Introduction
Fatigue is one of the most frequent and disabling
nonmotor problems and results in a negative impact on cognitive and
physical function. Chronic fatigue occurs with aging, depression,
diabetes and Parkinson's disease and is one of the most common
symptoms in primary care (1).
Kai-Xin-San (KXS), a traditional Chinese medicinal formula for
relieving psychological diseases, was originally prescribed by the
famous Chinese doctor Sun Si-Miao and, at present, continues to be
used to efficiently regulate central nervous system function, in
addition to treating anxiety and depression (2). Compositionally, KXS contains four
indigenous medicines: Ginseng (the root of Panax ginseng
C.A. Meyer), Fu Ling (the white part of Poria cocos F.A.
Wolf), Yuan Zhi (the root of Polygala tenuifolia Willd) and
Shi Chang-Pu (the rhizome of Acorus gramineus Solander).
Ginseng, as the principal active component in KXS, has been
traditionally used for the development of physical strength,
particularly in patients suffering from severe fatigue and hypoxia
(3,4). The predominant active components of
ginseng are ginseng saponins, also known as ginsenosides,
polysaccharides, peptides, polyacetylenic alcohols and fatty acids
(5).
In our previous study, KXS was shown to exert an
antidepressant-like effect in mice, as assessed using the forced
swim test (FST) and the tail suspension test (TST) (2). KXS may improve depressive behavior by
increasing the expression of phospho-cAMP response element-binding
protein (p-CREB) in CA1, CA3 and the dentate gyrus (DG) of the
hippocampus, as shown in chronically stressed rats (2,3).
Despite the popularity of KXS in the treatment of psychological
diseases, there is no scientific evidence concerning the potential
anti-fatigue effects of this formulation in animal models. Thus, in
the current study, the anti-fatigue action of KXS extract was
examined using the treadmill running test and the effects of KXS on
behavioral and biochemical markers for fatigue were assessed.
Specifically, the levels of hepatic and muscle glycogen, serum urea
nitrogen (SUN) and lactic dehydrogenase (LDH) were evaluated.
Material and methods
Plant material and extraction
All medicines formulating KXS (viz. radix of
P. ginseng, white part of P. cocos, radix of P.
tenuifolia and rhizome of A. gramineus) were purchased
from the Beijing Tong Ren Tang Group Co., Ltd. (Beijing, China) in
2009. The voucher specimens of the four plants, identified by
Professor Ping Liu and registered under the numbers NU-90111,
NU-82003, NU-79015 and NU-80617, respectively, were preserved at
the pharmacy of the Herbarium of Traditional Chinese Medicinal
(TCM), Chinese People's Liberation Army (PLA) General Hospital
(Beijing, China). The total extract was prepared in accordance with
our previous study (2). All the
experiments were completed between April and May 2010.
