Introduction
Hyperlipidemia has been indicated to be associated
with acute pancreatitis (AP) in 12–38% of cases worldwide, which
has been intensively studied since Speck noted the association
between hyperlipidemia and AP in 1865 (1). However, the exact mechanisms by which
hyperlipidemia affects the pathogenesis of AP remain uncertain and
there are currently no treatment methods for hyperlipidemic severe
acute pancreatitis (SAP).
Peroxisome proliferator-activated receptor-γ
(PPAR-γ) is one of the most intensively studied nuclear hormone
receptors of the last two decades. It is a ligand-activated
transcription factor that is expressed in various tissues and cell
types, including the pancreas, liver, kidney, adipose tissue and
colon (2). Natural ligands of
PPAR-γ include fatty acids, arachidonic acid metabolites and
prostaglandins. Synthetic ligands include certain nonsteroidal
anti-inflammatory agents and a series of antidiabetic agents known
as thiazolidinediones (TZDs), including troglitazone, pioglitazone
and rosiglitazone (3). PPAR-γ has
been shown to be important as a transcriptional mediator in lipid
and glucose homeostasis (4–6). A
number of studies have demonstrated that PPAR-γ agonists exert
potent anti-inflammatory and antioxidant properties. For example,
PPAR-γ ligands inhibit proinflammatory cytokine production and
macrophage activation, reduce the development of inflammation and
tissue injury associated with spinal cord trauma (7), and reduce spinal cord injury,
evolution of periodontitis and intestinal ischemia/reperfusion
injury (8–10). The present study aimed to evaluate
the therapeutic potential of the exogenous PPAR-γ ligand
rosiglitazone in rats with hyperlipidemic SAP and to examine its
effects on hyperlipidemia associated with a critical illness, in
order to explore the mechanism of action.
Materials and methods
Reagents
Rosiglitazone was obtained from Cayman Chemical Co.
(Ann Arbor, MI, USA) and GW9662 was purchased from Enzo Life
Sciences Inc. (Ann Arbor, MI, USA). Additionally, sodium
taurocholate (TCA) and dimethyl sulfoxide were purchased from
Sigma-Aldrich Co. (St. Louis, MO, USA).
Animals
Male specific pathogen-free Sprague Dawley rats
(weight, 150–200 g) were obtained from the Experimental Animal
Center of Hubei Academy of Medical Sciences (Wuhan, China). All
animal procedures were approved by the ethics committee of Wuhan
University (Wuhan, China) and performed in compliance with the EC
regulations and the NIH standards (Guide for the Care and Use of
Laboratory Animals, NIH publication, 85–23, revised 1996).
Animal grouping
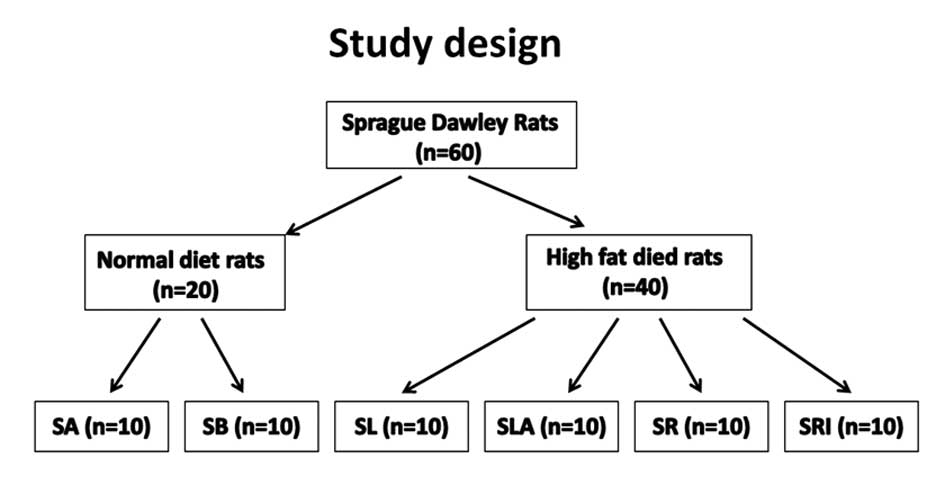
The experimental design is shown in Fig. 1. Sixty animals were randomly
divided into two groups, 40 rats received intragastric
administration of a high-fat diet (77% normal diet, 20% animal fat
and 3% cholesterol) for 2 weeks, which induced experimental
hyperlipidemia. The remaining rats received a normal diet and ate
freely. Rats receiving the normal diet were divided into 2 groups;
the sodium TCA group (SB group, n=10) and the control group (SA
group, n=10). The SB group was subjected to sodium TCA-induced SAP
and the SA group were injected with saline instead of sodium TCA.
Rats with hyperlipidemia were randomly divided into 4 groups: the
hyperlipidemia and sodium TCA group (SLA group, n=10); the
hyperlipidemia, rosiglitazone and sodium TCA group (SR group,
n=10); the hyperlipidemia, GW9662, rosiglitazone and sodium TCA
group (SRI group, n=10); and the hyperlipidemia group (SL group,
n=10). The SLA and SR groups were subjected to sodium TCA-induced
AP and the SR group was additionally treated with 10 mg/kg
rosiglitazone by intraperitoneal injection (IP) 1 h prior to sodium
TCA. The SRI group were treated in the same way as the SR group
with the additional administration of 0.3 mg/kg GW9662 by IP
injection 30 min prior to rosiglitazone. The SL group was treated
with saline only (Fig. 1).
