Introduction
Beauveria bassiana is a type of
entomopathogenic fungi used as an insecticide (1). In humans, this fungus has limited
toxicity. To the best of our knowledge, only a few cases of
keratitis due to Beauveria bassiana have been documented (1). Furthermore, entomopathogenic fungi
are known for their beneficial activities in various biological
fields (2). Previously,
entomopathogenic fungi, including Beauveria bassiana,
Cordyceps sinensis, Cordyceps militaris and
Paecilomyces, have been used to treat atopic dermatitis,
athlete’s foot and dandruff (3).
These fungi have also been shown to possess immunomodulatory,
anti-diabetic, anti-stress and anti-tumor activities (4); however, their cosmeceutical
properties are not adequate for use. Studies concerning the
whitening effects of fungal fermentation products have since been
initiated (5).
It is considered important to conduct eye irritancy,
skin irritancy and phototoxicity tests prior to obtaining approval
and authorization for the use of test compounds as functional
cosmetic ingredients. Since Draize developed a method for the
measurement of irritancy and toxicity of substances applied
topically to the skin and mucous membranes (6), numerous trials have been performed to
assess the cosmetic and cosmeceuticals effect of various products.
However, alternative testing methods, including an in vitro
3T3 NRU phototoxicity test and local lymph node assays (7), are increasingly being considered as
credible alternatives to animal models for evaluating functional
cosmetic ingredients, due to ethical concerns about animal use. A
number of potential applications are emerging for the use of
biochemicals present in insect extracts (or fractions thereof) as
cosmetics/cosmeceuticals. For this reason, we performed toxicity
tests to investigate whether S-(-)-10,11-dihydroxyfarnesic acid
methyl ester, a compound isolated from Beauveria bassiana
CS1029, has the potential to induce irritation of the ocular
mucosa. To the best of our knowledge, it has not been documented
whether insect extracts cause toxicity when the skin or eye lens is
exposed to them. Unwanted reactions to cosmetics are frequent in
patients with allergic contact dermatitis. Various adverse effects,
including acute/chronic toxicity, irritation and sensitization,
have been assessed using in vivo, in vitro, semi
in vivo and ex vivo animal models (8–10).
In the present study, we performed the eye
irritation test with a derivative of dihydroxyfarnesic acid
produced by Beauveria bassiana CS1029 using an in
vivo animal model. Various parameters were assessed to evaluate
the degree of eye irritation induced by the compound and determine
whether it is safe for development in cosmetic/cosmeceutical
applications.
Materials and methods
Animals and care
New Zealand white (NZW) rabbits (9-week-old males
weighing 2.1–2.4 kg) were purchased from Samtaco (Osan, Korea). The
animals were fed a commercial diet (Purina, Seoul, Korea) and water
ad libitum throughout the experiment. The study protocols
complied with the guidelines of the International Association for
the Study of Pain Committee for Research and Ethical Issues
(11) and the internal guidelines
of the Kyungpook National University Animal Ethical Committee were
strictly observed. All animals acclimated to the laboratory
environment for at least 1 week prior to commencement of the
experiment.
Isolation and preparation of
S-(-)-10,11-dihydroxyfarnesic acid methyl ester
S-(-)-10,11-dihydroxyfarnesic acid methyl ester was
produced by Beauveria bassiana CS1029. In brief, a
fermentation medium consisting of 3% sucrose, 2% corn steep liquor
(C4648; Sigma, St. Louis, MO, USA), 0.05% potassium phosphate
dibasic, 0.1% potassium phosphate monobasic and 0.05% magnesium
sulfate•6H2O was prepared in a 5-liter mini jar
fermenter (Hankook Fermenter, Seoul, Korea). The medium was then
sterilized at 121°C for 30 min and chilled prior to inoculation
with the seed culture of Beauveria bassiana CS1029 up to 5%.