Standardization of KXS
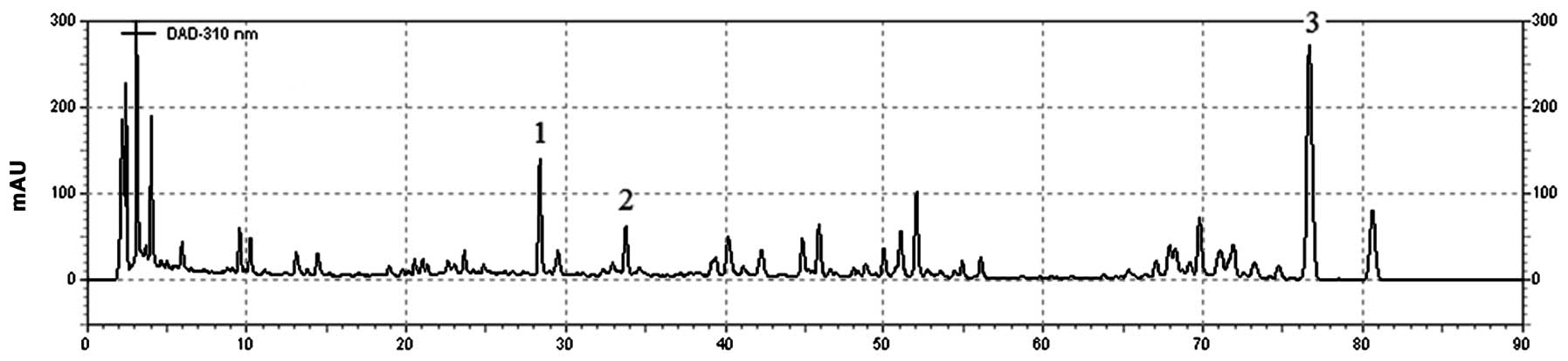
The chemical fingerprinting of the extracts was
analyzed. The chemicals used for the identification and
quantification of three compounds in the KXS extract were
tenuifoliside A,
1-O-(E)-benzoyl-[3-O-(E)-α-toluyl]-β-D-fructofuranosyl-(2→1)-[β-D-glucopyranosyl-(1→2)]-α-D-glucopyranoside
and ginsenoside Rb1, respectively.
KXS extract (28 mg) was dissolved in 1 ml methanol
and filtered through a 0.45 μm syringe filter, prior to
injection into the high-performance liquid chromatography (HPLC)
system. The HPLC system was composed of an L-2200 Autosampler
(Hitachi, Tokyo, Japan), an L-2130 pump (Hitachi), an ELSD 2000ES
evaporative light scattering detector (Alltech Medical Systems,
LLC, Cleveland, OH, USA) and an Agilent HC C18 (4.6×250.0 mm)
column (Agilent Technologies, Santa Clara, CA, USA). A gradient
solvent system of acetonitrile (A) and 0.65% ammonium acetate in
water (B) was used as follows: 5%A/95%B (start), 15%A/85%B (8 min),
20%A/80%B (35 min), 28%A/72%B (60 min), 35%A/65%B (70 min), 100%A
(74 min) and 5%A/95%B (75 min), at a flow rate of 1.0 ml/min
(Fig. 1).
Animals and drug administration
The experiments were performed using Sprague Dawley
(SD) rats (weight, 180-220 g). The rats used in this study were
cared for and treated humanely according to the ‘Guide for the Care
and Use of Laboratory Animals’ of the Shanghai Institute of
Material Medica. In this study, the mice were divided into six
groups, as follows: untreated control (UC), running control (RC),
RC treated with 13 mg/kg/day modafinil and RC treated with KXS at
dosages of 125, 250 and 500 mg/kg/day, respectively. The 10-12 mice
in each group were kept in a cage under standard laboratory
conditions (at a temperature of 20±1°C and a 12-h light/dark cycle)
with free access to food and water. All experiments were performed
from 9:00 a.m. to 4:00 p.m. Modafinil, administered at a dose of 13
mg/kg, served as a positive control in the tests. The solutions of
the tested samples were administered to the rats via gastric
intubation at different dosages once a day at 9:00 a.m. The control
groups received the same volume of the dosing vehicle (saline). The
rats also received a single dose of the treatment 60 min prior to
testing.
Treadmill running protocols
The physical exercise load applied in the present
study took the form of treadmill running on a motor-driven
treadmill. The treatments were administered once a day for four
weeks and 60 min prior to the running protocol. The rats of the RC
and treatment groups were forced to run on a treadmill for 20 min
once a day and the speed of the treadmill belt was gradually
increased from 15 m/min to 35 m/min (slope of 15°). A failure to
run caused the rat to slide off the moving belt and into a 15×15 cm
electric shock grid that delivered 1.2 mA of current at 3 Hz. On
the 30th day of the experiment, the time to exhaustion for
treadmill running was determined for the exercise groups.
