Introduction
The bulk of endovascular effort and innovation has
been the development of devices and techniques for opening stenotic
or occluded arteries. Although stenotic or occluded arteries
represent the bulk of vascular diseases, there are occasions when
closure or occlusion of artery is required. Examples of this
include pathological coronary artery fistula (CAF) and patent
ductus arteriosus (PDA). There are a numerous interventional
instruments that have been used to close CAFs and PDAs, including
detachable balloons, stainless steel coils, controlled-release PDA
coils, the Amplatzer PDA plug, and the Jostent
[polytetrafluoroethylene (PTFE)-covered stent-graft] (1–4).
Each of these devices shares a common interventional theme of
implanting thrombogenic or occlusive devices close the target
artery. In the current study, we investigated a novel technique for
the closure of coronary arteries using percutaneous transluminal
coronary radiofrequency closure (PTRFC). PTRFC utilizes a modified
angioplasty wire to deliver RF energy that addresses localized
injury and thrombosis, and results in closure of the target
artery.
Materials and methods
Materials
The guiding catheter (5F JL3.5, XB3.5), wire
(Wizdom™, ATW™), microballoon (U-Pass, 1.5/20 mm, 2.0–2.5/10 mm to
20 mm) and microcatheter (Mass Transit, prowler 10–14) were
provided by Johnson & Johnson (New Brunswick, NJ, USA). The RF
electric wire (CRW-Zcy), a modified angioplasty wire and the
extracorporeal RF adapter were designed and produced at the Guizhou
Provincial Cardiovascular Institute and the Cardiology Department
of Guizhou Provincial Hospital (Guiyang, China). The RF machine,
ILEAD 2000 was acquired from Chengdu Jinjang Equipment Co., Ltd.
(Chengdu, China).
Methods
In total, 13 healthy dogs, of either gender,
weighing 25–30 kg were used in this study. This study was performed
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (5). The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of the Cardiology Department of Guizhou
Provincial Hospital (Guiyang, China). The canines were anesthetized
with intravenous pentobarbital (3%, 30 mg/kg), and placed onto the
catheterization table in the supine position. Tracheas were
intubated and artificially ventilated in order to maintain a
physiological pH and pCO2, and pO2 levels.
Electrodes were placed over the limbs and the infra-xiphoid area
and connected to the multi-lead recorder for electrocardiogram
(ECG) recordings. A plate electrode (3×5 cm) was inserted
subcutaneously into the back in order to serve as an irrelevant RF
electrode. Heparin (100 U/kg) was subsequently injected into the
ear vein. The femoral artery was cannulated and a coronary
angiogram was performed using 5F JL3.5 catheters. The target
vessels for RF closure were small branches of the left anterior
descending (LAD) and circumflex (Cx) arteries, 1–2 mm in diameter.
The RF electric wire (CRW-Zcy, self-modified) insulated from the
microcatheter was inserted into the terminal end of the target
vessels via the coaxial microballoon catheter (U-Pass, 1.5/20,
2.0–2.5/10–20 mm), and 10–20 mm beyond the catheter tip. The
extracorporeal end of the CRW-Zcy was connected to the RF adapters
(self-modified) and the RF machine (ILEAD 2000). Once the blood
flow was successfully blocked by the inflated balloon or
microcatheter (Mass Transit, prowler 10–14), RF energy was
discharged to the terminal segment of the vessel via the CRW-Zcy
tip for the closure of the target vessel. The therapeutic dosage
was 20–30 W every 10–30 sec (according to the diameter of the
target artery) two or three times. Following the discharge of RF
energy, a slight resistance in the movement of the wire was noted,
indicating a fixed intravascular CRW-Zcy tip. Continuous and gentle
pulling on the wire resulted in a sudden release from the target
vessels, which freely moved the CRW-Zcy back into the
microcatheter. If the proximal aspects of the target vessel were
required to be closed, the same process was repeated in order to
close the opening of the proximal artery branch. CRW-Zcy was
subsequently removed and the microcatheter was placed back into the
main coronary artery for a second angiogram 5–10 min later.
