Introduction
Contrast-induced nephropathy (CIN) is a complex
syndrome of acute kidney injury that follows exposure to
intravascular contrast media. CIN is the third leading cause of
hospital-acquired renal failure (1) and is associated with poor outcomes,
particularly in patients undergoing primary percutaneous coronary
intervention (PCI) (2–4). Numerous risk factors for CIN have
been identified, including preexisting impairment of renal
function, diabetes mellitus with associated renal insufficiency,
nephrotoxic drugs, reduction of effective intravascular volume,
multiple myeloma, volume and timing of contrast administration and
advancing age (5). These risk
factors may be additive (5). Due
to the age-associated degradation in physiological functions and a
higher prevalence of chronic diseases, elderly patients have more
risk factors. Therefore, older age was shown to be a strong risk
factor for CIN (6). Although a
series of preventive measures have been developed, CIN remains a
major challenge encountered in elderly patients by interventional
cardiologists.
Hydration is generally regarded as an important
strategy for preventing CIN. However, the optimal therapy for
preventing CIN remains uncertain (7). Prostaglandin E1
(PGE1), a natural prostaglandin with numerous
pharmacologic effects, has been reported to have potential as an
agent for preventing CIN (8–9).
However, no data are currently available concerning the potential
effects of the combined use of hydration and alprostadil in the
prevention of CIN following PCI in elderly patients. Therefore, the
aim of the present study was to investigate the ability of a
combination of hydration and alprostadil to prevent CIN following
PCI in elderly patients.
Patients and methods
Patient population
The present study included elderly patients (aged
≥60 years) with coronary artery disease who were admitted to the
Affiliated Shantou Hospital of Sun Yat-sen University (Shantou,
China) between June 1, 2010 and January 31, 2012, and treated with
PCI. Patients were excluded from the present study when the
following criteria were met: i) refusal to participate in the
clinical trial, ii) refusal of PCI treatment, iii) use of any
nephrotoxic drugs during the perioperative period, iv) severe
hepatic and renal failure, v) serious infectious disease, vi) New
York Heart Association Functional Classification (NYHA) (10) class >3, vii) hemodynamic
instability (including systolic blood pressure <90 mmHg), viii)
coronary lesions below the threshold for clinical revascularization
therapy, ix) coronary lesions not suitable for PCI due to coronary
anatomy, and x) allergic reaction to contrast media and
alprostadil. The present study was approved by the Ethics Committee
of the Affiliated Shantou Hospital of Sun Yat-sen University, and
informed consent was obtained from all the patients.
Treatment regimens
According to the American Heart Association
(AHA)/American College of Cardiology (ACC) guidelines for secondary
prevention for patients with coronary artery disease (11), all the patients following admission
were treated with antiplatelet and other therapies, including
angiotensin-converting enzyme inhibitors/angiotensin II receptor
blockers (ARBs), β-blockers and statins.
All the patients underwent coronary angiography and
percutaneous transluminal coronary angioplasty and/or implantation
of stents according to the condition of the coronary artery.
According to interventional guidelines of coronary artery disease
(ACC/AHA 2005) (12), >70%
narrowing of local stenosis of the culprit lesions (lesions
directly responsible for the ischemia episode) is the threshold for
clinical revascularization therapy (13). Iopromide was the non-ionic,
monomeric, hypo-osmolar contrast media employed during PCI.
Following admission, the patients were randomly
allocated to one of three groups: i) the control group, where
treatment was based on the routine PCI without hydration; ii) the
hydration group, where routine hydration was performed with 1
ml/kg/h normal saline for 6 h prior to PCI and 12 h following PCI;
and iii) the hydration + alprostadil group, where, on the basis of
hydration, patients received alprostadil 10 μg (diluted with
100 ml normal saline) twice a day by intravenous drip for the 3
days following PCI.
Clinical and laboratory monitoring
The serum creatinine (SCr) concentration was
measured prior to PCI and then daily for three days following PCI.