Exhaustion was operationally defined as the third time a rat was no
longer able to keep pace with the speed of the tread-mill belt and
remained on a the electric shock grid for 2 sec rather than
running. For each trial, the total time of exhaustive running was
calculated and used as the best estimate of endurance running
capacity.
Blood and tissues collection
Following the behavioral test, rats were rapidly
decapitated to obtain venous blood. The serum was separated by
centrifugation at 1,800 × g and stored at −20ºC prior to being
assayed. Brain, liver and muscle tissues were removed rapidly on
the ice-plate. The tissues were washed with cold saline, blotted
dry and stored at −80°C prior to being assayed.
Measurement of biochemical parameters
associated with fatigue
Levels of β-endorphin in the brain, hepatic and
muscle glycogen, lactate dehydrogenase (LDH), serum urea nitrogen
(SUN), blood lactic acid (BLA), testosterone and malondialde-hyde
(MDA), and the activity of superoxide dismutase (SOD) in the serum
were determined using commercially available kits from the Nanjing
Jiancheng Bio-Company (Nanjing, China).
Statistical analysis
Data are presented as the mean ± standard deviation.
Overall differences according to the treatment were evaluated using
one-way analysis of variance (ANOVA), while differences between
groups were determined by analysis of variance and the Student's
t-test (6). P<0.05 or P<0.01
was considered to indicate a statistically significant