Results
Efficacy of target vessel closure
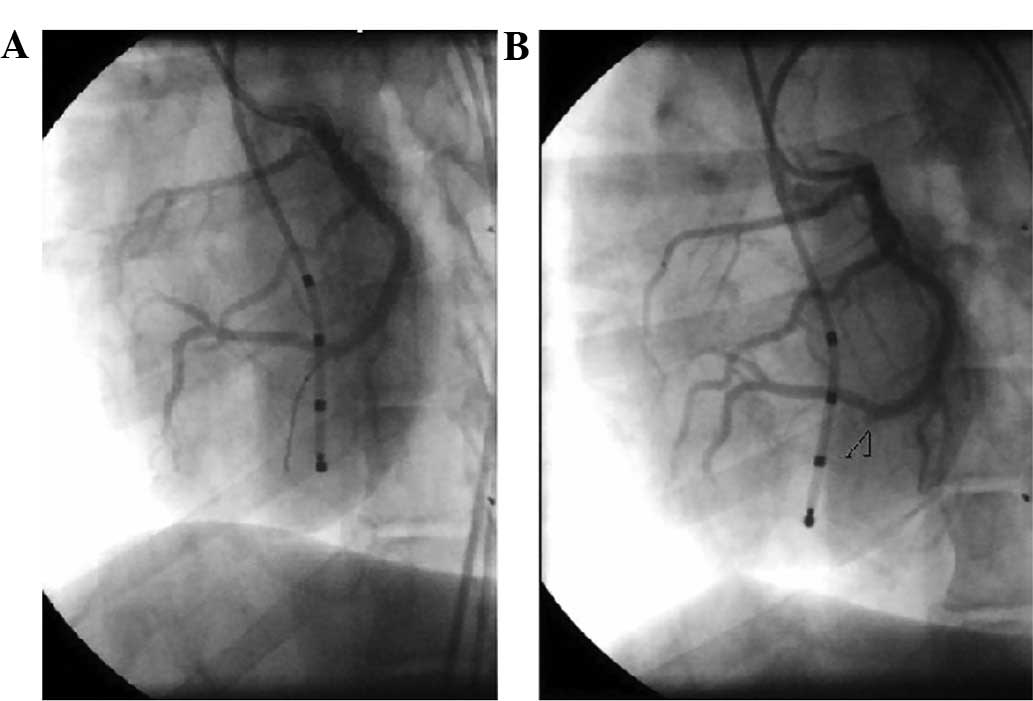
Post-PTRFC angiograms demonstrated a complete
closure in all 26 target vessels of the 13 dogs. The proximal end
of the closed target vessel was small and stub-like, which was
consistent with the area protected by the distal microcatheter.
Immediate angiography at the time of initial closure did not reveal
any evidence of residual flow (Fig.
1). The protected proximal vessel segment insulated by the
CRW-Zcy from the microcatheter appeared smooth with normal flow and
had no stricture of the lumen (Fig.