The serum lipid profile and fasting blood glucose (FBG) were also
measured during hospitalization. Transthoracic echocardiography was
performed prior to PCI to evaluate the left ventricular ejection
fraction (LVEF) in all the patients, and the results were recorded
and interpreted by experienced experts.
Definitions
CIN was defined as a relative increase of >25% or
an absolute increase of ≥0.5 mg/dl in SCr from the baseline value
72 h after exposure to the contrast medium (14). Creatinine clearance (Ccr) was
calculated by applying the Cockcroft-Gault formula to the SCr
(15): Ccr = (140 – age) × weight
(kg)/[SCr (mg/dl) × 72], with adjustment for female gender
(Ccrfemale = Ccr × 0.85).
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 (SPSS, Inc., Chicago. IL, USA). Continuous variables
following a symmetric distribution were presented as means ±
standanrd deviation (SD). Comparisons between means were performed
using Student’s t-test, while comparisons between frequencies were
performed using Chi-square or Fisher’s exact tests. Variables
following an asymmetric distribution were presented as medians and
interquartile ranges [M (P25, P75)] and were compared using the
Kruskal-Wallis nonparametric test. Categorical variables were
described as frequencies and analyzed by Chi-square or Fischer’s
exact tests. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
From June 1, 2010 to January 31, 2012, 85 elderly
patients were included in the present study after exclusions. All
the included patients completed the full clinical trial. The
patients were randomly allocated to the control, hydration and
hydration + alprostadil groups. In the hydration + alprostadil
group, two patients were observed to have mild drug-related
phlebitis. Moreover, no cardiovascular hypotensive side-effects
were observed. The baseline clinical, laboratory and procedural
characteristics of the study population are shown in Table I.
 | Table I.Baseline clinical, laboratory and
procedural characteristics of the patients included in the present
study. |
Table I.
Baseline clinical, laboratory and
procedural characteristics of the patients included in the present
study.
| Characteristic | Group
| P-value |
|---|
| Control (n=22) | Hydration (n=28) | Hydration +
alprostadil (n=35) |
|---|
| Mean age (years) | 71±8 | 69±6 | 70±7 | 0.540 |
| Male, n | 15 | 19 | 26 | 0.822 |
| Scr (mol/l) | 88.27±27.40 | 76.82±19.45 | 83.63±23.59 | 0.277 |
| Ccr (ml/min) | 63.59±20.07 | 66.54±18.29 | 64.07±20.38 | 0.759 |
| LDL-C (mmol/l) | 2.95±0.96 | 2.93±0.97 | 2.94±1.08 | 0.997 |
| FBG (mmol/l) | 6.61±2.68 | 7.40±3.04 | 6.75±1.67 | 0.424 |
| LVEF ≤ 45%, n
(%) | 4 (18.18) | 5 (17.86) | 6 (17.14) | 0.994 |
| Past history |
| Hypertension, n
(%) | 12 (54.55) | 13 (46.43) | 15 (42.86) | 0.688 |
| Diabetes mellitus,
n (%) | 7 (31.82) | 13 (46.43) | 14 (40.00) | 0.578 |
| Renal
insufficiency, n (%) | 1 (4.55) | 3 (10.71) | 6 (17.14) | 0.348 |
| Combined
medications |
| ACEIs/ARBs, n
(%) | 20 (90.91) | 25 (89.29) | 29 (82.86) | 0.618 |
| Diuretic agents, n
(%) | 2 (9.09) | 5 (17.86) | 5 (14.29) | 0.676 |
| Mean contrast
volume (ml) | 134.09±36.99 | 123.57±37.14 | 133.71±32.46 | 0.400 |
SCr
SCr values (mean ± SD) measured at the four time
points specified in this study are shown in Table II. The mean SCr of the three groups
increased gradually during the three days following PCI. The
highest SCr value was observed in the control group. However, in
the remaining two groups (hydration and hydration + alprostadil
groups), the SCr values were only mildly increased. The mean SCr
values at baseline did not differ significantly following analysis
of variance (P=0.277). However, Kruskal-Wallis test comparisons of
SCr values on the 3rd day after PCI demonstrated a significant (Chi
square=10.527, P=0.005) difference among the three groups.
 | Table II.Time course of serum creatinine (SCr)
(mean ± SD, μmol/l). |
Table II.
