Introduction
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), are syndromes of acute
respiratory failure that result from a variety of direct or
indirect injuries to the lungs. When ALI or ARDS occur, there is a
large influx of activated neutrophils into the lungs,
proinflammatory mediators are produced and lung epithelial and
endothelial surfaces are severely damaged (1). Although significant advances have
been made in the treatment of these syndromes, mortality associated
with ALI/ARDS remains high, at 30–70% (2). The complex pathogenesis of ALI makes
animal models a necessity in the study of this disorder, and these
models are numerous according to their insults of initiation,
maintenance and host organisms utilized. Moreover, interpreting and
applying the mechanistic data from animal models in the context of
patients is challenging (3), and
there is uncertainty as to which model best reflects the true
situation in humans. However, numerous workshop participants have
suggested that the two-hit model may be more appropriate for
reflecting the common comorbidities and risk factors that are
present in patients with ALI (4,5). The
two-hit phenomenon indicates that an initial insult primes
inflammatory cells so that a second insult results in an
exaggerated response. However, other researchers consider that the
one-hit model is more effective than the two-hit model, due to the
reproducibility, rapid onset of clinical symptoms and lack of
expense. Similarly, a previous study suggested that the
introduction of a second hit has no impact on inflammation or
increased lung injury (6).
Therefore, based on previous findings, the present study aimed to
investigate and compare the two models using
[18F]fluorodeoxyglucose (FDG) micro-positron emission
tomography (microPET) to evaluate the inflammatory response in the
lungs in these models.
Materials and methods
Animals
Male Sprague Dawley rats (Grade II; weight, 180–210
g) were purchased from the Animal Center of Zhejiang University
School of Medicine (Hangzhou, China). All animals were housed in
air-filtered, temperature-controlled units with access to food and
water ad libitum. All experimental protocols were approved
by the animal care committee of Zhejiang University School of
Medicine and the Principles of Laboratory Animal Care (NIH
publication no. 86–23, revised 1985) were followed.
Experimental protocol
The rats were randomly divided into four groups; the
rats in the lipopolysaccharide (LPS; n=10) and LPS-HCl (n=10)
groups were challenged by the intraperitoneal (IP) administration
of 5 mg/kg LPS (Escherichia coli, serotype 0111, B4;
Sigma-Aldrich, St. Louis, MO, USA), while the rats in the normal
saline (NS) control (n=3) and HCl (n=10) groups received an IP
injection of 1 ml/kg normal saline solution. After 16 h, all
animals were anesthetized with an IP injection of 40 mg/kg sodium
pentobarbital and placed in a 60° inclined position. The femoral
artery was cannulated and connected to a pressure transducer to
record the arterial pressure and heart rate on a polygraph recorder
(Shenzhen Mindray Bio-Medical Electronics Co. Ltd., Shenzhen,
China). The trachea was surgically exposed, and the rats in the HCl
and LPS-HCl groups received a direct intratracheal injection of 0.5
ml/kg HCl (pH=1.2), while rats in the NS control and LPS groups
received the same volume of normal saline solution. Blood gas
samples (0.3 ml) were obtained 30, 90 and 240 min following acid
instillation (IT) and the lost blood was replaced by the same
volume of saline solution. Samples were analyzed using a blood gas
analyzer (Omni C; Roche Diagnostics, Indianapolis, IN, USA).
microPET examination
All rats underwent microPET examination 210 min
following HCl IT. PET was performed using a microPET R4 rodent
model scanner (CTI Concorde Microsystems Inc., Knoxville, TN, USA)
that was equipped with microPET Manager for data acquisition in a
list mode and ASI Pro VM™ software for preparing sinograms and
image reconstruction. The scanner contained a computer-controlled
bed and 10.8-cm transaxial and 7.8-cm axial fields of view (FOV)
with an image resolution of <1.8 mm. FDG was prepared with a
specific activity of 500 Ci/mmol in the Department of Nuclear
Medicine, Zhejiang University School of Medicine (Hangzhou, China).
Rats woke up 210 min after the instillation, so rats were
reanesthetized in order to undergo microPET examination. Prior to
examination, the rats were reanesthetized and injected with 10.56
MBq (0.3 mCi) FDG through the cannulation. After 30 min, the rats
were placed under the central FOV of the microPET R4 scanner and
underwent a 10 min static examination. The images were
reconstructed by a maximum a posteriori probability algorithm. The
ratio of the regions of interest (ROI) in the right lung to the
muscle were calculated for each scan using ASI Pro VM. These ROIs
were drawn and placed by one of the authors who had extensive
experience in manual ROI definition and was blinded to the results.