Induction of SAP and tissue
procurement
Twelve hours prior to the start of the experiment,
rats were deprived of food but allowed access to water ad
libitum. The SAP model was induced by the method by Paszkowski
et al (11), with
improvements. The rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (3 ml/kg). SAP was induced by the
retrograde infusion of 5% sodium TCA (1 ml/kg; Sigma-Aldrich Co.)
into the ampulla of Vater, transduodenally, using an Angiocath with
a standardized pressure-controlled infusion rate under laparotomy.
Following infusion, the section of the bile-pancreatic duct
entering the duodenum was clipped by a noninvasive Angiocath for 5
min. After checking for bile leakage, the opening in the duodenum
lateral wall was sutured. The abdomen was closed, and the rats
recovered from the anesthetic and were allowed access to water. All
rats were sacrificed by exsanguination 12 h following the induction
of pancreatitis, and blood samples were obtained by direct
intra-cardiac puncture. The pancreas was removed immediately and
the head of the pancreas was fixed in formaldehyde. Tissue sections
were paraffin-embedded and continual sections were cut. Portions of
this organ were frozen in liquid nitrogen and stored at −80°C until
assayed.
Serum assay
Serum amylase (AMY) activity was measured using an
automatic biochemistry analyzer (Olympus Optical Co., Ltd., Tokyo,
Japan). Serum triglyceride (TG) and total cholesterol (TC)
concentrations were measured in triplicate using commercially
available colorimetric assay kits (Diagnosticum Rt, Budapest,
Hungary) adapted to 96-well plates, as described previously
(12). The accuracy of the assays
was monitored using standard lipid controls (Sentinel, Milan,
Italy).
Histopathological examination
Continuous sections of paraffin-embedded pancreatic
tissue were stained with hematoxylin and eosin for pathological
examination. Morphometric documentation for pancreatic sections
using a light microscope (Olympus Optical Co., Ltd., Tokyo, Japan)
were evaluated by two independent pathologists, each of whom was
blinded to the other’s assessment and recorded their findings on an
evaluation form for pancreatic injury. The evaluation of pancreatic
injury, including the graded assessment of pancreatic edema,
vascular edema, fat necrosis, acinar necrosis and calcification,
was determined by the histological score of pancreatic injury
(13).
Determination of intercellular adhesion
molecule-1 (ICAM-1) and tumor necrosis factor-α (TNF-α) protein
expression by western blot analysis
Frozen pancreatic tissue was mechanically
homogenized in 1 ml ice-cold extraction buffer (50 mM Tris-HCl, pH
7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM
ethylene diamine tetraacetic acid; 1 mM phenylmethylsulfonyl
fluoride; 0.1% sodium dodecylsulfate and 1 μg/ml each of
aprotinin and leupeptin). Tissue samples were incubated on ice for
30 min, then centrifuged at 13,000 × g at 4°C for 30 min, and the
supernatant was collected and stored at −80°C. The protein
concentration of each sample was determined using the Bradford
method with bovine serum albumin as the standard. Protein samples
(40 μg) were electro-phoresed using sodium dodecyl
sulfate-polyacrylamide gels at 100 V for 120 min. The separated
proteins were transferred to a nitrocellulose membrane. Moreover,
the membrane was blocked with blocking buffer [Tris-buffered saline
(TBS) containing 5% non-fat dry milk and 0.1% Tween-20] for 120 min
at room temperature, then washed three times for 10 min each in TBS
with 0.1% Tween-20 and incubated with the primary antibodies. The
primary antibodies used were goat polyclonal anti-rat ICAM-1
(1:600; Santa Cruz Biotechnology Inc., CA, USA) antibody, rabbit
polyclonal anti-rat TNF-α antibody (1:1,000; Abcam, MA, USA) and
rabbit polyclonal anti-rat β-actin antibody (1:1,000; Cell
Signaling Technology Inc., Danvers, MA, USA), and were stored
overnight at 4°C. Tissue samples were washed three times for 10 min
each with TBS containing 0.05% Tween-20 (TBST) and the membranes
were incubated with secondary antibodies, horseradish peroxidase
(HRP)-conjugated goat anti-rabbit or rabbit anti-goat
immunoglobulin G (1:3,000, Pierce Biotechnology, Rockford, IL, USA)
for 1 h at room temperature. Following repeated washings with TBST,
the antibody-antigen complex was detected with enhanced
chemiluminescence reagent (Immobilon Western HRP Substrate;
Millipore Corp., Bedford, MA, USA) and scanned for densitometric
analysis using a bio-image analysis system (Bio-Rad Laboratories
Inc., Baltimore, MD, USA) for quantification. The results from each
experimental group are expressed as the relative integrated
intensity of ICAM-1 and TNF-α compared with the β-actin band
densities in the same batch.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed with SPSS for Windows, version
17.0 (SPSS, Inc., Chicago, IL, USA). Means of the different groups
were compared using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Serum lipids
Following the 2-week high-fat diet period, the rats
with hyperlipidemia were heavier than the rats that had received a
normal diet, although not significantly so. However, the 2-week
high-fat diet significantly increased the serum cholesterol and TG
levels from 1.72±0.13 and 0.61±0.12 mmol/l to 10.86±1.47 and
1.24±0.28 mmol/l, respectively (P<0.05; Table I).
 | Table I.Serum AMY, TC, TG, HDL and LDL
levels. |
Table I.