Time course of serum creatinine (SCr)
(mean ± SD, μmol/l).
| Time point | Group
|
|---|
| Control | Hydration | Hydration +
alprostadil |
|---|
| Baseline | 88.27±27.4 | 76.82±19.45 | 83.63±23.59 |
| 1st day after
PCI | 95.63±31.21 | 79.79±23.38 | 83.47±26.46 |
| 2nd day after
PCI | 99.23±34.20 | 83.41±21.80 | 85.43±23.70 |
| 3rd day after
PCI | 107.48±30.45 | 87.11±22.72 | 86.49±21.86 |
Ccr
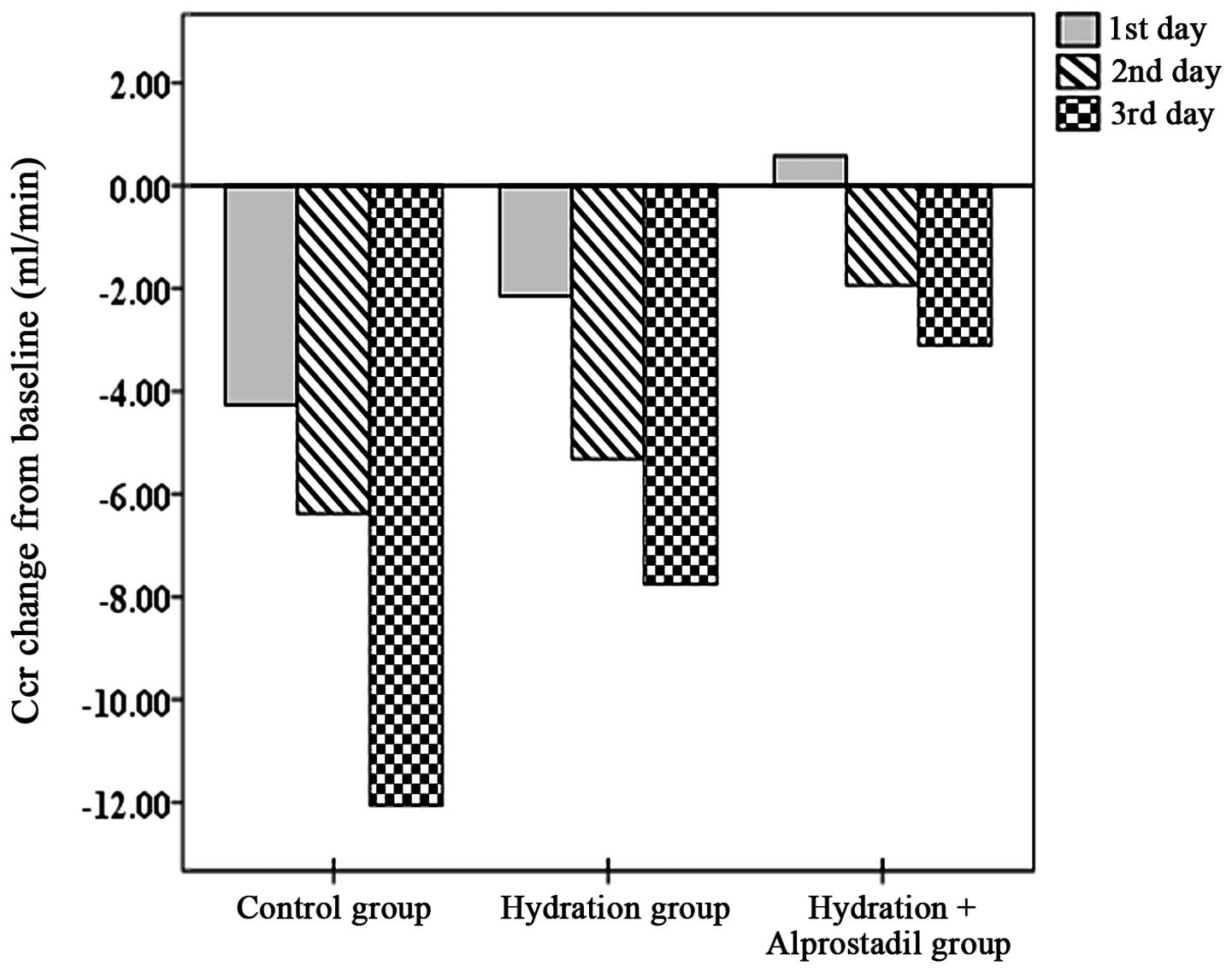
The mean changes in Ccr from baseline for the three
groups are shown in Fig. 1. On the
first day after PCI, the Ccr in the control group had decreased
moderately. The reduction in the Ccr was less evident in the
remaining two groups. However, no statistically significant
difference was observed in the mean changes of Ccr between the
control and hydration groups. A statistically significant
difference was observed between the control and hydration +
alprostadil groups. On the second day after PCI, the mean changes
in Ccr from baseline for the three groups were similar to those on
the first day after PCI. No statistically significant difference
was observed in the mean changes of Ccr between the control and
hydration groups. However, the difference was statistically
significant between the control and hydration + alprostadil groups.
On the third day after PCI, the Ccr values among the three groups
were statistically significant from baseline. Notably, the lowest
reduction in the Ccr value was observed in the hydration +
alprostadil group, while the highest reduction was observed in the
control group. The Ccr value in the hydration group was decreased
to a lesser extent compared with that in the control group
(P<0.05). A smaller reduction in the Ccr value was observed in
the hydration + alprostadil group than in the hydration group
(P<0.01).
Incidence of CIN
In the present study, 10 patients developed CIN
following PCI and the overall incidence reached 11.76%. There were
six patients in the control group, three in the hydration group and
one in the hydration + alprostadil group that developed CIN. The
incidence of CIN in the three groups was 27.27, 10.71 and 2.86%,
respectively. The Kruskal-Wallis test comparisons of the incidence