Corrections for dead time (time after each event during which the
system is not able to record another event), random scattering and
attenuation were performed for all scans.
Histology
At the end of the experiment, all rats were killed
with an overdose of pentobarbital sodium. The left lung was placed
in 4% formalin, embedded in paraffin and stained with hematoxylin
and eosin. According to an arbitrary four-grade scale (7), all sections were examined and graded
by a pathologist who was unaware of the experimental conditions of
each animal. Briefly, the sections were assessed with regard to the
airway epithelial necrosis, intra-alveolar edema, hyaline
membranes, hemorrhage and recruitment of inflammatory cells to the
air spaces. Each characteristic was scored between 0 and 3 (0,
absent; 1, mild; 2, moderate; 3, prominent).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using SPSS statistical software, version 17.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance with
repeated measurement analysis was used to compare samples obtained
at several time points from the same animals. An independent
samples t-test was used to determine which groups were
significantly different. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood gas analysis
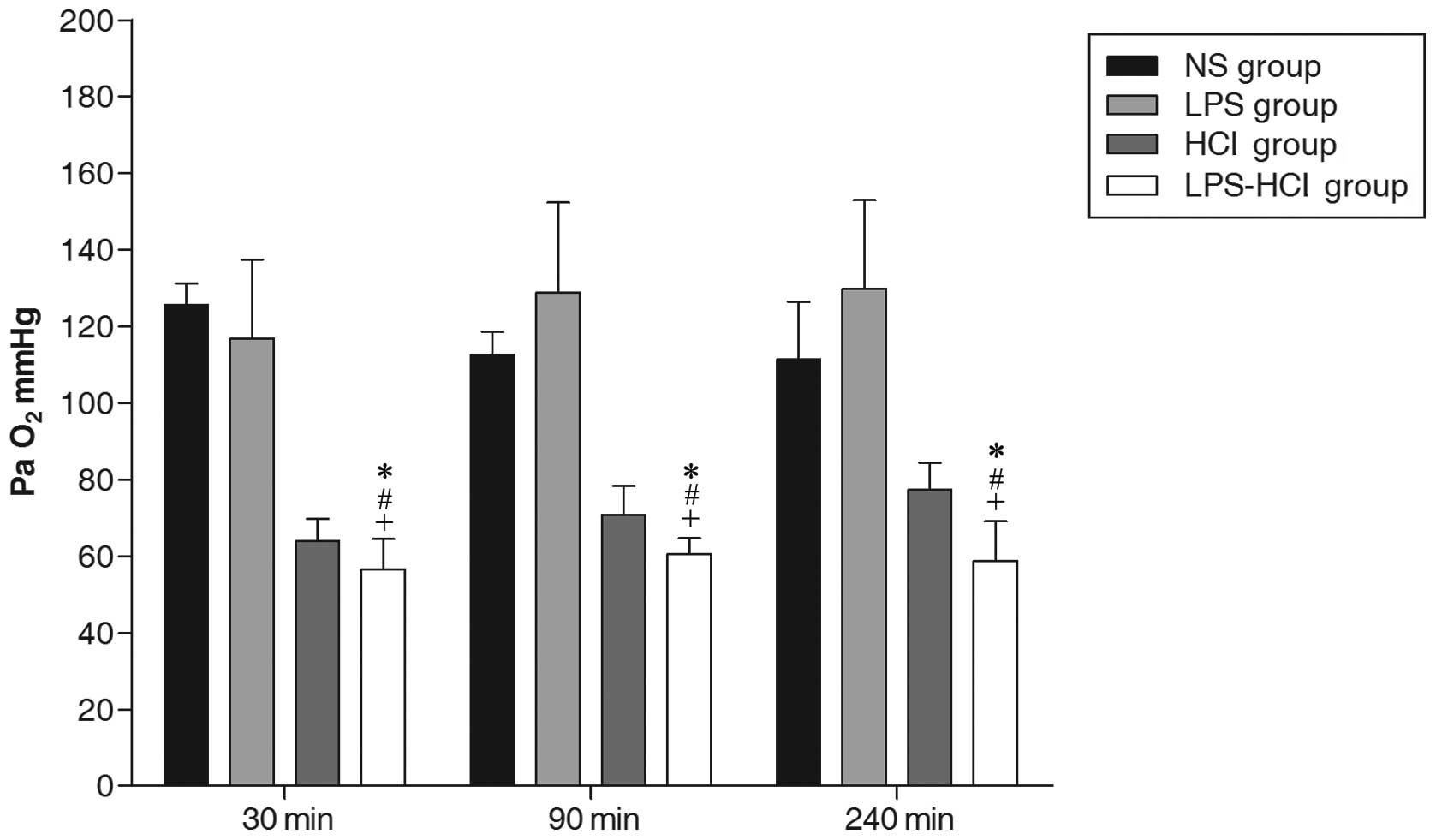
Arterial PaO2
The arterial PaO2 in the NS (116.54±10.97
mmHg) and LPS (125.20±22.49 mmHg) groups remained normal throughout
this experiment. As expected, the PaO2 showed an initial
decline in the HCl and LPS-HCl groups following acid IT. The
average PaO2 was 58.67±7.77 mmHg in the LPS-HCl group
and 70.68±8.67 mmHg in the HCl group (P<0.01). At the end point
of the experiment, the mean PaO2 in the HCl group
(77.29±7.15 mmHg) was higher compared with that of the LPS-HCl
group (58.81±10.27 mmHg) and the difference between the two groups
was statistically significant (P<0.01; Fig. 1).
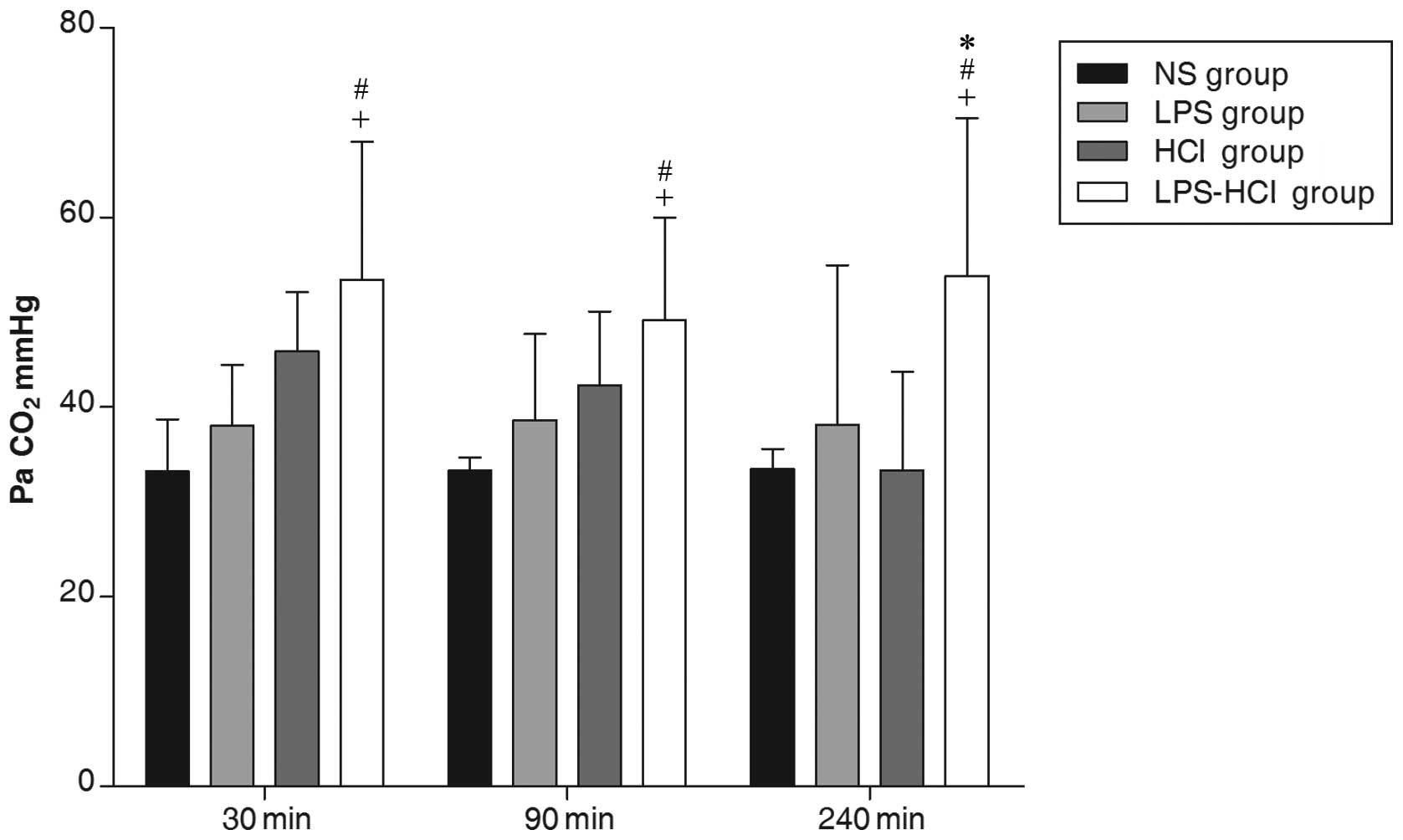
Arterial PaCO2
The arterial PaCO2 in the LPS-HCl group
(52.14±13.86 mmHg) was significantly higher compared with those in
the HCl (41.17±9.18 mmHg), LPS (38.25±11.24 mmHg) and NS control
(33.32±3.02 mmHg) groups (P<0.05; Fig. 2).