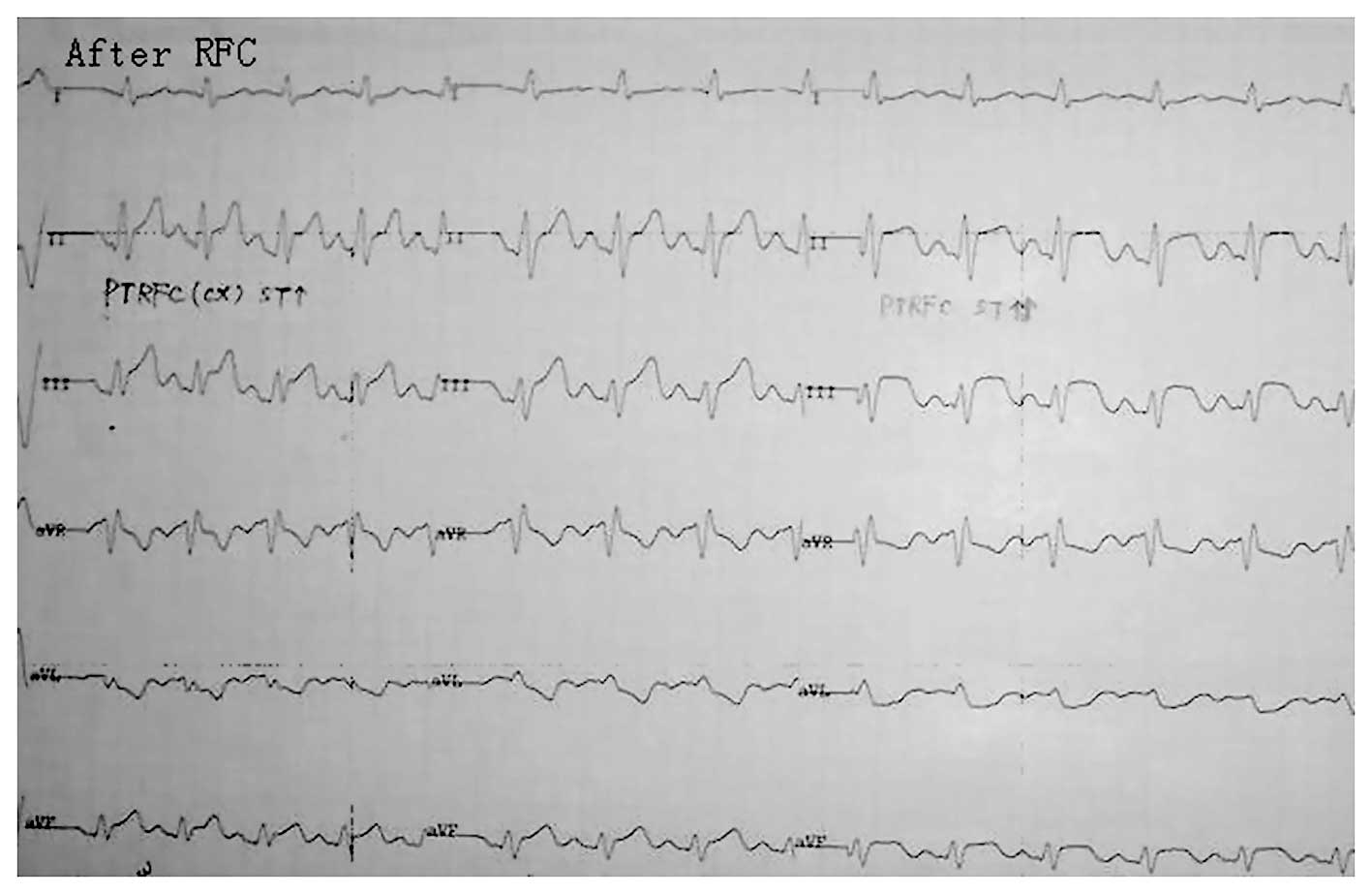
2). The ECG at the time of PTRFC revealed an acute current
injury corresponding to the myocardial area supplied by the target
vessel (Fig. 3). No evidence of
diffused ischemia consistent with a large area of vasospasm or
ventricular dysrhythmia was observed. Certain cases exhibited
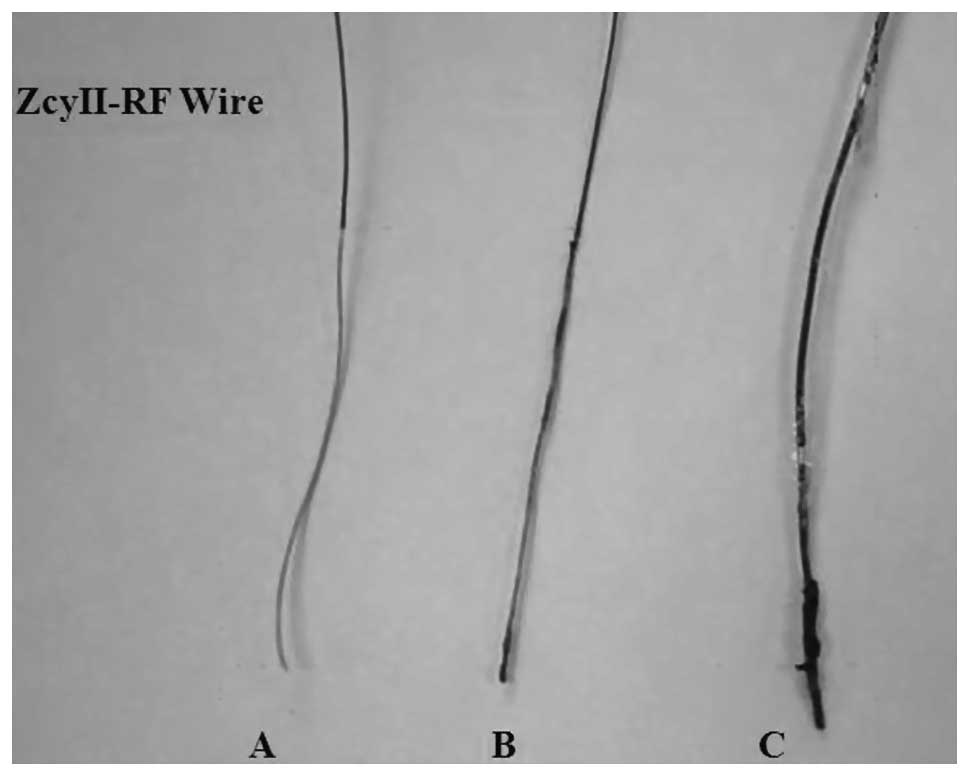
promising clinical outcomes through PTRFC. All wires were inspected
following the procedure and were noted to be intact, with no
evidence of structural damage. Carbonization was noted on the tips