Fermentation was performed for 3 days. The fermentation broth was
then centrifuged at 10,000 × g for 30 min and the supernatant was
added to the following columns as previously described (12). Briefly, the precipitate was applied
to an HP chromatography column and high-performance liquid
chromatography (HPLC) was performed using a reverse column (Waters,
Milford, MA, USA) with a detector at 254 nm (Waters 2998 Photodiode
Array detector). A peak was identified as
S-(-)-10,11-dihydroxyfarnesic acid methyl ester by nuclear magnetic
resonance (NMR) and mass spectroscopy as previously described
(12). A voucher specimen
(#2009-Bb) of the methyl ester obtained from Beauveria
bassiana CS1029 was deposited in the Laboratory of Food Enzyme
Biotechnology, Kyungpook National University, Korea.
Eye irritation test
S-(-)-10,11-dihydroxyfarnesic acid methyl ester (100
mg/100 μl) was dripped into the eyes of each NZW rabbit
(n=3) which were held open with clips at the lid. As a positive
control, 10% sodium dioctyl sulfosuccinate solution was applied.
Progressive damage to the rabbit eye was monitored every day for 5
days. Potential reactions to the methyl ester included swelling of
the eyelid, iris inflammation, ulceration and hemorrhage (13,14).
In brief, the eye lens mucosa was assessed for localized
irritation. Saline was used as the control. The conjunctival sac in
the right eye of each rabbit was treated with the undiluted methyl
ester (0.1 ml), negative control (saline) or positive control (10%
sodium dioctyl sulfosuccinate). After applying the solutions once
for 2 sec, the eyes were washed with saline. The undiluted methyl
ester (0.1 ml) was administered once under the eyelid, which was
slightly pulled away from the eyeball to form a space to allow easy
delivery into the conjunctival sac. The cornea, iris and
conjunctiva were then examined daily (for 1, 2, 3, 4 and 5 days) to
evaluate acute irritation of the lens mucosa.
Analysis of irritancy
The development of eye lesions was monitored by
comparison of the treated eye with the left eye that was not
treated with the test substance, as previously described (15). On days 1, 2, 3, 4 and 5 after
application of the methyl ester or positive control, the following
variables were evaluated with the naked eye: corneal opacity and
turbidity, reaction of the iris, conjunctival edema and ocular
discharge. Irritation of the eye lens mucosa was evaluated based on
redness or ocular lesion development by clinical examiners under
the direction of a veterinarian from the Center of Laboratory
Animal and Care, Kyungpook National University, Korea.
Results and Discussion
While screening natural resources for active
components exhibiting whitening activities that may be used in
cosmetics, we identified that Beauveria bassiana CS1029
produces a potent compound into the medium during liquid culturing.
The compound was identified to be S-(-)-10,11-dihydroxyfarnesic
acid methyl ester and was observed to display anti-tyrosinase
activity in vitro and in vivo (12) (data not shown). We subsequently
determined whether the agent was capable of ameliorating conditions
associated with skin inflammation, including atopic dermatitis
(12).
Biomaterials derived from insects and
insect-symbiotic fungi may be obtained using a variety of methods,
including supercritical extraction, microbial fermentation,
biotransformation and chemical modification. Certain biomaterials
may be converted into cosmetic, cosmeceutical or neutraceutical
ingredients. This prompted our investigation in which we
investigated an anti-tyrosinase agent derived from medicinal insect
extracts and identified that it exhibited a potent whitening
activity (12). To determine
whether the agent was toxic or non-toxic and suitable to serve as a
cosmetic ingredient, we performed an acute toxicity
investigation.