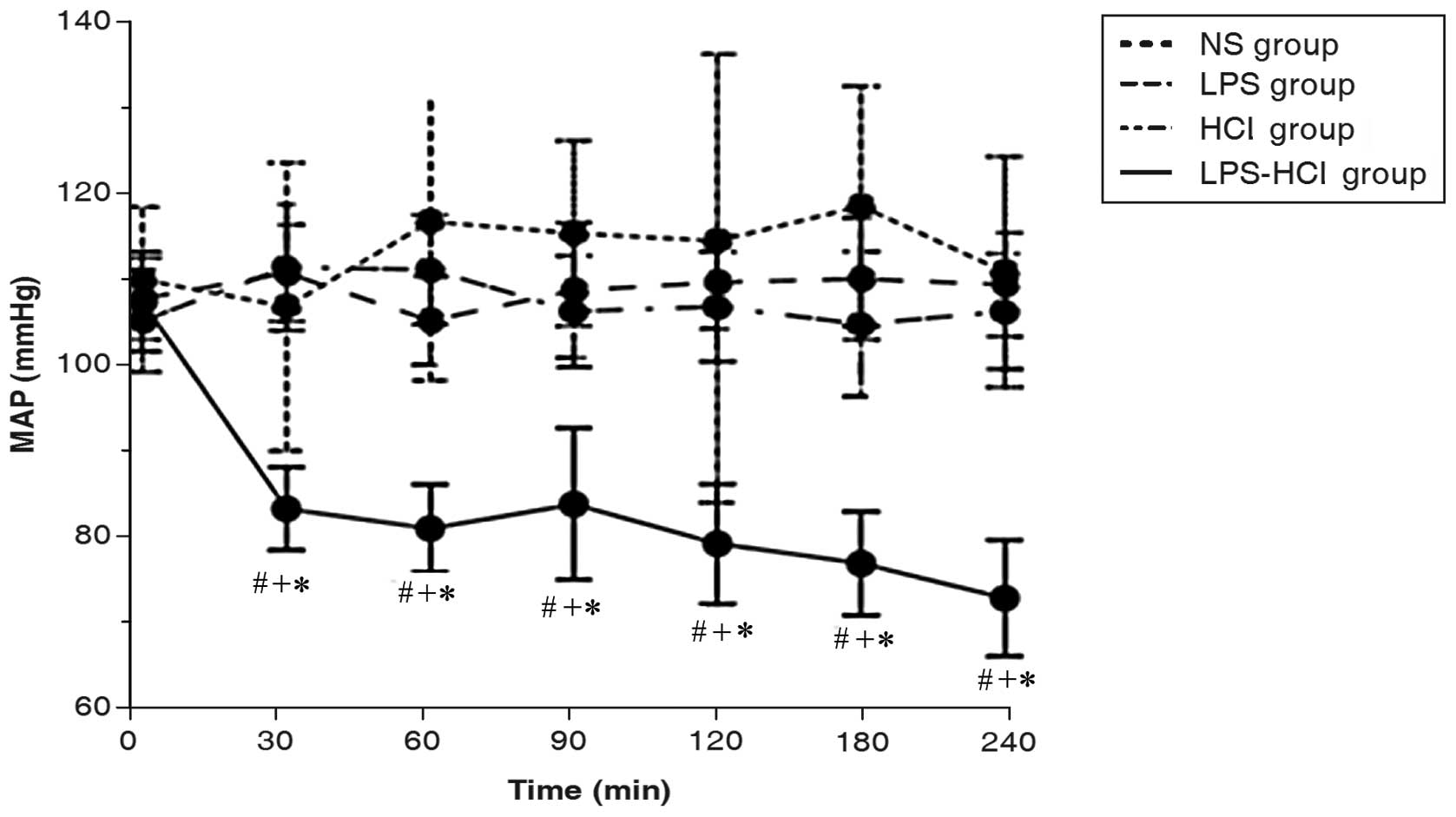
Mean arterial pressure (MAP)
Basal measurements of the MAP among the four groups
were not different. The MAP in the LPS-HCl group decreased markedly
while the MAP in the LPS, HCl and NS control groups remained
stable. The MAP of the LPS-HCl group was significantly different
compared with those of the other three groups at the subsequent
time points (P<0.001; Fig.