Serum AMY, TC, TG, HDL and LDL
levels.
| Group | No. of rats | AMY (U/l) | TC (mmol/l) | TG (mmol/l) | HDL (mmol/l) | LDL (mmol/l) |
|---|
| SA | 10 | 1200.0±0.0 | 1.72±0.13 | 0.61±0.12 | 0.82±0.04 | 0.66±0.04 |
| SL | 10 | 1139.3±35.6 | 10.86±1.47a | 1.24±0.28a | 1.61±0.11a | 7.85±1.06a |
| SB | 10 |
5922.2±925.9a | 2.82±0.24 | 0.54±0.08 | 0.97±0.09 | 1.76±0.14 |
| SLA | 10 |
6501.9±3771.0b,c | 10.42±0.95c | 1.36±0.13c | 1.64±0.07c | 6.31±0.72c |
| SR | 10 |
2006.9±331.9b,d |
4.36±0.99b,d |
0.58±0.12b,d | 1.29±0.17 | 8.21±0.50 |
| SRI | 10 |
5892.2±474.3e | 11.08±1.05e | 1.58±0.12e | 1.19±0.07 | 6.40±0.76 |
Histological analysis of pancreatic
tissue
Representative histological sections of pancreatic
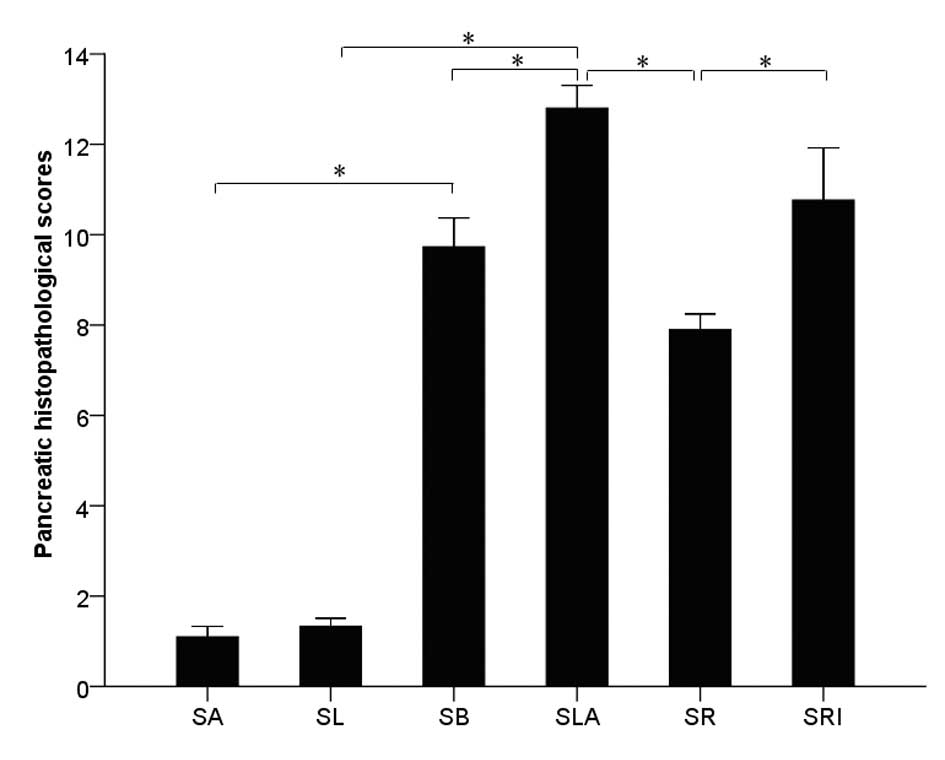
tissue are shown in Fig. 2. The
histological severity of pancreatitis was measured with a validated
scale (pancreatitis score) based on the degree of edema,
inflammatory cell infiltration, hemorrhage and necrosis. No
pathological injury in the rats of the SA and SL groups was
observed. The total pancreatitis score of the SLA group was
significantly higher compared with that of the SB group
(P<0.05). Pretreatment with rosiglitazone significantly reduced
the inflammatory changes in the SA group compared with those of the
SR group (P<0.05); however, the total pathological scores of the
SLA and SRI groups were not significantly different (P>0.05;
Fig. 3).