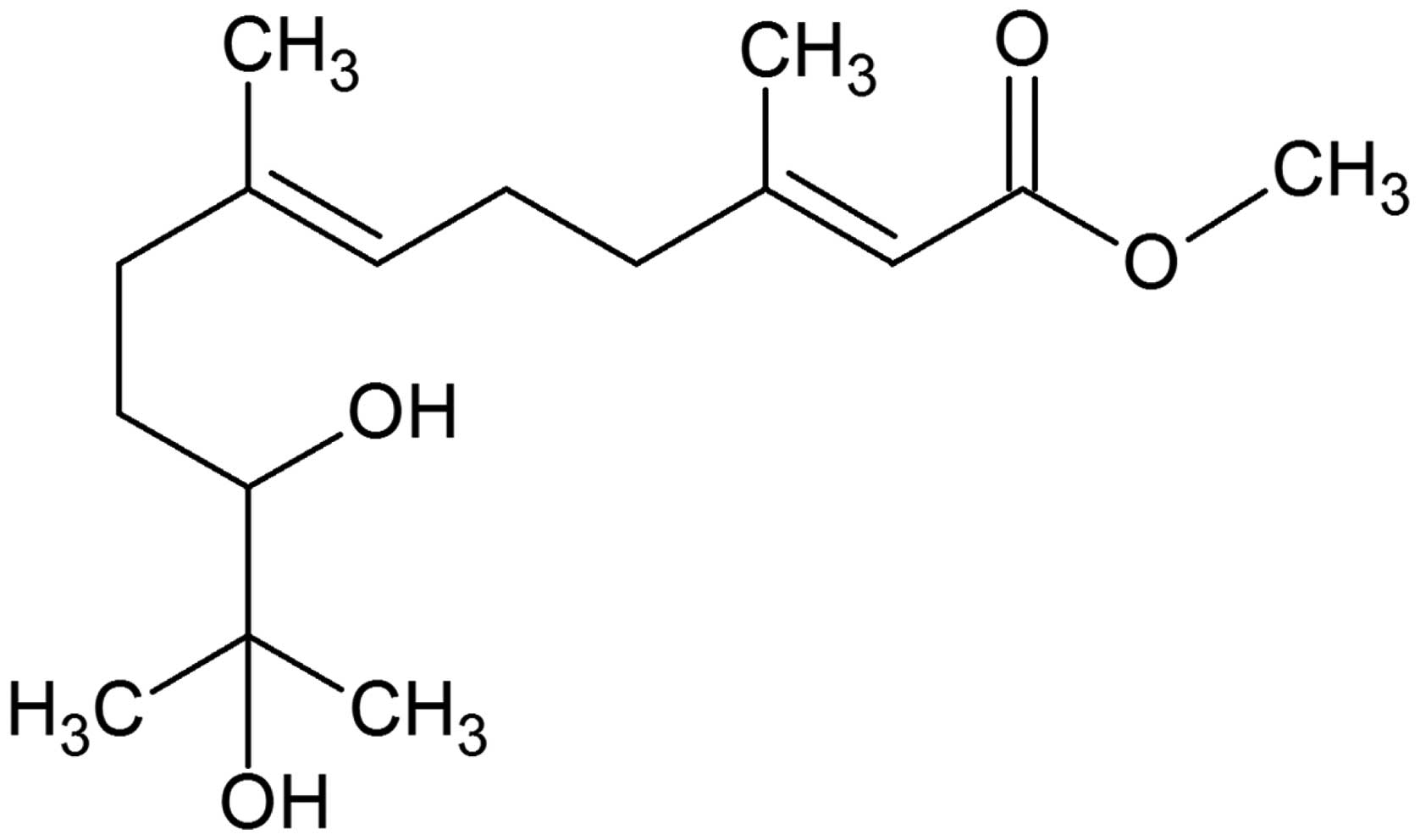
In the present study, S-(-)-10,11-dihydroxyfarnesic
acid methyl ester (Fig. 1; final
concentration, 100 mg/100 μl) was administered to rabbit
eyes and eye irritation data was obtained to determine whether the
compound is safe to use. When saline was administered as the
control, no adverse symptoms were observed around the pupil or
whites of the eye (data not shown). The Draize eye irritation test
used in the current study is strictly observational and is not
considered to reflect the degree of irritation in humans adequately
(13). This technique is,
therefore, generally considered crude, imprecise and unreliable;
however, it is inexpensive, time-saving and produces potentially
convincing data. A number of scientists are seeking alternative
testing methods to avoid animal ethics issues.
