
the cerebral embolic model of rabbits. No To Shinkei. 50:347–354.
1998.(In Japanese).
|
|
19.
|
Overgaard K, Sereghy T, Pedersen H and
Boysen G: Neuroprotection with NBQX and thrombolysis with rt-PA in
rat embolic stroke. Neurol Res. 15:344–349. 1993.PubMed/NCBI
|
|
20.
|
Marder VJ, Chute DJ, Starkman S, et al:
Analysis of thrombi retrieved from cerebral arteries of patients
with acute ischemic stroke. Stroke. 37:2086–2093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Overgaard K, Sereghy T, Pedersen H and
Boysen G: Effect of delayed thrombolysis with rt-PA in a rat
embolic stroke model. J Cereb Blood Flow Metab. 14:472–477. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Benes V, Zabramski JM, Boston M, Puca A
and Spetzler RF: Effect of intra-arterial tissue plasminogen
activator and urokinase on autologous arterial emboli in the
cerebral circulation of rabbits. Stroke. 21:1594–1599.
1990.PubMed/NCBI
|
|
23.
|
Busch E, Krüger K and Hossmann KA:
Improved model of thromboembolic stroke and rt-PA induced
reperfusion in the rat. Brain Res. 778:16–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hamilton MG, Lee JS, Cummings PJ and
Zabramski JM: A comparison of intra-arterial and intravenous
tissue-type plasminogen activator on autologous arterial emboli in
the cerebral circulation of rabbits [corrected]. Stroke.
25:651–656. 1994.PubMed/NCBI
|
|
25.
|
Lee JS, Hamilton MG and Zabramski JM:
Variations in the anatomy of the rabbit cervical carotid artery.
Stroke. 25:501–503. 1994. View Article : Google Scholar : PubMed/NCBI
|