of these wires consistent with the coagulative process of PTRFC
(Fig. 4).
High-dose effect
A high current safety test was performed on all
dogs. In this experiment, a single continuous RF discharge of 60 W
for 120 sec was performed. There was no evidence of diffused
vasospasm or cardiac arrhythmia during this experiment. Pathologic
analysis revealed coagulative necrosis for 2–3 mm surrounding the
target vessel and the myocardium.
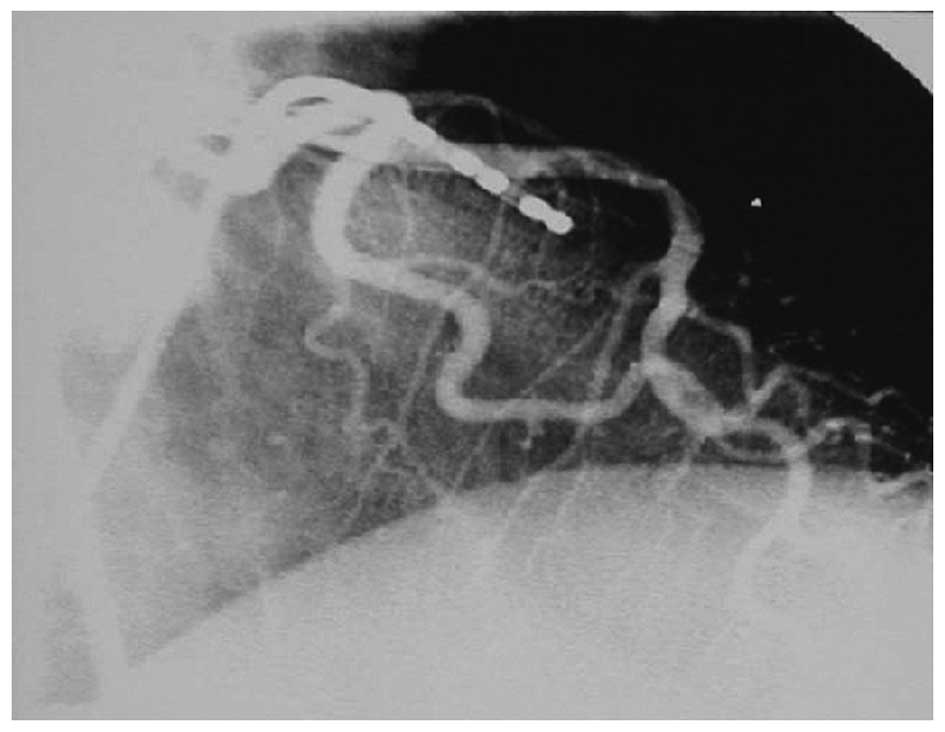
Delayed angiograms
Follow-up angiograms were performed on two dogs two
weeks after the PTRFC. Revascularization was not observed.