In the present study, we precisely evaluated the
symptoms of toxicity using the following criteria: swelling,
inflammation and lesions on the eye lens. Initially, 24 h after
treatment with the methyl ester, morphological changes of the
eyelid and ocular mucosa membranes were examined. Ocular lesions in
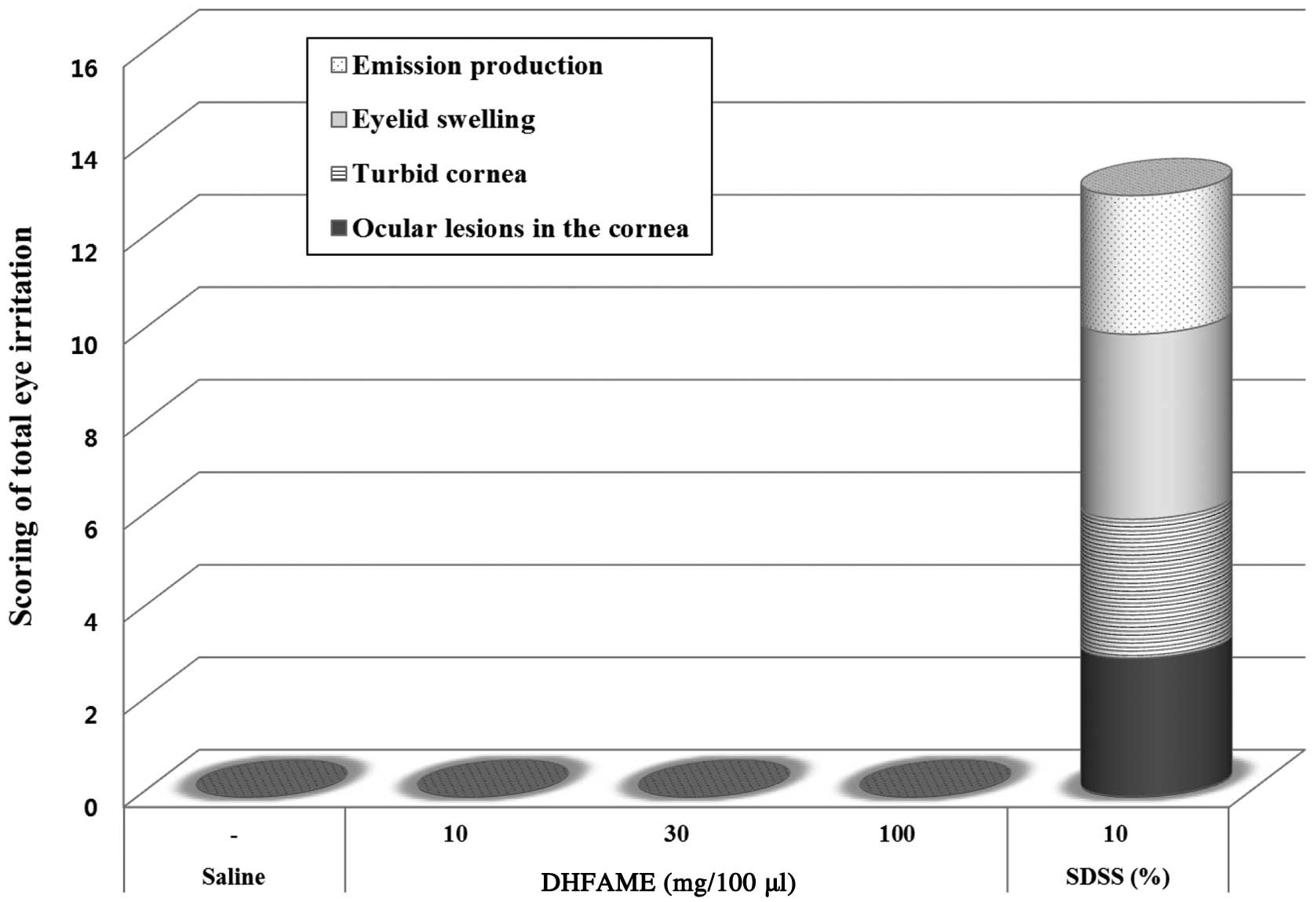
the cornea (represented by the black section in Fig. 2) were scored as follows: 0, no
suppuration or haze; 1, slight opacity compared with normal
transparency; 2, semi-transparent; 3, no observation at the end of
the pupil size; and 4, an opaque and turbid cornea but unaffected
iris. The methyl ester did not induce ocular lesions in the cornea
(Fig. 2) or any other symptoms
(similar to saline). Scores for turbid cornea size (lined section
in Fig. 2) were as follows: 0, no
turbidity; 1, 1/4 or less; 2, greater than 1/4 to no more than 1/2;
3, greater than 1/2 to no more than 3/4 in size; and 4, greater
than 3/4 to the entire cornea affected. The turbid cornea size was
not affected by the methyl ester (Fig.