difference.
Results
HPLC fingerprint
Chromatograms of the KXS extract and its three major
compounds are shown in Fig. 1. As
the main component, the concentration of tenuifoliside A was
9.99±0.12 mg/g, while the concentrations of
1-O-(E)-benzoyl-[3-O-(E)-α-toluyl]-β-D-fructofuranosyl-(2→1)-[β-D-glucopyranosyl-(1→2)]-α-D-glucopyranoside
and ginsenoside Rb1 were 16.71±0.16 and 5.79±0.04 mg/g,
respectively.
Effects of KXS in the treadmill running
test
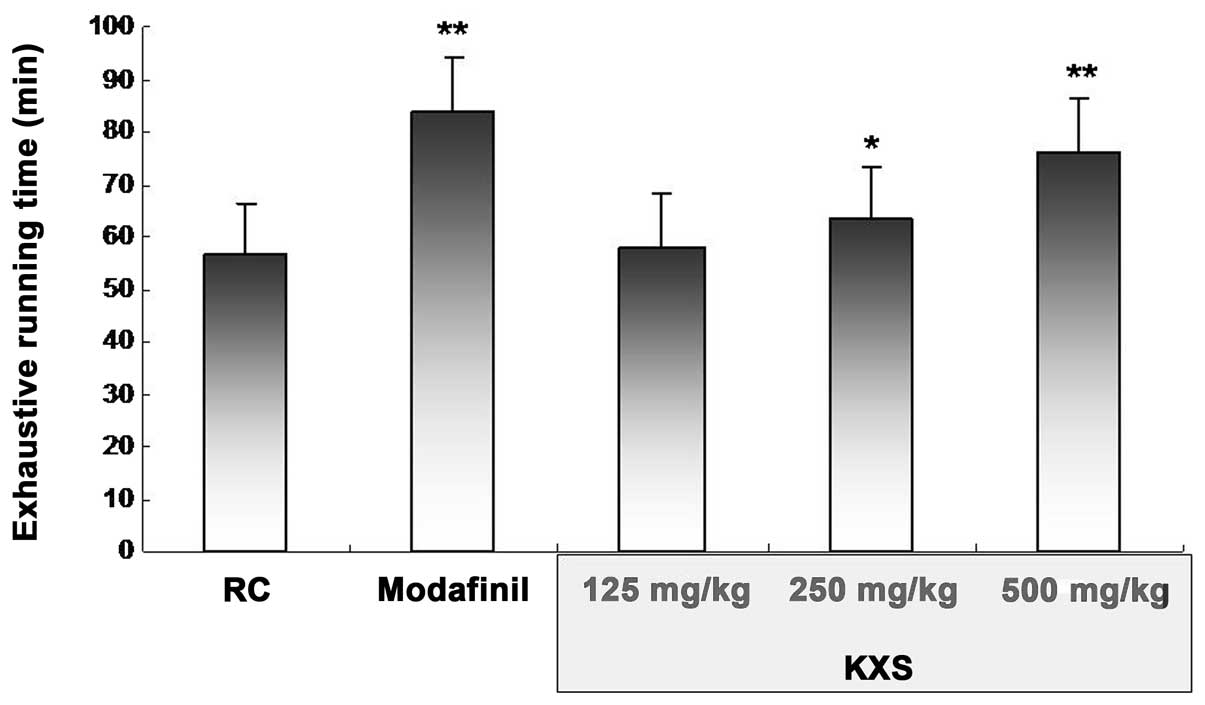
As shown in Fig. 2,
in comparison with the RC group, 250 and 500 mg/kg KXS induced
marked increases in exhaustive running time in the treadmill
running test, with the most efficacious results demonstrated at a
dose of 500 mg/kg (76.28±2.56 versus 56.40±3.61 min in the RC
group, P<0.01)
Effects of KXS on biochemical parameters
of energy metabolism
As shown in Table
I, exposure to the treadmill running test led to increases in
the LDH, SUN, BLA and β-endorphin levels in the brain or serum, and
decreased hepatic/muscle glycogen and testosterone levels compared
with the UC group. All these effects were inhibited by 500 mg/kg
KXS, which showed good effects on all biochemical parameters,
especially β-endorphin and testosterone. Modafinil significantly
affected only the levels of SUN, β-endorphin and testosterone.
 | Table I.Effects of Kai-Xin-San (KXS) on the
biochemical parameters involved in energy metabolism. |
Table I.
Effects of Kai-Xin-San (KXS) on the