Pathological findings
Canine hearts were examined in five of the dogs two
days following the procedure by pathological tests. In the
protected section, the myocardium and blood vessels were normal
(Fig. 5Aa and Ab). In the thrombus
section, mixed thrombus engorgement, blocked vessel cavities in the
epicardium and myocardial layer, destroyed inner membranes of blood
vessels, and atrophic smooth muscles of the middle membrane were
evident (Fig. 5Ba); incomplete
blockage of a few small blood vessels, swollen neighboring
myocardial cells and roundish red granular degeneration in the cell
plasma were also observed. A section of the myocardium was swollen,
had merged together, and resembled ‘hot, solidifying’ necrosis
(Fig. 5Bb). In the damaged
section, there was almost complete destruction of the blood vessel
wall structure, an excessive quantity of red blood cells (Fig. 5Ca), bleeding between the
neighboring myocardia, swollen myocardial cells with disappearing
transverse lines, dissolved and disappearing nuclear breaks, and
myocardial necrosis (Fig.
5Cb).
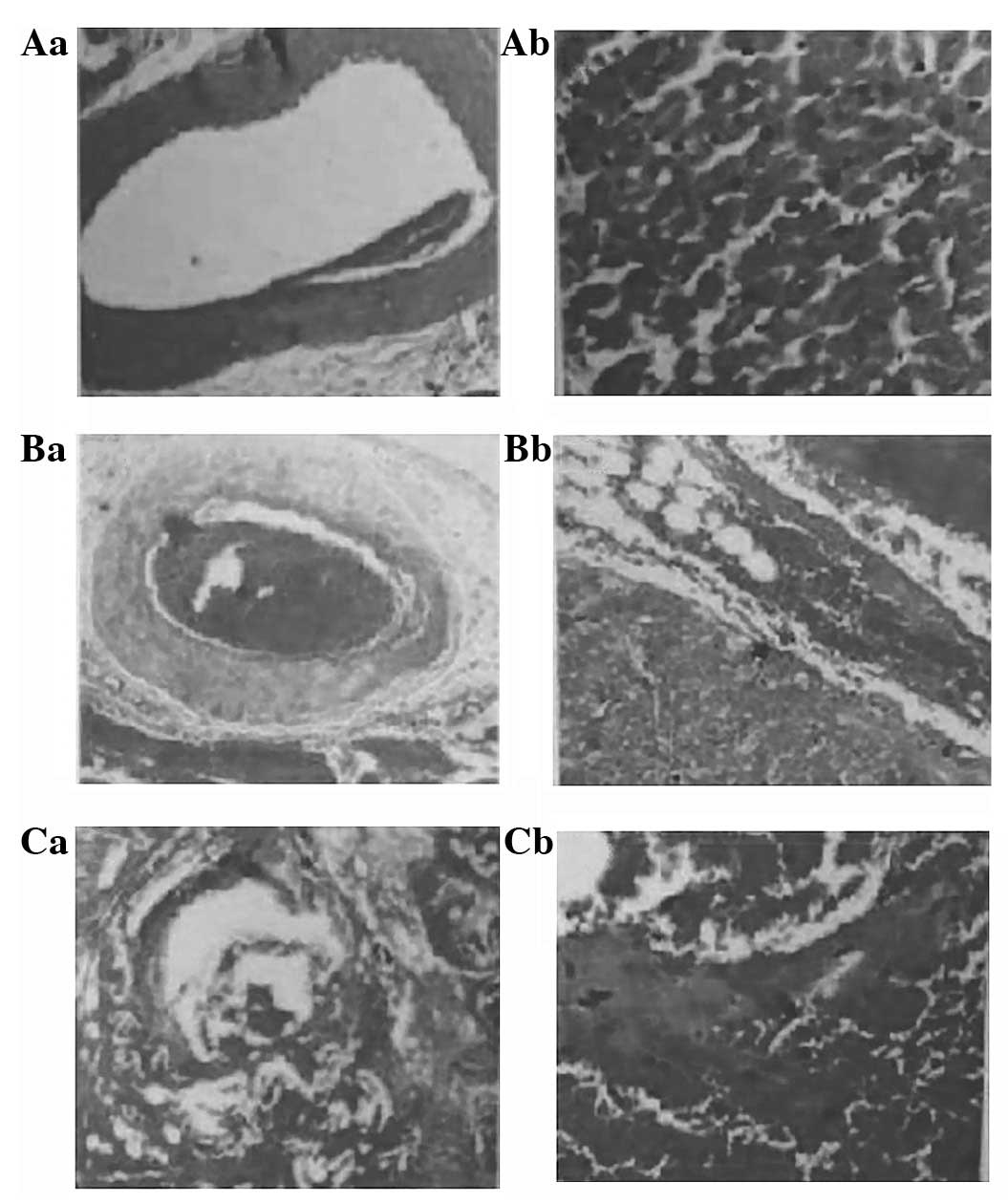 | Figure 5.Pathological findings following
percutaneous transluminal radio-frequency closure (PTRFC).