2). The effects of the methyl ester on eyelid swelling were
also examined. Eyelid swelling (grey section in Fig. 2) was scored as follows: 0, no
swelling; 1, slightly swollen; 2, significant swelling of the
eyelid resulting in partial abduction; 3, swelling affecting
approximately half of the eyelid; and 4, more than half of the
eyelid is swollen. Using this scoring system, we confirmed that
eyelids treated with the methyl ester were not significantly
swollen (Fig. 2). Production of
ocular discharge (dotted section in Fig. 2) was analyzed according to the
following scale: 0, no discharge; 1, a small amount of moistness
around the eyelashes; 2, wet discharge; and 3, a large area around
the eye, eyelid and/or eyelashes containing wet discharge. No
discharge was observed in the eye or eyelid/eyelashes following
treatment with the methyl ester (Fig.
2). Overall, we did not detect any changes or damage induced by
the methyl ester; this was in contrast with sodium dioctyl
sulfosuccinate, which caused severe symptoms of toxicity (Fig. 2) based on our clinical
observations.
The criteria for determining whether other
parameters are associated with acute eye irritation were also
assessed. Observations made at different time intervals (day 1, 2,
3, 4 and 5) demonstrated that the methyl ester did not lead to the
development of ocular lesions in the cornea (Fig. 3), increase the turbid cornea size
(data not shown), eyelid swelling (data not shown) or emission
production (data not shown), whereas sodium dioctyl sulfosuccinate
produced severe effects (Fig. 3)
on days 1, 2, 3, 4 and 5.
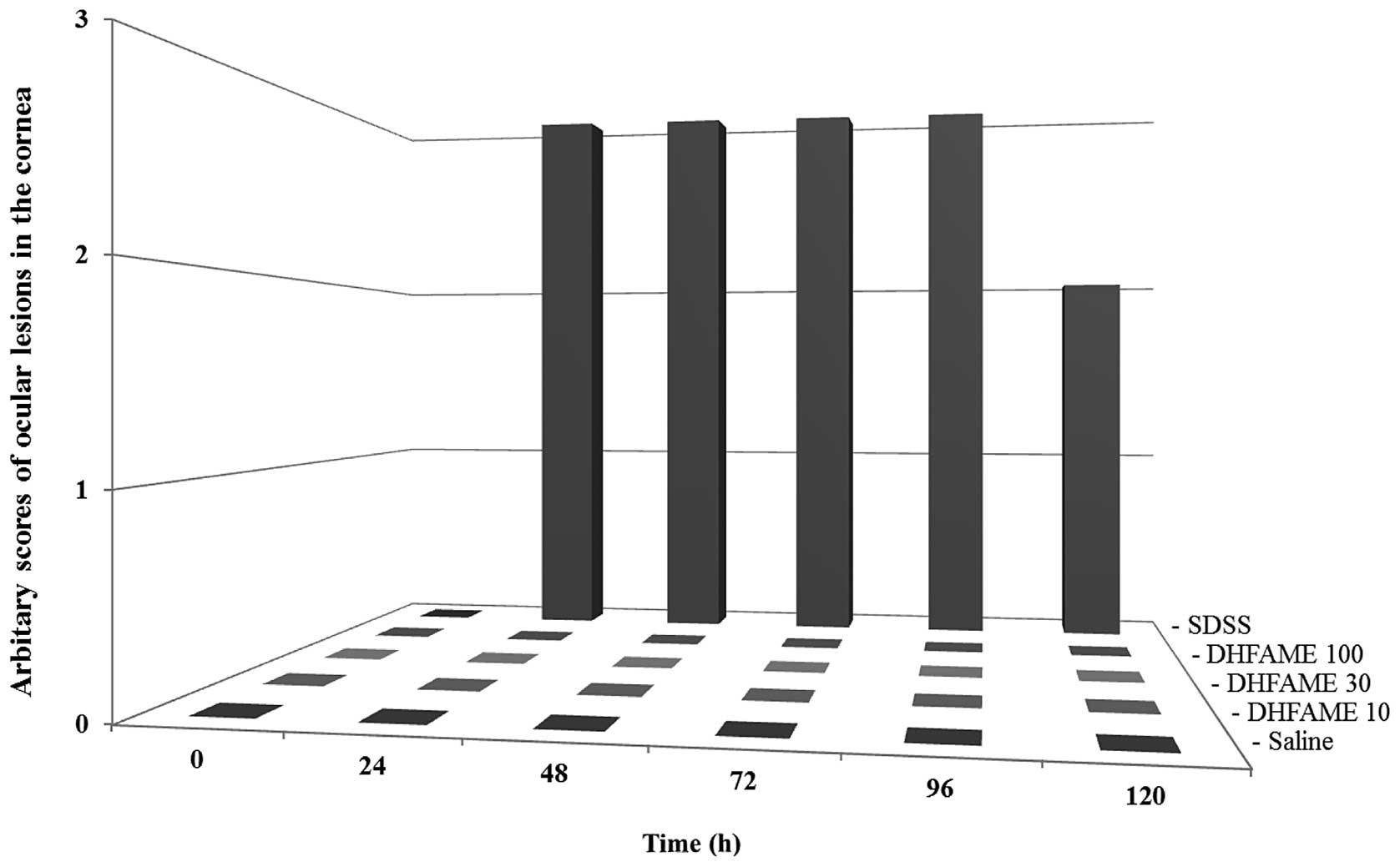 | Figure 3.Development of ocular lesions in the