biochemical parameters involved in energy metabolism.
| Group | Dose (mg/kg) | LDH (U/l) | SUN (nmol/l) | BLA (nmol/l) | β-endorphin
(ng/l) | Testosterone
(nmol/l) | Hepatic glycogen
(mg/g) | Muscle glycogen
(mg/g) |
|---|
| UC | - | 2600.86±200.90 | 3.39±0.16 | 5.71±0.15 | 451.61±31.41 | 16.07±1.25 | 30.10±1.45 | 1.41±0.11 |
| RC | - |
3140.52±170.43a | 7.26±0.55a | 10.33±0.95a | 582.06±42.79a | 14.28±0.58b | 20.71±2.05a | 1.15±0.10a |
| Modafinil | 13 | 3184.48±275.85 | 6.30±0.24d | 9.98±0.76 | 445.99±29.52d | 15.81±0.65d | 20.13±2.86 | 1.22±0.16 |
| KXS | 125 |
2434.07±250.78d | 6.54±0.53c | 7.36±0.59d | 456.11±26.90 | 16.96±0.53d | 23.64±0.82d | 1.44±0.16d |
| KXS | 250 | 3119.83±180.14 | 7.21±0.28 | 10.64±0.70 | 537.07±26.32 | 16.35±0.55d | 18.83±1.95 | 1.32±0.34 |
| KXS | 500 | 2983.62±228.89 | 6.75±0.20 | 8.56±1.18d | 431.37±20.24d | 17.35±0.72d | 23.17±1.96c | 1.52±0.11d |
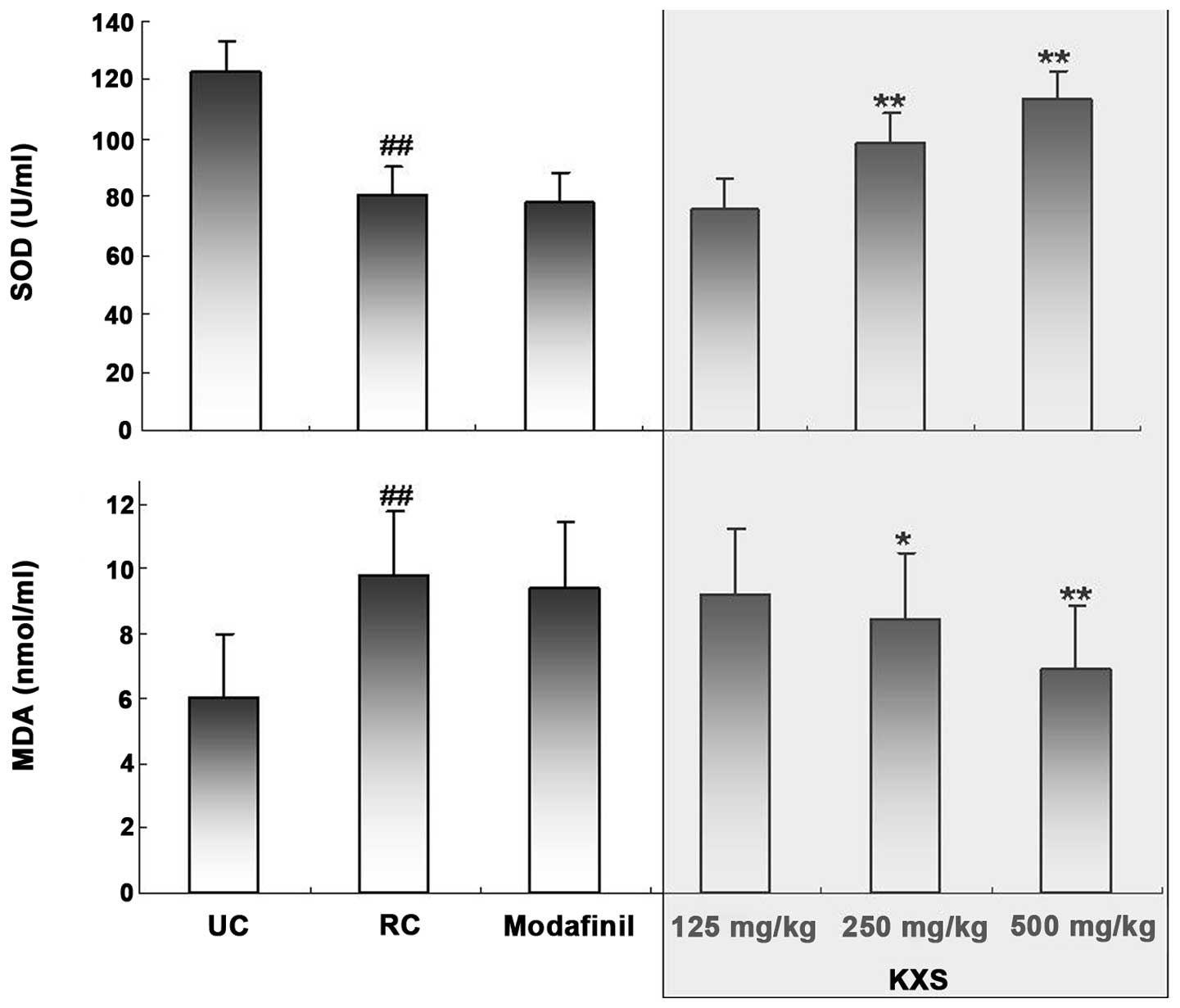
Effects of KXS on SOD activity and MDA
level
The serum total SOD activity and MDA level were
measured at the end of the treadmill running test (Fig. 3). Exhaustive running induced a
significant reduction in total SOD activity (P<0.01) and a
significant increase in the level of MDA (P<0.01) compared with
those in the UC group. KXS treatment dose-dependently increased the
total SOD activity and decreased the MDA level; however, modafinil
treatment did not demonstrate any significant effects on either
variable.