Protected sections; (Aa) the blood vessel and (Ab) the myocardium
are normal. Thrombus section: (Ba) mixed thrombus engorgement,
blocked vessel cavities in the epicardium and myocardial layer,
destroyed inner membranes of blood vessels, and atrophic smooth
muscles of the middle membrane; (Bb) incomplete blockage of a few
small blood vessels, swollen neighboring myocardial cells, and
granular degeneration in the cell plasma. A section of the
myocardium was swollen, had merged together, and resembled ‘hot,
solidifying’ necrosis. Damaged section: (Ca) almost complete
destruction of the vessel wall structure. (Cb) Bleeding between the
neighboring myocardium, swollen myocardial cells with disappearing
horizontal lines, dissolved and disappearing nuclear breaks, and
myocardial necrosis. |
Discussion
This study investigated the use of PTRFC to close
selective coronary artery branches in dogs. The advanced techniques
and instruments of percutaneous transluminal coronary angioplasty
(PTCA)/RF ablation were combined to perform PTRFC in 26 selective
branches of the canine LAD and Cx coronary arteries with the
successful instant closure of all target vessels. Angiograms were
conducted for two canines (four vessels) two weeks after the
procedure and no revascularization was found. Pathological
examination demonstrated that therapeutic and high doses (for the
safety test) of RF discharge did not result in injury to the target
vessels and the adjacent myocardium, except for closure of the
selected small branches and the consequent localized myocardial
infarction of the corresponding area. Limited experimental data
proved that the PTRFC mechanism for closing the vessel involved
thrombosis secondary to intima injury, in addition to coagulative
tissue necrosis due to heated gasification by the RF current. The
microcatheter insulated the RF current for protection. The
introduction of CRW-Zcy in this study prevented the uninvolved
vessels/myocardium from being injured when selective closure of
small arteries and localized myocardial infarction were performed.
Furthermore, blockage of blood flow in the target vessels by a
microballoon/microcatheter reduced RF energy loss by contraflow in
the target site, thus improving the efficacy of PTRFC.
The interventional process of PTRFC is valuable
clinically as PTRFC may be applied for CAF closure. The presence of
a pathological vessel and blood/pressure stealing (shunt from left
to right) (6) leads to the
occurrence of multiple serious complications with an increase in
age. Complications include heart failure, myocardial ischemia,
myocardial infarction, infectious endocarditis, the formation of
aneurysms and ruptures, and embolisms (7–10).
Therefore, symptomatic or large-shunt CAF or CAF with coronary
aneurysm should be treated as quickly as possible upon diagnosis
(11–16). The conventional approach of
ligating or suturing by opening the chest previously had an
important role in CAF treatment (11,12).
Recent advances in interventional cardiology make it possible to
block the fistula with various interventional instruments, such as
detachable balloons, stainless steel coils, controlled-release
coils, controlled-release PDA coils, Amplatzer PDA plug and Jostent
(PTFE-covered stent-graft). Provided the instruments are
appropriately selected, the rate of success for CAF closure is as
high as 97%, and an interventional approach is predicted to replace
conventional surgery as a first-choice treatment for CAF in the
majority of circumstances (1–4).