cornea due to acute eye irritation over time. Scoring standards are
described in Materials and methods. Observations were made at 0,
24, 48, 72, 96 and 120 h after treatment with various reagents.
Arbitrary scores for measuring eye irritation (assessed by ocular
lesions in the cornea) over time are shown. Data shown are
representative results of five independent observations. SDSS,
sodium dioctyl sulfosuccinate. DHFAME,
S-(-)-10,11-dihydroxyfarnesic acid methyl ester. Lane 1, saline;
lane 2, DHFAME (10 mg/100 μl); lane 3, DHFAME (30 mg/100
μl); lane 4, DHFAME (100 mg/100 μl); lane 5,
SDSS. |
Certain toxic effects may be revealed by other
safety tests; therefore, we are unable to exclude the possibility
of potential toxicity based on acute, sub-acute or chronic safety
tests (16–18).
In summary, the dihydroxyfarnesic acid methyl ester
produced by Beauveria bassiana CS1029 did not induce
symptoms of acute eye irritation (haze, swelling, redness or
discharge from the ocular lens mucosa) in rabbits. This compound
may therefore be suitable for use in the development of cosmetic or
cosmeceutical products. Additionally, no toxic effects were
observed in the eye irritation test. Future studies using
alternative methods to assess eye and skin irritation, as well as
phototoxicity in vitro and in vivo, are required to
more fully understand the long-term effects and safety of this
compound for used in cosmetics.
Acknowledgements
The authors thank Ms Min-A Kim, Mr
Chi-Ryeol Yoo and Mr Dong-Yoon Nam for their technical assistance.
This study was supported by The Kyungpook National University
Research Fund, 2012.