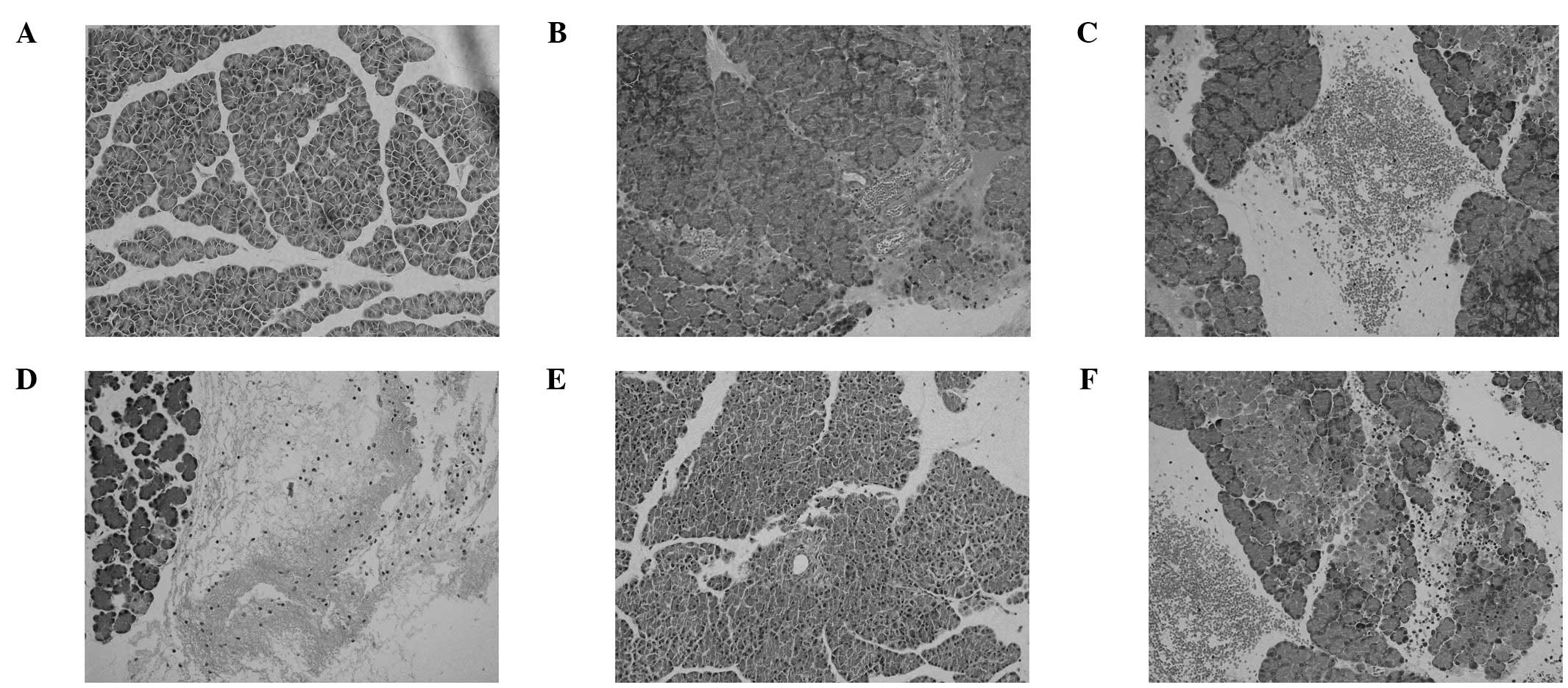 | Figure 2.Morphological changes of pancreatitis.
Hematoxylin and eosin-stained sections were examined under a light
microscope (original magnification, x200) in groups (A) SA, (B) SL,
(C) SB, (D) SLA, (E) SR and (F) SRI. SA, normal diet + saline; SB,
normal diet + sodium taurocholate; SL, hyperlipidemia + saline;
SLA, hyperlipidemia + sodium taurocholate; SR, hyperlipidemia +
rosiglitazone + sodium taurocholate; SRI, hyperlipidemia + GW9662 +
rosiglitazone + sodium taurocholate. |
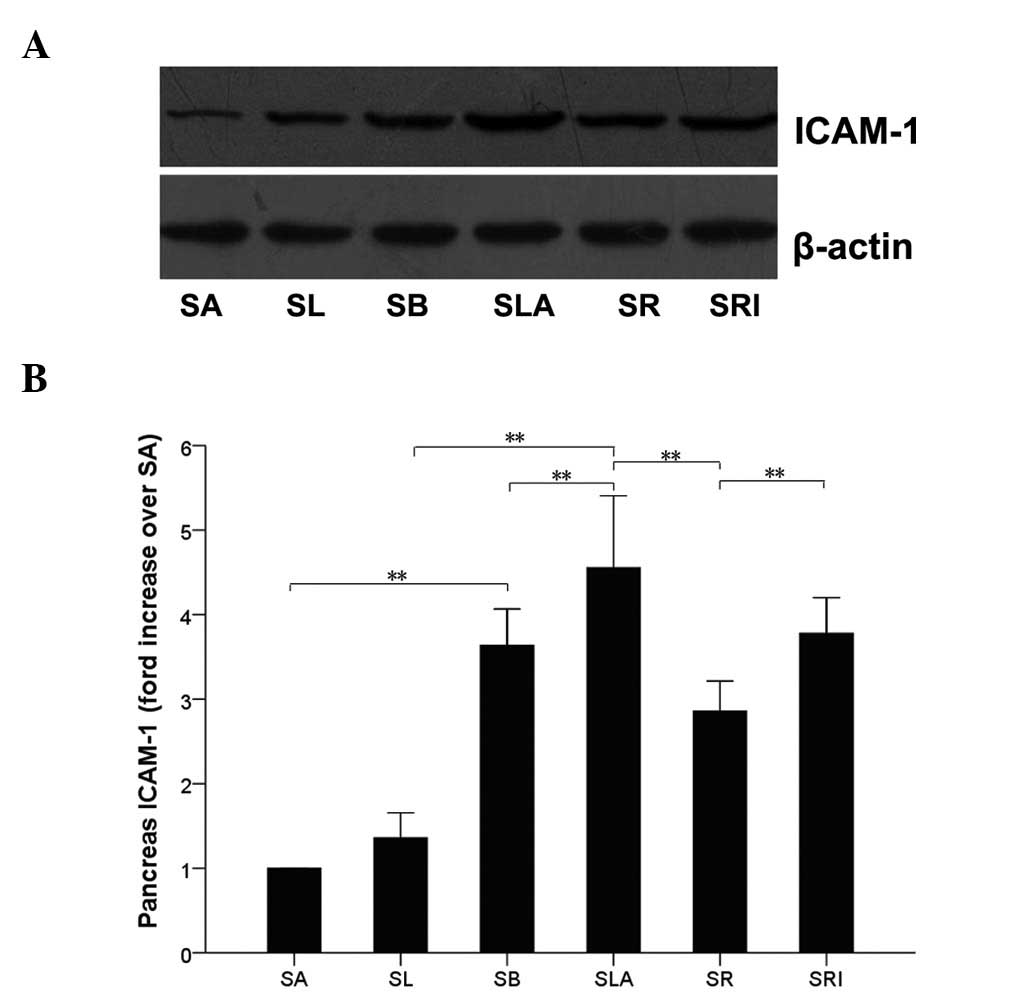
ICAM-1 protein expression
In a rat model of SAP similar to that used in the
present study (14), the protein
expression level of ICAM-1 in the pancreas peaked at 12 h. In the
present study, western blot analysis of pancreatic tissues also
identified ICAM-1 expression at 12 h following the induction of SAP
(Fig. 4). The expression level of
ICAM-1 in the pancreatic tissues was significantly higher in the
SLA group than in the SL group (P<0.01), and higher in the SB
group than in the SA group. The ICAM-1 protein expression level was
greatly reduced in the SR group compared with that of the SLA group
(P<0.01), and showed no significant difference when compared
with those of the SA and SL groups (P>0.05). Furthermore, no
significant differences were observed between the SLA and SRI
groups with regard to ICAM-1 protein expression (P>0.05).