3).
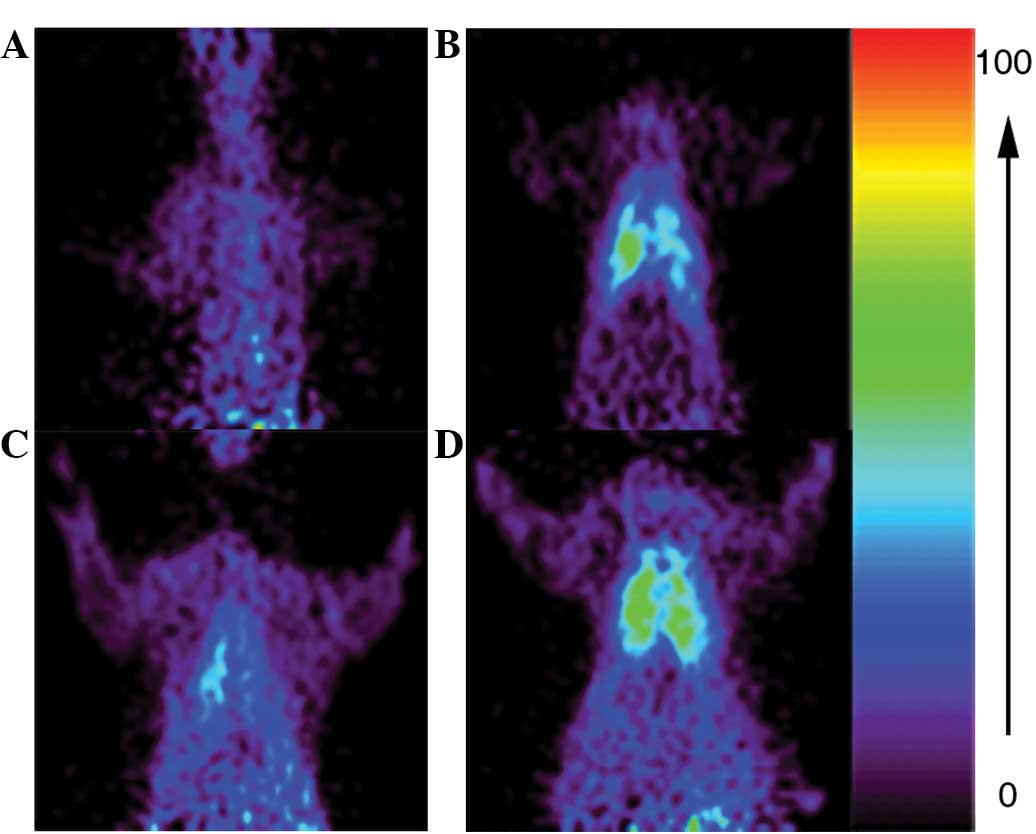
microPET
The ratios of mean [18F]FDG uptake in the
lungs to that in the muscle tissue were compared among the
different groups of rats and the ratio in the LPS-HCl group
(9.00±1.41) was observed to be significantly higher than the
uptakes in the LPS (4.01±0.60) and HCl (3.33±0.55) groups
(P<0.01; Fig. 4).