References
|
1.
|
Figueira L, Pinheiro D, Moreira R, Pinto
E, Simões J, Camisa E, Torrão L, Palmares J and Falcão-Reis F:
Beauveria bassiana keratitis in bullous keratopathy:
antifungal sensitivity testing and management. Eur J Ophthalmol.
22:814–818. 2012.
|
|
2.
|
Pedrini N, Crespo R and Juárez MP:
Biochemistry of insect epicuticle degradation by entomopathogenic
fungi. Comp Biochem Physiol C Toxicol Pharmacol. 146:124–137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhou X, Gong Z, Su Y, Lin J and Tang K:
Cordyceps fungi: natural products, pharmacological functions and
developmental products. J Pharm Pharmacol. 61:279–291. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tomoda H and Doi T: Discovery and
combinatorial synthesis of fungal metabolites beauveriolides, novel
antiatherosclerotic agents. Acc Chem Res. 41:32–39. 2008.PubMed/NCBI
|
|
5.
|
Liu L, Liu Y, Li J, Du G and Chen J:
Microbial production of hyaluronic acid: current state, challenges,
and perspectives. Microb Cell Fact. 10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Draize JH: Appraisal of the Safety of
Chemicals in Foods, drugs and cosmetics. Association of Food and
Drug Officials of the United States; CA: pp. 46–59. 1959
|
|
7.
|
Nigam PK: Adverse reactions to cosmetics
and methods of testing. Indian J Dermatol Venereol Leprol.
75:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tavaszi J, Budai P, Pálovics A and
Kismányoki A: An alternative test battery in detecting ocular
irritancy of agrochemicals. Commun Agric Appl Biol Sci. 73:891–895.
2008.PubMed/NCBI
|
|
9.
|
Scott L, Eskes C, Hoffmann S, Adriaens E,
et al: A proposed eye irritation testing strategy to reduce and
replace in vivo studies using bottom-up and top-down approaches.
Toxicol In vitro. 24:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Osborne R, Perkins MA and Roberts DA:
Development and intralaboratory evaluation of an in vitro human
cell-based test to aid ocular irritancy assessments. Fundam Appl
Toxicol. 28:139–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nam SH, Yoon CS, Jeon JY, Lee SH, Lee KG,
Yeo JH and Hwang JS: Composition exhibiting melanin-inhibiting
activity. KR Patent 10-1239631. Filed March 28, 2011; issued
February 27, 2013.
|
|
13.
|
Draize JH, Woodard G and Calvery HO:
Methods for the study of irritation and toxicity of substances
applied topically to the skin and mucous membranes. J Pharmacol Exp
Ther. 82:377–390. 1944.
|
|
14.
|
Aoshima H, Saitoh Y, Ito S, Yamana S and
Miwa N: Safety evaluation of highly purified fullernenes (HPFs):
based on screening of eye and skin damage. J Toxicol Sci.
34:555–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Son HU, Yoon EK, Cha YS, Kim MA, Shin YK,
Kim JM, Choi YH and Lee SH: Comparison of the toxicity of aqueous
and ethanol fraction of Angelica keiskei leaf using the eye
irritancy test. Exp Ther Med. 4:820–824. 2012.PubMed/NCBI
|
|
16.
|
Korting HC, Herzinger T, Hartinger A,
Kerscher M, Angerpointner T and Maibach HI: Discrimination of the
irritancy potential of surfactants in vitro by two cytotoxicity
assays using normal human keratinocytes, HaCaT cells and 3T3 mouse
fibro-blasts: correlation with in vivo data from a soap chamber
assay. J Dermatol Sci. 7:119–129. 1994. View Article : Google Scholar
|
|
17.
|
Tardiff RG, Hubner RP and Graves CG:
Harmonization of thresholds for primary skin irritation from
results of human repeated insult patch tests and laboratory animal
skin irritation tests. J Appl Toxicol. 23:279–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Basketter DA and Kimber I: Skin
irritation, false positives and the local lymph node assay: a
guideline issue? Regul Toxicol Pharmacol. 61:137–140. 2011.
View Article : Google Scholar : PubMed/NCBI
|