However, the expression of ICAM-1 in the pancreatic tissues was
significantly increased in the SLA group compared with that of the
SB group (P<0.05; Fig. 4).
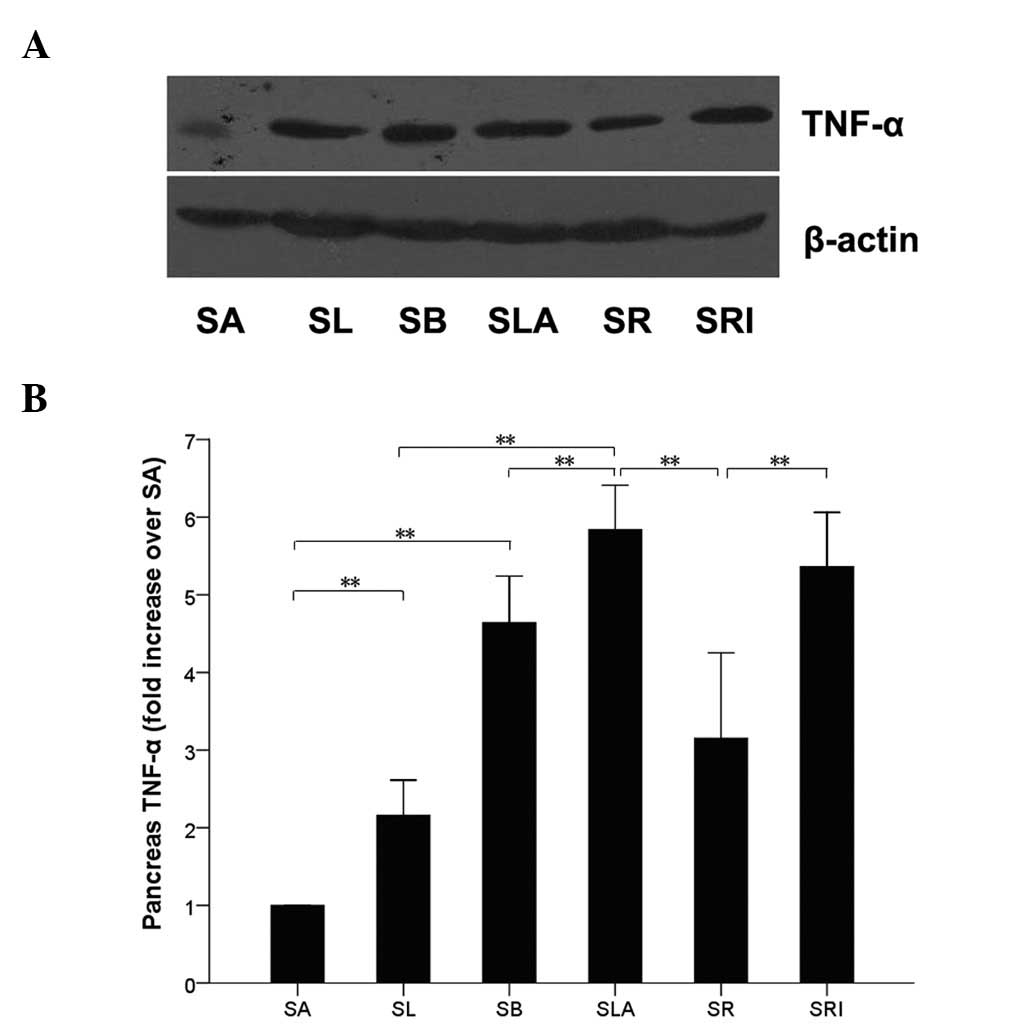
TNF-α protein expression
Western blot analysis of the pancreatic tissue
(Fig. 5) identified that the
protein expression level of TNF-α was significantly higher in the
SLA group than in the SL group (P<0.01), and higher in the SB
group than in the SA group. The TNF-α protein level was greatly
reduced in the SR group compared with that in the SLA group
(P<0.01), and showed no difference when compared with those of
the SA and SL groups (P>0.05). Furthermore, no significant
difference in TNF-α expression between the SLA and SRI groups was
observed (P>0.05). However, TNF-α protein expression levels were
significantly increased in the SLA group compared with those of the
SB group (P<0.05; Fig. 5).
Discussion
The present study demonstrated that a high-fat diet
did not damage the exocrine pancreas; however, it aggravated the
severity of sodium TCA-induced SAP. The discrepancies between the
results of this study and those from previous studies may be
explained by methodological differences (15–17).
In isolated ex vivo perfused dog pancreas, hyperlipidemia
was observed to induce histological and serological alterations of
acute pancreatitis (15).
Endogenous hyperlipidemia was observed to intensify the course of
acute edematous and necrotizing pancreatitis in the rat (16), while exogenous triglycerides
increased the pancreatic damage in acute edematous and necrotizing
pancreatitis, initiated via different pathogenetic pathways in the
isolated perfused pancreas (17).