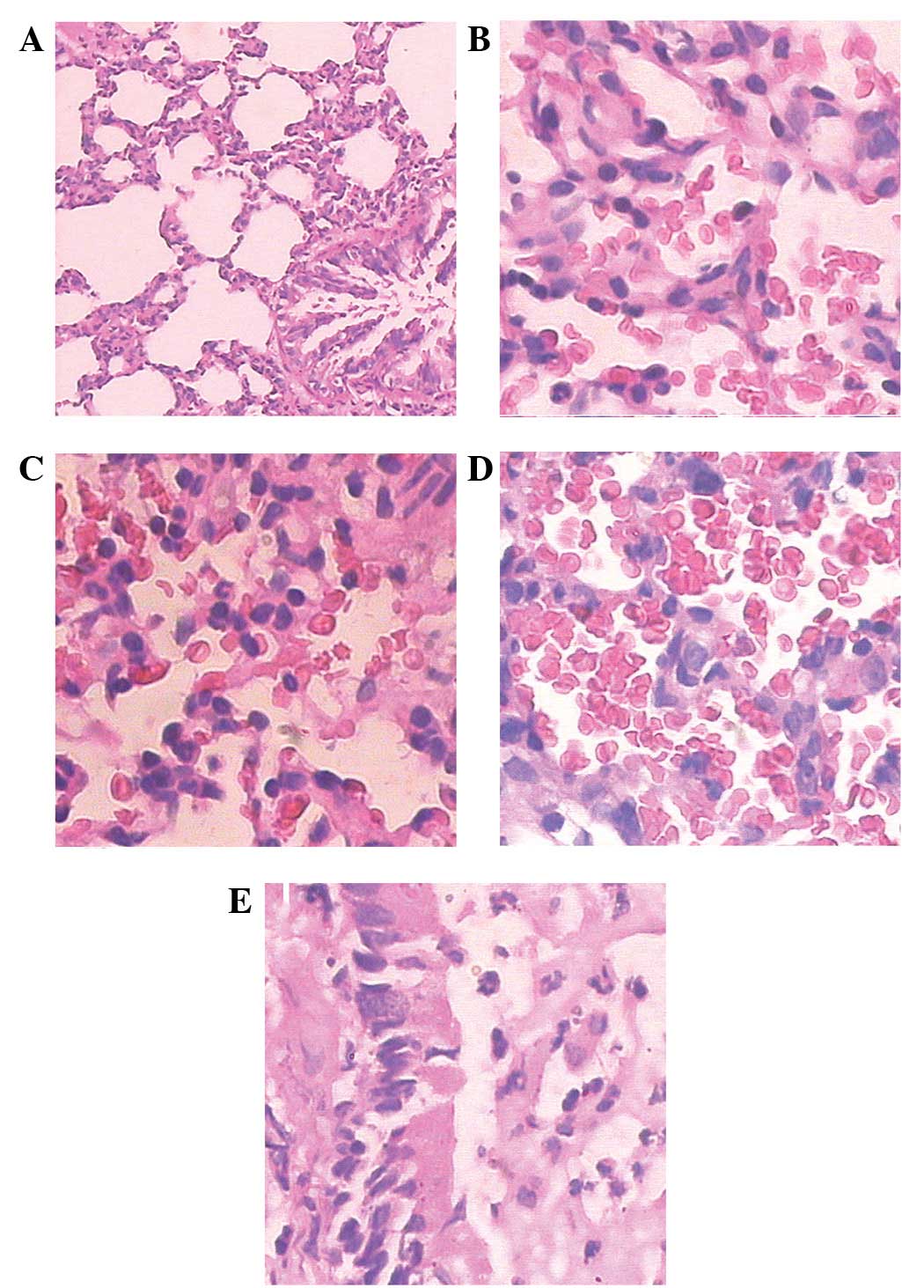
Histology
Histological examination showed that lung injuries
of varying degrees occurred in all animals that had received LPS
injection, HCl IT or both. Neutrophil infiltration and hemorrhage
were present in all animals of the LPS, HCl and LPS-HCl groups,
however, they were more prominent in the LPS-HCl group. Hyaline
membranes were common in the LPS and LPS-HCl groups but were rare
in the HCl group. Alveolar edema and airway epithelial necrosis
were common and prominent in the HCl and LPS-HCl groups, while
there were inconsistent findings in the LPS group. Briefly, the
mean lung injury score in the LPS-HCl group (12.7±0.95) was
significantly higher compared with the scores in the HCl
(8.40±1.26) and LPS (7.00±0.82) groups (P<0.001; Fig. 5).