Discussion
Fatigue often occurs in aging, cancer, depression,
HIV infection, multiple sclerosis and Parkinson's disease (7). However, there are very few
pharmacological drugs or therapies available for the treatment of
fatigue (1). Natural products may
be used to improve athletic ability, postpone fatigue and
accelerate the elimination of fatigue in human beings, with few
side-effects (8). A direct measure
of an anti-fatigue effect is the increase in exercise tolerance.
Running to exhaustion is an experimental exercise model used to
evaluate anti-fatigue effects and the endurance capacity of
rodents, and gives a high reproducibility (9,10).
The present study demonstrated that KXS exhibited anti-fatigue
effects in the treadmill running test through the adjustment of one
behavioral and several biochemical markers for fatigue. An
HPLC-fingerprint was used as a tool to ensure the standardization
of the KXS extract. Rats treated with KXS demonstrated significant
increases in exhaustive running time. Moreover, the effects of KXS
in the treadmill running test were accompanied by an attenuation of
the fatigue-induced effects on the physiological markers relevant
for fatigue.
One possible explanation for the anti-fatigue effect
observed following treatment with KXS may involve glycogen
mobilization during exercise. There are numerous biochemical
parameters that are associated with glycogen mobilization during
exercise and fatigue. LDH catalyzes the interconversion of pyruvate
and lactate, with levels increasing during exercise (11). Serum urea nitrogen, which is the
product of energy metabolism when moving, is a sensitive index used
to evaluate the bearing capability when human bodies suffer from a
physical load. The less an animal is adapted to exercise, the more
LDH and SUN levels increase (12).
Mild androgen deficiency may account for increased fatigue and
testosterone replacement has been shown to improve these
abnormalities (13,5). In addition, a lack of a β-endorphin
response has been demonstrated to be beneficial to endurance
exercise (14). Moreover, the
liver/muscle glycogen and BLA levels are also sensitive parameters
associated with fatigue. The depletion of these stores of glycogen
may be detrimental to an animal's capacity to function and a high
level of lactic acid may lead to a reduction in the pH of the
muscle tissue and the blood during high-intensity exercise
(15).
These seven biochemical parameters exhibited
significant differences in this exhaustive running test. The high
and middle-doses of KXS reduced the levels of LDH, SUN, BLA and
β-endorphin and increased the levels of hepatic/muscle glycogen and
testosterone in fatigued rats. The results showed that the
anti-fatigue activity of KXS may have been correlated with an
improvement in the metabolic control of exercise and the activation
of energy metabolism. This was consistent with the study by Wang
et al (5). Another possible
explanation for the anti-fatigue effect observed following
treatment with KXS may involve its antioxidant activity during
exercise. In a previous assay, enhanced oxidative stress was
observed in a rat model of fatigue and depression, predominantly
expressed by a significant increase in the MDA level and a
significant reduction in SOD activity (16). The present data were consistent
with these studies. Our results also indicate that KXS is able to
increase SOD activity and reduce lipid oxidation in exhaustive
running models, thereby showing marked antioxidant effects. The
results suggest that the anti-fatigue effect of KXS most likely
occurred through the protection of the corpuscular membrane by the
prevention of lipid oxidation.
In conclusion, the present study demonstrated the
anti-fatigue activity of the KXS formulation in the treadmill
running test. The biochemical mechanisms of the anti-fatigue
effects demonstrated by KXS may be due to its antioxidant activity
and the improvement in the metabolic control of exercise and the
activation of energy metabolism. Further studies regarding the
isolation of the major bioactive components of KXS responsible for
the observed effects and the precise site and the mechanism of
action are required.
Acknowledgements
The authors would like to express
thanks for the financial support from the Foundation from the
Ministry of Science and Technology of China (grant no.
2008ZXJ09004-028).