However, there have been occasional occurrences of embolisms
(2–4), fistula dissection (3), and the dislocation of instruments
(4) into the main trunk, resulting
in fatalities (10). Furthermore,
coronary arteries with particularly small diameters are unsuitable
for closure by certain instruments, such as coils (1). To the best of our knowledge, the
current study succeeded for the first time in effectively and
safely closing vessels that are 1–2 mm in diameter with PTRFC, a
new approach for interventional treatment. PTRFC has been applied
clinically to close two CAF vessels in a 66-year-old female who had
presented with angina with exertion for two years. The procedure
was successful and the clinical symptoms disappeared. The ECG
improved and no complications occurred. Further investigations are
required in order to define the exact vessel size and indication
for PTRFC, compare it with other interventional approaches, and
determine its advantages and disadvantages.
Hypertrophic obstructive cardiomyopathy (HOCM) is
one of the indications for PTRFC. Since Sigwart (17) treated idiopathic hypertrophic
subaortic stenosis (IHSS) by percutaneous transcatheter
septomyocardial ablation (PTSMA) with chemical agents in 1995, an
increasing number of studies have been published regarding this
technique, demonstrating satisfactory recent/mid-term outcomes and
a promising future for its application (13–16,18–23).
Although improvements have been made in terms of methodology,
instrument quality, rate of success, mortality and degree of
complication, there have been cases of severe complications, such
as three branch heart block, complete atrioventricular block
(19–23), pulmonary embolism (19), non-flow of the LAD artery, and
ventricular fibrillation requiring conversion defibrillation
(22). One of the major causes of
these complications is injury of the myocardium by the
uncontrollable infiltration of ethanol. However, PTRFC may prevent
these complications.
RF ablation, based on endocardial mapping, is
unhelpful for ventricular tachycardia (VT) when the origin is the
subepicardial layer (24–26). The tree branch-like microcoronary
arteries and veins distributed over the ventricular myocardium
provide an anatomical basis for electrophysiological mapping and
destruction of the pathological local myocardial segments.
Investigations using electrophysiological mapping for VT origin via
microcoronary veins followed by successful catheter RF ablation
have been performed (24–26). Therefore, PTRFC may be used for
treating patients with VT.
The findings in our study indicated that PTRFC is a
novel interventional approach for the effective and safe closure of
small pathological coronary arteries. Further investigations are
required in order to define the exact indication of PTRFC, to
compare its advantages and disadvantages with other interventional
approaches, and to improve the different properties of the
interventional instruments for clinical purposes.
References
|
1.
|
Hsieh KS, Huang TC and Lee CL: Coronary
artery fistulas in neonates, infants, and children: clinical
findings and outcome. Pediatr Cardiol. 23:415–419. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Qureshi SA and Tynan M: Catheter closure
of coronary artery fistulas. J Interv Cardiol. 14:299–307. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Armsby LR, Keane JF, Sherwood MC, Forbess
JM, Perry SB and Lock JE: Management of coronary artery fistulae.
Patient selection and results of transcatheter closure. J Am Coll
Cardiol. 39:1026–1032. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Okubo M, Nykanen D and Benson LN: Outcomes
of transcatheter embolization in the treatment of coronary artery
fistulas. Catheter Cardiovasc Interv. 52:510–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington: 2011
|
|
6.
|
Rajs J, Brodin LA, Hertzfeld I and Larsen
FF: Death related to coronary artery fistula after rupture of an
aneurysm to the coronary sinus. Am J Forensic Med Pathol. 22:58–61.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cotton JL: Diagnosis of a left coronary
artery to right ventricular fistula with progression to spontaneous
closure. J Am Soc Echocardiogr. 13:225–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wong KT and Menahem S: Coronary arterial
fistulas in childhood. Cardiol Young. 10:15–20. 2000.
|
|
9.
|
Hirose H, Amano A, Yoshida S, et al:
Coronary artery aneurysm associated with fistula in adults:
collective review and a case report. Ann Thorac Cardiovasc Surg.
5:258–264. 1999.PubMed/NCBI
|
|
10.
|
Dorros G, Thota V, Ramireddy K and Joseph
G: Catheter-based techniques for closure of coronary fistulae.
Catheter Cardiovasc Interv. 46:143–150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang S, Wu Q, Hu S, et al: Surgical
treatment of 52 patients with congenital coronary artery fistulas.