Discussion
Although PET with [18F]FDG has become an
established diagnostic tool in oncology in clinical practice, FDG
PET is also emerging as a promising imaging technique for
infectious and inflammatory diseases (8–10).
[18F]FDG-labeled leukocyte PET/computed tomography (CT)
has a high sensitivity and specificity for the diagnosis of
infection. It may also accurately localize the foci of infection
and the source of a fever of undetermined origin, thereby guiding
additional testing (11). In ALI,
the predominant inflammatory cells are neutrophils, and the
adhesion and activation of neutrophils are important for the
development of ALI (12,13). The activated neutrophils also take
up [18F]FDG at an accelerated rate following
[18F]FDG injection, thus generating a PET imaging
signal. [18F]FDG PET/CT is a useful tool for evaluating
pulmonary lesions, as FDG uptake may be quantified. The present
study used FDG quantification to assess activated neutrophils and
obtain satisfactory results.
Animal models mimicking human ALI have been useful
for providing valuable information regarding the mechanisms
underlying the pathogenesis of this injury. An increasing number of
studies have suggested that LPS administration induces an
inflammatory response in the lung in animal models (14–16)
these effects are induced by the direct injury of endothelial cells
and by the activation of inflammatory cells, and has been
considered to be an appropriate model for ALI experiments (17). However, the LPS-induced lung injury
model in animals has its limitations in reflecting the true
situation in human patients with ALI or ARDS. Clinically, the
development of ALI and ARDS is complex and it is rare for these
conditions to be caused by only a single instigating factor
(18). The occurrence of ALI
increases with multiple risk factors and direct pulmonary disorders
(such as pneumonia, aspiration or pulmonary contusion) and indirect
pulmonary risk factors (such as sepsis or multiple trauma) are
well-known risk constellations (19).
A synergistic response has previously been shown in
the two-hit model. Studies have demonstrated that in animal models,
neutrophil recruitment to the lungs is enhanced when hemorrhagic
shock is followed by LPS treatment and when sepsis is followed by
direct lung injury with immune complexes or LPS (20,21,22).
In the present study, [18F]FDG microPET showed that rats
in the LPS-HCl group had a significant influx of activated
neutrophils into the lungs, whereas rats in the LPS and HCl groups
exhibited lower level of influx. In addition, pretreatment with LPS
significantly increased and prolonged the reduction in arterial
PaO2. These results indicate that the IP injection of
LPS greatly increased the inflammatory response to acid IT and
caused the rats to become more susceptible to ALI.
The histological examination results also
demonstrated that LPS pretreatment significantly magnified the
inflammatory response to acid aspiration. Neutrophil infiltration,
hemorrhage, hyaline membranes, alveolar edema and airway epithelial
necrosis were observed to be common and prominent in the two-hit
model; however, there were inconsistent findings in the LPS and HCl
groups. The results showed that the uptake of [18F]FDG
examined by microPET positively correlated with the
histopathological lung injury score (R=0.831, P<0.01). Thus, we
conclude that by using [18F]FDG PET, it is possible to
accurately assess the degree of lung injury.
The findings of the present study may be explained
by the two-hit model. In this model, an initial insult primes the
host to generate an amplified response to a second insult. The
initial hit (LPS IP injection) predisposed the rats to produce an
augmented inflammatory response to the second hit (acid IT). The
same dose of LPS or HCl alone only resulted in a localized
inflammatory response. However, the combination of LPS and HCl
generated a generalized inflammatory response that was detected in
both lungs.
As infection is often associated with other risk
factors in the clinic, this two-hit ALI model reflects a potential
clinical situation more effectively than a one-hit model.
Therefore, by closely paralleling the clinical development of
pulmonary injury, the two-hit model may be invaluable for the study
of human ALI.
Acknowledgements
This project was supported by the
Specialized Research Fund for the Doctoral Program of Higher
Education of China (grant no. 20110101120125) and Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY12H15005).
References
|
1.
|
Jeyaseelan S, Chu HW, Young SK, Freeman MW
and Worthen GS: Distinct roles of pattern recognition receptors
CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun.