of CIN demonstrated a significant difference among the three groups
(Chi square=7.802, P=0.05).
Discussion
To the best of our knowledge, this is the first
study to provide information regarding the potential effects of the
combined use of hydration and alprostadil in the prevention of CIN
following PCI in elderly patients. Our findings demonstrate that
the combined use of hydration and alprostadil is more effective in
preventing CIN in elderly patients undergoing PCI compared with
hydration alone.
Older age is an important risk factor for CIN since
elderly people often suffer from age-related diseases (6). These diseases contribute to an
increased incidence of CIN. Therefore, it is important to prevent
CIN in this population. Many preventive strategies for reducing the
incidence of CIN have been reported. However, such strategies,
except for intravenous hydration, have yielded disappointing
outcomes (1,16). Previous studies (8,9) have
shown that the intravenous administration of PGE1 is
beneficial in reducing the incidence of CIN. The main mechanism is
considered to relate to a reduction in the levels of prostaglandins
when CIN develops, which causes a shift in the physiologic
vasoconstriction/vasodilatation balance. Alprostadil, an exogenous
form of PGE1, is suggested to cause renal vasodilation
and active renal artery perfusion, and to counteract
contrast-induced renal tubular epithelial cell toxicity (9,17).
In the present study, the effectiveness of hydration
combined with alprostadil in the prevention of CIN in elderly
patients undergoing PCI was investigated. The Ccr values in the
hydration group were not significantly different in the first and
second day after PCI compared with those in the control group.
However, on the third day after PCI, the Ccr in the control group
decreased more markedly compared with that in the hydration group.
The incidence of CIN in the control and hydration groups was 27.27
and 10.71%, respectively. This finding demonstrated that hydration
was effective in reducing the incidence of CIN. However, the
incidence of CIN was still higher compared with that in the general
population (18). Therefore,
hydration alone was not sufficient in preventing CIN in elderly
patients undergoing PCI.