PPAR-γ is a transcription factor belonging to the
nuclear hormone receptor superfamily (18). PPAR-γ activation usually regulates
lipid metabolism, glucose homeostasis and influences cell
proliferation and differentiation (19–21).
In addition, studies have demonstrated that PPAR-γ ligands exhibit
anti-inflammatory effects by modulating the production of
inflammatory mediators (22,23).
Therefore, PPAR-γ ligands may have therapeutic effects on
inflammatory diseases, including SAP. The TZDs pioglitazone and
rosiglitazone have been approved by the US Food and Drug
Administration to control blood glucose levels in patients with
type 2 diabetes. TZDs have also been demonstrated to be beneficial
in several inflammatory diseases. Rosiglitazone has shown to have
protective effects against hyperoxia-induced lung injury (24). Therefore, the anti-inflammatory
effects of TZDs may be a general characteristic of PPAR-γ
activation, as this phenomenon has been indicated in certain models
of critical diseases (25,26). The PPAR-γ natural agonist
15d-prostaglandin J2 was shown to exhibit protective effects in the
brain against ischemia-reperfusion injury (27) and to reduce the development of
inflammation in mice with acute lung injury induced by
lipopolysaccharides (28).
In the present study, the effects of using
rosiglitazone as a therapeutic agent to treat rats with
hyperlipidemic SAP were evaluated. The results showed that
rosiglitazone not only decreased the severity of pancreatic damage
but also significantly decreased the levels of serum AMY, TC and
TG, and inhibited the release of the proinflammatory cytokines
TNF-α and ICAM-1 in pancreatic tissue. This study has demonstrated
that rosiglitazone represents a potential therapeutic strategy for
the treatment of hyperlipidemic SAP.
In this study, rats of the SR group were
intraperitoneally treated with 10 mg/kg rosiglitazone 1 h prior to
the induction of SAP, with the aim of preventing inflammation and
hyperlipidemic effects. However, the SRI group who were treated
with PPAR-γ antagonist GW9662, showed a significant increase in
tissue damage compared with that observed in the SL and SR groups.
This suggested that PPAR-γ activation by its endogenous ligands is
an essential anti-inflammatory event that may be enhanced by
providing exogenous agonists. The histological score of pancreatic
injury from each group supports the hypothesis that rosiglitazone
exerts protective effects in rats with hyperlipidemic SAP.
PPAR-γ activation regulates lipid metabolism and
glucose homeostasis, as well as affecting cell proliferation and
differentiation (19–21). The definite mechanisms by which
PPAR-γ ligands affect hyperlipidemic SAP remain unclear. Previous
studies have demonstrated that the effects of PPAR-γ may be
attributed to the inhibition of proinflammatory transcription
factors, such as activator protein-1, signal transducers and
activators of transcription and nuclear factor-κB (NF-κB) (29), modulation of p38 mitogen-activated
protein kinase activity and partitioning of the corepressor of
B-cell lymphoma 6 (BCL-6) (30).
Lim et al (31) identified
that baicalin-induced PPAR-γ expression inhibited age-related
inflammation through blocking proinflammatory NF-κB activation. In
a direct interaction model, PPAR-γ is actively exported from the
nucleus into the cytosol through interaction with NF-κB, which
results in an alteration in the expression of proinflammatory
genes, including TNF-α, vascular cell adhesion protein-1,
interleukin-1β (IL-1β) and IL-6 (32). Therefore, PPAR-γ ligands may exert
potent anti-inflammatory properties by inhibiting NF-κB activation.
Moreover, NF-κB is important for the inflammatory response of AP
and the intervention against NF-κB activation eliminates the
induced overexpression of inflammatory cytokines, TNF-α and
ICAM-1.
ICAM-1, an inducible cell transmembrane glycoprotein
of the immunoglobulin supergene family, usually expressed at low
levels on the surface of endothelial cells, acts as an important
component in the inflammatory response for recruitment of
leukocytes to sites of inflammation. Several studies have
demonstrated that ICAM-1 is upregulated during inflammation,
asthma, rheumatoid arthritis and lung injury (33–36),
and the expression of ICAM-1 is mediated by various inflammatory
cytokines, particularly TNF-α (37). Furthermore, ICAM-1 is known to be
upregulated in AP and it recruits neutrophils into the pancreas and
distant organs, which inhibits the development of the disease
(38,39). In the present study, ICAM-1 protein
expression was significantly increased in the pancreatic tissue of
rats in the SB and SLA groups. In addition, pretreatment with
rosiglitazone markedly attenuated the expression of ICAM-1 in the
pancreas tissues of rats in the SR group. Cuzzocrea et al
also identified that rosiglitazone attenuates the severity of acute
inflammation through the reduction of ICAM-1 protein expression in
the pancreatic tissue of cerulein-treated mice (40).
A previous study has indicated that, among various
inflammatory mediators related to SAP, TNF-α is key in the
pathogenesis of SAP (41). It is
secreted from severely damaged acinar cells, macrophages and
monocytes, provides the regulation of cellular apoptosis and
increases the sequestration of pancreatic leukocytes. Various
studies have shown that the level of TNF-α, which is a
proinflammatory cytokine, is increased in models of AP (42–44).
Results from the present study support these previous findings.
TNF-α levels in the SB and SLA groups were significantly higher
compared with those of the SA and SL groups. Therefore, prevention
of TNF-α activity has a beneficial effect on the severity of AP
(23,44). In the present study, the
prophylactic administration of rosiglitazone markedly reduced TNF-α
expression in the pancreas. Moreover, there were no significant
differences in the level of TNF-α between the SR and SRI groups,
which may indicate a further suppressive effect of
rosiglitazone.