73:1754–1763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Isik AF, Kati I, Bayram I and Ozbek HA: A
new agent for treatment of acute respiratory distress syndrome:
thymoquinone. An experimental study in a rat model. Eur J
Cardiothorac Surg. 28:301–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Hu P, Wang X, Haitsma JJ, Furmli S, Masoom
H, Liu M, et al: Microarray meta-analysis identifies acute lung
injury biomarkers in donor lungs that predict development of
primary graft failure in recipients. PLoS One. 7:e455062012.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matthay MA, Zimmerman GA, Esmon C,
Bhattacharya J, Coller B, Doerschuk CM, et al: Future research
directions in acute lung injury: summary of a National Heart, Lung,
and Blood Institute working group. Am J Respir Crit Care Med.
167:1027–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chen KB, Lee CY, Lee JJ, Tsai PS and Huang
CJ: Platonin mitigates lung injury in a two-hit model of
hemorrhage/resuscitation and endotoxemia in rats. J Trauma Acute
Care Surg. 72:660–670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Domenici L, Pieri L, Gallè MB, Romagnoli P
and Adembri C: Evolution of endotoxin-induced lung injury in the
rat beyond the acute phase. Pathobiology. 71:59–69. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lu KW, William Taeusch H, Robertson B,
Goerke J and Clements JA: Polymer-surfactant treatment of
meconium-induced acute lung injury. Am J Respir Crit Care Med.
162:623–628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rodrigues RS, Miller PR, Bozza FA,
Marchiori E, Zimmerman GA, Hoffman JM and Morton KA: FDG-PET in
patients at risk for acute respiratory distress syndrome: a
preliminary report. Intensive Care Med. 34:2273–2278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rodrigues RS, Carvalho AR, Morton KA and
Bozza FA: (18)-F-fluorodeoxyglucose positron emission
tomography/computed tomography study in acute lung injury/acute
respiratory distress syndrome. Crit Care Med. 38:347–348. 2010.
View Article : Google Scholar
|
|
10.
|
Dittrich AS, Winkler T, Wellman T, de
Prost N, Musch G, Harris RS and Vidal Melo MF: Modeling
18F-FDG kinetics during acute lung injury: experimental
data and estimation errors. PLoS One. 7:e475882012.PubMed/NCBI
|
|
11.
|
Love C, Tomas MB, Tronco GG and Palestro
CJ: FDG PET of infection and inflammation. Radiographics.
25:1357–1368. 2005. View Article : Google Scholar
|
|
12.
|
Cepkova M and Matthay MA: Pharmacotherapy
of acute lung injury and the acute respiratory distress syndrome. J
Intensive Care Med. 21:119–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Abraham E: Neutrophils and acute lung
injury. Crit Care Med. 31(Suppl): S195–S199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Huang TY, Tsai PS, Wang TY, Huang CL and
Huang CJ: Hyperbaric oxygen attenuation of
lipopolysaccharide-induced acute lung injury involves heme
oxygenase-1. Acta Anaesthesiol Scand. 49:1293–1301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Reutershan J, Morris MA, Burcin TL, Smith
DF, Chang D, Saprito MS and Ley K: Critical role of endothelial
CXCR2 in LPS-induced neutrophil migration into the lung. J Clin
Invest. 116:695–702. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Chu CH, David Liu D, Hsu YH, Lee KC and
Chen HI: Propofol exerts protective effects on the acute lung
injury induced by endotoxin in rats. Pulm Pharmacol Ther.
20:503–512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lu MY, Kang BH, Wan FJ, Chen CS and Huang
KL: Hyperbaric oxygen attenuates lipopolysaccharide-induced acute
lung injury. Intensive Care Med. 28:636–641. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lang JD and Hickman-Davis JM: One-hit,
two-hit...is there really any benefit? Clin Exp Immunol.
141:211–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Steinberg J, Halter J, Schiller H, Gatto L
and Nieman G: The development of acute respiratory distress
syndrome after gut ischemia/reperfusion injury followed by fecal
peritonitis in pigs: a clinically relevant model. Shock.
23:129–137. 2005. View Article : Google Scholar
|
|
21.
|
Fan J, Marshall JC, Jimenez M, Shek PN,
Zagorski J and Rotstein OD: Hemorrhagic shock primes for increased
expression of cytokine-induced neutrophil chemoattractant in the
lung: role in pulmonary inflammation following lipopolysaccharide.
J Immunol. 161:440–447. 1998.
|
|
22.
|
Zhou R, Hu DY, Liu LM and Zhou XW:
Protective effects of apocynin on ‘two-hit’ injury induced by
hemorrhagic shock and lipopolysaccharide. Acta Pharmacol Sin.
23:1023–1028. 2002.
|