Chin Med J (Engl). 114:752–755. 2001.PubMed/NCBI
|
|
12.
|
Maeda M, Konagai N, Yano H, et al:
Surgical treatment of coronary artery-pulmonary artery fistula with
coronary aneurysm. Kyobu Geka. 54:1033–1037. 2001.(In
Japanese).
|
|
13.
|
Seggewiss H, Gleichmann U, Faber L,
Fassbender D, Schmidt HK and Strick S: Percutaneous transluminal
septal myocardial ablation in hypertrophic obstructive
cardiomyopathy: acute results and 3-month follow-up in 25 patients.
J Am Coll Cardiol. 31:252–258. 1988. View Article : Google Scholar
|
|
14.
|
Kazmierczak J, Kornacewixz-Jach Z, Kisly
M, Gil R and Wojtarowicz A: Electrocardiographic changes after
alcohol septal ablation in hypertrophic obstructive cardiomyopathy.
Heart. 80:257–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tsuchikane E, Nakamura T, Yamazaki K, et
al: Transluminal percutaneous septal myocardial ablation in a
patient with hypertrophic obstructive cardiomyopathy. Jpn Circ J.
63:537–540. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gspar J, Martínez-Ríos MA, Vonderwaide C,
et al: Pericardium-covered stent for septal myocardial ablation in
hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Intev.
47:73–79. 1999.PubMed/NCBI
|
|
17.
|
Sigwart U: Non-surgical myocardial
reduction for hypertrophic obstructive cardiomyopathy. Lancet.
346:211–214. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Faber L, Seggewiss H, Ziemssen P and
Gleichmann U: Intraprocedural myocardial contrast echocardiography
as a routine procedure in percutaneous transluminal septal
myocardial ablation: detection of threatening myocardial necrosis
distant from the septal target area. Catheter Cardiovasc Interv.
47:462–466. 1999. View Article : Google Scholar
|
|
19.
|
Faber L, Seggewiss H and Gleichmann U:
Percutaneous transluminal septal myocardial ablation in
hypertrophic obstructive cardiomyopathy: results with respect to
intraprocedural myocardial contrast echocardiography. Circulation.
98:2415–2421. 1998. View Article : Google Scholar
|
|
20.
|
Seggewiss H, Faber L and Gleichmann U:
Percutaneous transluminal septal ablation hypertrophic obstructive
cardiomyopathy. Thorac Cardiovasc Surg. 47:94–100. 1999. View Article : Google Scholar
|
|
21.
|
Gietzen FH, Leuner CJ, Raute-Kreinsen U,
et al: Acute and long-term results after transcoronary ablation of
septal hypertrophy (TASH). Catheter interventional treatment for
hypertrophic obstructive cardiomyopathy. Eur Heart J. 20:1342–1354.
1999. View Article : Google Scholar
|
|
22.
|
Kim JJ, Lee CW, Park SW, et al:
Improvement in exercise capacity and exercise blood pressure
response after transcoronary alcohol ablation of septal hypertrophy
in hypertrophic cardiomyopathy. Am J Cardiol. 83:1220–1223. 1999.
View Article : Google Scholar
|
|
23.
|
Kuhn H, Gietzen FH, Schäfers M, et al:
Changes in the left ventricular outflow tract after transcoronary
ablation of septal hypertrophy (TASH) for hypertrophic obstructive
cardiomyopathy as assessed by transoesophageal echocardiography and
by measuring myocardial glucose utilization and perfusion. Eur
Heart J. 20:1808–1817. 1999.
|
|
24.
|
Stellbrink C, Diem B, Schauerte P, Ziegert
K and Hanrath P: Transcoronary venous radiofrequency catheter
ablation of ventricular tachycardia. J Cardiovasc Electrophysiol.
8:916–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
de Paola AA, Melo WD, Távora MZ and
Martinez EE: Angiographic and electrophysiological substrstes for
ventricular tachycardia mapping through the coronary veins. Heart.
79:59–63. 1998.PubMed/NCBI
|
|
26.
|
Dubuc M, Talajic M, Roy D, Thibault B,
Leung TK and Friedman PL: Feasibility of cardiac cryoablation using
a transvenous steerable electrode catheter. J Interv Card
Electrophysiol. 2:285–292. 1998. View Article : Google Scholar : PubMed/NCBI
|