In the present study, the combined use of hydration
and alprostadil showed an evident protective effect on renal
function from the first day after PCI. The influence of the
contrast medium on Ccr in the hydration + alprostadil group was
limited. However, the Ccr levels in the control and hydration
groups were differentially reduced, particularly in the control
group. A clear advantage was observed following the combined use of
hydration and alprostadil after the third day following PCI, when
compared with the hydration group. Finally, the incidence of CIN in
the hydration and hydration + alprostadil groups was 10.71 and
2.86%, respectively. Our findings indicate that the combined use of
hydration and alprostadil is more effective in reducing the
incidence of CIN. Therefore, hydration and alprostadil are
suggested to act synergistically to protect renal function.
Studies by Koch et al (8) and Sketch et al (9) concerning the duration and dosage of
intravenously administered PGE1 obtained similar
results. In these studies, PGE1 administration was
implemented 1 h prior to contrast exposure and was continued for a
total of 6 h. The time interval was chosen based on the knowledge
of the half-life of contrast media excretion (19). The most effective dosage used was
20 ng/kg/min (8,9). However, concerning the clinical
characteristics of CIN, according to the literature, SCr usually
increases 24 h after contrast exposure and peaks within 48–72 h
(14,20). In the present study, based on the
above-mentioned clinical characteristics of CIN, alprostadil was
administered by intravenous drip for the 3 days following PCI.
Finally, our study confirmed the clear effect of the combined use
of hydration and alprostadil in reducing the incidence of CIN in
elderly patients undergoing PCI.
Alprostadil is known to lower blood pressure via
vasodilation. Therefore, alprostadil is suggested to aggravate
contrast media nephrotoxicity by a severe drop in blood pressure.
According to the study by Koch et al (8), there was no severe drop in blood
pressure when the protective effect of PGE1 reached a
peak at a dosage of 20 ng/kg/min. In the present study, patients
were treated with 10 μg alprostadil (diluted with 100 ml
normal saline) twice a day by intravenous drip for the 3 days
following PCI. No severe drop in blood pressure was observed, with
the exception of mild drug-related phlebitis. This result may be
attributed to the following factors: i) the dose of alprostadil
used was relatively low; and ii) hydration may increase the blood
volume and help prevent hypotension. Therefore, the findings of our
study also suggest that alprostadil, when combined with hydration,
protects renal function more effectively.
In conclusion, the present study showed that the
combined use of hydration and alprostadil significantly reduce the
incidence of CIN in elderly patients undergoing PCI. Hydration and
alprostadil are suggested to act synergistically to protect renal
function. The combined use of hydration and alprostadil is more
effective in the prevention of CIN in elderly patients undergoing
PCI compared with hydration alone.
However, the present study has several limitations.
Firstly, the number of each group of patients was limited.
Secondly, data were collected from a single center, and, finally,
this was not a double-blind study. Therefore, the results provided
by this study should be confirmed by a larger double-blind
multi-center study.
References
|
1.
|
Maeder M, Klein M, Fehr T and Rickli H:
Contrast nephropathy: review focusing on prevention. J Am Coll
Cardiol. 44:1763–1771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Marenzi G, Assanelli E, Campodonico J, et
al: Contrast volume during primary percutaneous coronary
intervention and subsequent contrast-induced nephropathy and
mortality. Ann Intern Med. 150:170–177. 2009.PubMed/NCBI
|
|
3.
|
Ma G, Yu D, Cai Z, et al: Contrast-induced
nephropathy in postmenopausal women undergoing percutaneous
coronary intervention for acute myocardial infarction. Tohoku J Exp
Med. 221:211–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Uyarel H, Cam N, Ergelen M, et al:
Contrast-induced nephropathy in patients undergoing primary
angioplasty for acute myocardial infarction: incidence, a simple
risk score, and prognosis. Arch Med Sci. 4:550–558. 2009.
|
|
5.