References
|
1.
|
Uthayathas S, Karuppagounder SS, Tamer SI,
Parameshwaran K, Degim T, Suppiramaniam V and Dhanasekaran M:
Evaluation of neuroprotective and anti-fatigue effects of
sildenafil. Life Sci. 81:988–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hu Y, Liu P, Guo DH, Rahman K, Wang DX,
Chen ML and Xie TT: Behavioral and biochemical effects of
Kaixin-San, a traditional Chinese medicinal empirical formula. Drug
Dev Res. 69:267–271. 2008. View Article : Google Scholar
|
|
3.
|
Wang JL LP WD, Tu HH and Chen GY: Effects
of kaixinsan on behavior and expression of p-CREB in hippocampus of
chronic stress rats. Zhongguo Zhong Yao Za Zhi. 32:1555–1558.
2007.(In Chinese).
|
|
4.
|
Zhu L, Wu XM, Yang L, Du F and Qian ZM:
Up-regulation of HIF-1alpha expression induced by ginkgolides in
hypoxic neurons. Brain Res. 1166:1–8. 2007.PubMed/NCBI
|
|
5.
|
Wang JJ, Shieh MJ, Kuo SL, Lee CL and Pan
TM: Effect of red mold rice on antifatigue and exercise-related
changes in lipid peroxidation in endurance exercise. Appl Microbiol
Biotechnol. 70:247–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ordoñez AA, Ordoñez RM, Zampini IC and
Isla MI: Design and quality control of a pharmaceutical formulation
containing natural products with antibacterial, antifungal
properties. Int J Pharm. 378:51–58. 2009.PubMed/NCBI
|
|
7.
|
Tharakan B, Dhanasekaran M, Brown-Borg HM
and Manyam BV: Trichopus zeylanicus combats fatigue without
amphetamine-mimetic activity. Phytother Res. 20:165–168. 2006.
View Article : Google Scholar
|
|
8.
|
Kim KM, Yu KW, Kang DH and Suh HJ:
Anti-stress and anti-fatigue effect of fermented rice bran.
Phytother Res. 16:700–702. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Foley TE, Greenwood BN, Day HE, Koch LG,
Britton SL and Fleshner M: Elevated central monoamine receptor mRNA
in rats bred for high endurance capacity: implications for central
fatigue. Behav Brain Res. 174:132–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lacerda AC, Marubayashi U, Balthazar CH,
Leite LH and Coimbra CC: Central nitric oxide inhibition modifies
metabolic adjustments induced by exercise in rats. Neurosci Lett.
410:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Koob GF: A role for GABA mechanisms in the
motivational effects of alcohol. Biochem Pharmacol. 68:1515–1525.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang Y, Yao X, Bao B and Zhang Y:
Anti-fatigue activity of a triterpenoid-rich extract from Chinese
bamboo shavings (Caulis bamfusae in taeniam). Phytother Res.
20:872–876. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Felipe AM, Rincão VP, Benati FJ, Linhares
RE, Galina KJ, de Toledo CE, et al: Antiviral effect of Guazuma
ulmifolia and Stryphnodendron adstringens on poliovirus
and bovine herpesvirus. Biol Pharm Bull. 29:1092–1095. 2006.
|
|
14.
|
Meyer WR, Muoio D and Hackney TC: Effect
of sex steroids on beta-endorphin levels at rest and during
submaximal treadmill exercise in anovulatory and ovulatory runners.
Fertil Steril. 71:1085–1091. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Okazaki Y, Ito Y, Kyo K and Tateishi T:
Corrosion resistance and corrosion fatigue strength of new titanium
alloys for medical implants without V and Al. Mater Sci Eng A
Struct Mater. 213:138–147. 1996. View Article : Google Scholar
|
|
16.
|
Wang J, Li S, Fan Y, Chen Y, Liu D, Cheng
H, et al: Anti-fatigue activity of the water-soluble
polysaccharides isolated from Panax ginseng C. A. Meyer. J
Ethnopharmacol. 130:421–423. 2010. View Article : Google Scholar : PubMed/NCBI
|