In summary, the present study demonstrated that
rosiglitazone, a specific PPAR-γ ligand, markedly reduced the
severity of pancreatic injury in rats with hyperlipidemic SAP. The
results of this study suggest a potential role of rosiglitazone as
a therapeutic agent against hyperlipidemic SAP. However, there were
several limitations to this study. As rosiglitazone pretreatment is
likely to be unsuitable for use in clinical practice, further
studies are required to confirm the effects of rosiglitazone
treatment on hyperlipidemic SAP.
Acknowledgements
The authors would like to thank the
Department of Central Laboratory, Renmin Hospital of Wuhan
University (Wuhan, China) for providing relevant experimental
facilities and technical support. This study was supported by the
Self-research Program for Doctoral Candidates of Wuhan University
in 2012 (grant no. 2012302020202) and by the National Natural
Sciences Foundation of China (grant no. 81070368).
References
|
1.
|
Gan SI, Edwards AL, Symonds CJ and Beck
PL: Hypertriglyceridemia-induced pancreatitis: A case-based review.
World J Gastroentrol. 12:7197–7202. 2006.PubMed/NCBI
|
|
2.
|
Dubuquoy L, Rousseaux C, Thuru X, et al:
PPARgamma as a new therapeutic in inflammatory bowel diseases. Gut.
55:1341–1349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kapadia R, Yi JH and Vemuganti R:
Mechanisms of anti-inflammatory and neuroprotective actions of
PPAR-gamma agonists. Front Biosci. 13:1813–1826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma:
adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994.
|
|
5.
|
Nolan JJ, Ludvik B, Beerdsen P, Joyce M
and Olefsky J: Improvement in glucose tolerance and insulin
resistance in obese subjects treated with troglitazone. New Engl J
Med. 331:1188–1193. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lehrke M and Lazar MA: The many faces of
PPARgamma. Cell. 123:993–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Zhang Q, Hu W, Meng B and Tang T: PPARγ
agonist rosiglitazone is neuroprotective after traumatic spinal
cord injury via anti-inflammatory in adult rats. Neurol Res.
32:852–859. 2010.
|
|
8.
|
Esposito E and Cuzzocrea S: Targeting the
peroxisome proliferator-activated receptors (PPARs) in spinal cord
injury. Expert Opin Ther Targets. 15:943–959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Di Paola R, Mazzon E, Maiere D, et al:
Rosiglitazone reduces the evolution of experimental periodontitis
in the rat. J Dent Res. 85:156–161. 2006.PubMed/NCBI
|
|
10.
|
Cuzzocrea S, Pisano B, Dugo L, et al:
Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2, ligands of
the peroxisome proliferator-activated receptor-gamma (PPAR-gamma),
reduce ischaemia/reperfusion injury of the gut. Br J Pharmacol.
140:366–376. 2003.
|
|
11.
|
Paszkowski AS, Rau B, Mayer JM, Möller P
and Beger HG: Therapeutic application of caspase
1/interleukin-1beta-converting enzyme inhibitor decreases the death
rate in severe acute experimental pancreatitis. Ann Surg.
235:68–76. 2002. View Article : Google Scholar
|
|
12.
|
Bjelik A, Bereczki E, Gonda S, et al:
Human apoB overexpression and a high-cholesterol diet differently
modify the brain APP metabolism in the transgenic mouse model of
atherosclerosis. Neurochem Int. 49:393–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Grewal HP, Mohey el Din A, Gaber L, Kotb M
and Gaber AO: Amelioration of the physiologic and biochemical
changes of acute pancreatitis using an anti-TNF-alpha polyclonal
antibody. Am J Surg. 167:214–219. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Werner J, Z’graggen K, Fernández-del
Castillo C, et al: Specific therapy for local and systemic
complications of acute pancreatitis with monoclonal antibodies
against ICAM-1. Ann Surg. 229:834–842. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Saharia P, Margolis S, Zuidema GD and
Cameron JL: Acute pancreatitis with hyperlipidemia: Studies with an
isolated perfused canine pancreas. Surgery. 82:60–67. 1977.
|
|
16.
|
Hofbauer B, Friess H, Weber A, et al:
Hyperlipaemia intensifies the course of acute oedematous and acute
necrotising pancreatitis in the rat. Gut. 38:753–758. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kimura W and Mössner J: Role of
hypertriglyceridemia in the pathogenesis of experimental acute
pancreatitis in rats. Int J Pancreatol. 20:177–184. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Blanquart C, Barbier O, Fruchart JC,
Staels B and Glineur C: Peroxisome proliferator-activated
receptors: regulation of transcriptional activities and roles in
inflammation. J Steroid Biochem Mol Biol. 85:267–273. 2003.
View Article : Google Scholar
|
|
19.
|
Escher P and Wahli W: Peroxisome
proliferator-activated receptors: insight into multiple cellular
functions. Mutat Res. 448:121–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mrówka P and Głodkowska-Mrówka E:
Structure, function and role of peroxisome proliferator-activated
receptor-gamma-PPARγ. Postepy Bilol Komorki. 38:629–651. 2011.
|
|
21.
|
Sun X, Tang Y, Tan X, et al: Activation of
peroxisome proliferator-activated receptor-γ by rosiglitazone
improves lipid homeostasis at the adipose tissue-liver axis in
ethanol-fed mice. Am J Physiol Gastrointest Liver Physiol.