|
Gleeson TG and Bulugahapitiya S:
Contrast-induced nephropathy. AJR Am J Roentgenol. 183:1673–1689.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sudarsky D and Nikolsky E:
Contrast-induced nephropathy in interventional cardiology. Int J
Nephrol Renovasc Dis. 4:85–99. 2011.PubMed/NCBI
|
|
7.
|
Bachórzewska-Gajewska H, Małyszko J,
Sitniewska E, Małyszko J and Dobrzycki S: Prevention of
contrast-induced nephropathy in patients undergoing percutaneous
coronary interventions in everyday clinical practice. Arch Med Sci.
2:256–261. 2006.PubMed/NCBI
|
|
8.
|
Koch JA, Plum J, Grabensee B and Mödder U;
PGE1 Study Group: Prostaglandin E1: a new agent for the
prevention of renal dysfunction in high risk patients caused by
radiocontrast media? Nephrol Dial Transplant. 15:43–49. 2000.
|
|
9.
|
Sketch MH Jr, Whelton A, Schollmayer E,
Koch JA, Bernink PJ, Woltering F and Brinker J; Prostaglandin E1
Study Group: Prevention of contrast media-induced renal dysfunction
with prostaglandin E1: a randomized, double-blind,
placebo-controlled study. Am J Ther. 8:155–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
The Criteria Committee of the New York
Heart Association: Nomenclature and Criteria for Diagnosis of
Diseases of the Heart and Great Vessels. 9th edition. Little, Brown
& Co.; Boston, MA, USA: pp. 253–256. 1994
|
|
11.
|
National Heart, Lung, and Blood Institute;
Smith SC Jr, Allen J, Blair SN, et al AHA; ACC: AHA/ACC guidelines
for secondary prevention for patients with coronary and other
atherosclerotic vascular disease: 2006 update endorsed by the
National Heart, Lung, and Blood Institute. J Am Coll Cardiol.
47:2130–2139. 2006. View Article : Google Scholar
|
|
12.
|
Smith SC Jr, Feldman TE, Hirshfeld JW Jr,
et al American College of Cardiology/American Heart Association
Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee
to Update 2001 Guidelines for Percutaneous Coronary Intervention:
ACC/AHA/SCAI 2005 guideline update for percutaneous coronary
intervention: a report of the American College of
Cardiology/American Heart Association Task Force on Practice
Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001
Guidelines for Percutaneous Coronary Intervention). Circulation.
113:e166–e286. 2006.
|
|
13.
|
Stadius ML and Alderman EL: Coronary
artery revascularization. Critical need for, and consequences of,
objective angiographic assessment of lesion severity. Circulation.
82:2231–2234. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mehran R and Nikolsky E: Contrast-induced
nephropathy: definition, epidemiology, and patients at risk. Kidney
Int Suppl. 100:S11–S15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from serum creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
McCullough PA: Contrast-induced acute
kidney injury. J Am Coll Cardiol. 51:1419–1428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Dołegowska B, Pikuła E, Safranow K,
Olszewska M, Jakubowska K, Chlubek D and Gutowski P: Metabolism of
eicosanoids and their action on renal function during ischaemia and
reperfusion: the effect of alprostadil. Prostaglandins Leukot
Essent Fatty Acids. 75:403–411. 2006.PubMed/NCBI
|
|
18.
|
Lasser EC, Lyon SG and Berry CC: Reports
on contrast media reactions: analysis of data from reports to the
U.S. Food and Drug Administration. Radiology. 203:605–610. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cronin RE: Radiocontrast media-induced
acute renal failure. Diseases of the Kidney. Schrier RW and
Gottschalk CW: (Hrsg).Little, Brown & Co.; Boston: pp.
1197–1198. 1993
|
|
20.
|
Thomsen HS: How to avoid CIN: guidelines
from the European Society of Urogenital Radiology. Nephrol Dial
Transplant. 20(Suppl 1): i18–i22. 2005. View Article : Google Scholar : PubMed/NCBI
|