302:G548–G557. 2012.
|
|
22.
|
Kouidhi S, Seugnet I, Decherf S, Guissouma
H, Elgaaied AB, Demeneix B and Clerget-Friodevaux MS: Peroxisome
proliferator-activated receptor-gamma (PPARgamma) modulates
hypothalamic Trh regulation in vivo. Mol Cell Endocrinol.
317:44–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chen C, Xu S, Wang WX, Ding YM, Yu KH,
Wang B and Chen XY: Rosiglitazone attenuates the severity of sodium
taurocholate-induced acute pancreatitis and pancreatitis-associated
lung injury. Arch Med Res. 40:79–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cai Q and Xu MY: Protective effect of
rosiglitazone against hyperoxia-induced lung injury in neonatal
rats. Zhongguo Dang Dai Er Ke Za Zhi. 14:301–305. 2012.(In
Chinese).
|
|
25.
|
Bassaganya-Riera J, Viladomiu M, Pedragosa
M, et al: Probiotic bacteria produce conjugated linoleic acid
locally in the gut that targets macrophage PPAR γ to suppress
colitis. PLoS One. 7:e312382012.PubMed/NCBI
|
|
26.
|
Buss Zda S, Medeiros YS and Fröde TS:
PPAR-gamma agonist rosiglitazone attenuates the inflammation caused
by carrageenan in the mouse model of pleurisy. Inflammation.
35:280–288. 2012.PubMed/NCBI
|
|
27.
|
Lin TN, Cheung WM, Wu JS, et al:
15d-Prostaglandin J2 protects brain from ischemia-reperfusion
injury. Arterioscl Throm Vasc Biol. 26:481–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Inoue K, Takano H, Yanagisawa R, et al:
Effect of 15-deoxy-delta 12,14-prostaglandin J2 on acute lung
injury induced by lipopolysaccharide in mice. Eur J Pharmacol.
481:261–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-gamma is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ricote M and Glass CK: PPARs and molecular
mechanisms of transrepression. Biochim Biophys Acta. 1771:926–935.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lim HA, Lee EK, Kim JM, et al: PPARγ
activation by baicalin suppresses NF-κB-mediated inflammation in
aged rat kidney. Biogerontology. 13:133–145. 2012.
|
|
32.
|
Kelly D, Campbell JI, King TP, et al:
Commensal anaerobic gut bacteria attenuate inflammation by
regulating nuclear-cytoplasmic shuttling of PPAR-gamma and ReIA.
Nat Immunol. 5:104–112. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Babu SK, Puddicombe SM, Arshad HH, et al:
Tumor necrosis factor alpha (TNF-α) autoregulates its expression
and induces adhesion molecule expression in asthma. Clin Immunol.
140:18–25. 2011.
|
|
34.
|
Hsu WY, Chao YW, Tsai YL, et al: Resistin
induces monocyte-endothelial cell adhesion by increasing ICAM-1 and
VCAM-1 expression in endothelial cells via p38MAPK-dependent
pathway. J Cell Physiol. 226:2181–2188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Yang CM, Luo SF, Hsieh HL, et al:
Interleukin-1beta induces ICAM-1 expression enhancing leukocyte
adhesion in human rheumatoid arthritis synovial fibroblasts:
Involvement of ERK, JNK, AP-1, and NF-kappaB. J Cell Physiol.
224:516–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Zhang XD, Hou JF, Qin XJ, et al:
Pentoxifylline inhibits intercellular adhesion molecule-1 (ICAM-1)
and lung injury in experimental phosgene-exposure rats. Inhal
Toxicol. 22:889–895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Bernot D, Peiretti F, Canault M,
Juhan-Vague I and Nalbone G: Upregulation of TNF-alpha-induced
ICAM-1 surface expression by adenylate cyclase-dependent pathway in
human endothelial cells. J Cell Physiol. 202:434–441. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Frossard JL, Saluja A, Bhagat L, et al:
The role of intercellular adhesion molecule 1 and neutrophils in
acute pancreatitis and pancreatitis-associated lung injury.
Gastroenterology. 116:694–701. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Lundberg AH, Granger DN, Russell J, et al:
Temporal correlation of tumor necrosis factor-alpha release,
upregulation of pulmonary ICAM-1 and VCAM-1, neutrophil
sequestration, and lung injury in diet-induced pancreatitis. J
Gastrointest Surg. 4:248–257. 2000. View Article : Google Scholar
|
|
40.
|
Cuzzocrea S, Pisano B, Dugo L, et al:
Rosiglitazone, a ligand of the peroxisome proliferator-activated
receptor-gamma, reduces acute pancreatitis induced by cerulein.
Intensive Care Med. 30:951–956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Laveda R, Martinez J, Munoz C, et al:
Different profile of cytokine synthesis according to the severity
of acute pancreatitis. World J Gastroenterol. 11:5309–5313.
2005.PubMed/NCBI
|
|
42.
|
Zhang XP, Ye Q, Jiang XG, et al:
Preparation method of an ideal model of multiple organ injury of
rat with severe acute pancreatitis. World J Gastroentrol.
13:4566–4573. 2007.PubMed/NCBI
|
|
43.
|
Gulcubuk A, Altunatmaz K, Sonmez K, et al:
Effects of curcumin on tumour necrosis factor-alpha and
interleukin-6 in the late phase of experimental acute pancreatitis.
J Vet Med A Physiol Pathol Clin Med. 53:49–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Gulben K, Ozdemir H, Berberoğlu U, et al:
Melatonin modulates the severity of taurocholate-induced acute
pancreatitis in the rat. Dig Dis Sci. 55:941–946. 2010. View Article : Google Scholar : PubMed/NCBI
|