Introduction
Colorectal carcinoma is a malignant tumor of the
digestive tract that significantly threatens human health. It has
been reported that ~60% of colorectal carcinomas arise from
conventional adenomas, 35% from serrated adenomas and the remaining
5% from Lynch syndrome (1). In
1990, Longacre and Fenoglio-Preiser (2) described as ‘serrated adenomas’ a type
of adenomas that are characterized by serrated architecture and
dysplastic epithelium of the conventional adenomas. Serrated
adenomas are considered to be preponderantly distributed in the
cecum, rectum and sigmoid colon, with an intramucosal carcinoma
incidence of 10%. Due to the advances in the investigation of
serrated lesions, their clinical pathology and molecular genetics
have been presented in detail in the WHO Classification of Tumors
of the Digestive System (2010 version). A colorectal serrated
lesion is defined in the new WHO Classification as a group of
lesions characterized by serrated epithelial architecture
including: hyperplastic polyp (HP), sessile serrated adenoma/polyp
(SSA/P) and traditional serrated adenoma (TSA). Serrated
adenocarcinoma is a recently described, distinct subtype of
colorectal carcinoma (3,4). HP is the most common serrated lesion,
which commonly occurs in distal colon and rectum with an incidence
of 10–12.5%, which consists of 80–90% of all the serrated lesions
(5). HP is characterized by the
serrated profile of 1/3–1/2 of the upper glandular crypt and small
cell nuclei that are regularly aligned close to the basement
membrane (6,7). Moreover, the cells in HP are not
characterized by atypia. TSA has a serrated characteristic
structure and a morphological cytology that may be adequately used
for the diagnosis of dysplasia (8). Ectopic crypt is a definitive TSA
feature, which is a type of crypt foci that is not adjacent to the
muscularis mucosa (9). SSA
predominantly occurs in the proximal colon and it generally appears
as a flat or sessile (broad-base) polyp that protrudes slightly.
Under a microscope, the crypt of SSA is mainly characterized by a
serrated morphology, which is closely adjacent to the muscularis
mucosa. In addition, crypt expansion and deformity are observed.
The bottom of the crypt broadens and appears as inverted T- or
L-shaped branches (10). The
complex gland of SSA may show dysplasia and pseudoinvasion
(11).
HP is generally considered a type of benign lesion.
Similarly to serrated precancerous lesions, SSA and TSA may
progress to cancerous lesions through the serrated molecular
genetic and epigenetic pathways, namely serrated neoplasia
pathways. A recent study hypothesized that two serrated neoplasia
pathways are involved in cancer development (1). The first is the sessile serrated
neoplasia pathway with a mutated BRAF gene. The serrated lesion
usually develops into serrated microvesicular HP (MVHP) and SSA/P
accompanied by CpG island promoter methylation, which may lead to
the silencing of some genes. When the human MutL homolog 1 (hMLH1)
gene is silenced, cell atypia rapidly results in progression to
SSA, followed by carcinogenesis. The second pathway, termed the
traditional serrated pathway, is considered to be unrelated to the
BRAF gene mutation; however, it is suggested to be involved in KRAS
mutation. This pathway involves goblet-cell rich HP (GCHP) and TSA.
Currently, there is limited knowledge concerning GCHP. Although it
has been suggested that TSA is able to develop into cancer, the
types of cancer that TSA develops into remain controversial. It has
been reported that TSA has the potential to develop into
microsatellite instability-low (MSI-L) colorectal cancer following
methylation of the MTMG gene (1).
However, further studies concerning the molecular mechanisms
underlying the TSA-induced serrated neoplasia pathway are
required.
Few cases with carcinogenesis of serrated lesions
have been reported in countries other than China, and there is
limited knowledge on the carcinogenesis of serrated lesions in
China. In the present study, the medical files of 5,347 patients
with polyps or adenomas were collected from five hospitals in
Beijing and Hubei (China) during a 5-year period, along with 258
cases with serrated lesions that were screened, including 16 cases
with serrated lesions associated with invasive carcinoma/HIN. The
clinicopathological characteristics of the 16 cases were
investigated and immunohistochemistry was performed. The aim was to
aid further understanding of the carcinogenesis of the serrated
lesions in order to provide evidence regarding the colorectal
carcinogenesis pathways and the management of clinical prognosis of
the patients.
Subjects and methods
Subjects
A total of 5,347 patients with polyps or adenomas
were included in this study from October 2002 to September 2009.
Patient diagnosis was performed following pathological examinations
of the colon and rectum in the General Hospital of Beijing PLA
Military Region (Beijing, China), 252 Hospital of Chinese PLA
(Baoding, China), Navy General Hospital (Beijing, China), Julu
County Hospital (Hubei, China) and Dongzhimen Hospital affiliated
to Beijing University of Chinese Medicine (Beijing, China). All the
polyp and adenoma sections were retrospectively reviewed. The
sections were reviewed by three pathologists in 4–5 rounds using
WHO Criteria to screen 258 cases of polyps and adenomas with
serrated features (serrated lesions). Further histological
diagnosis and classification was conducted in 258 cases with
serrated lesions. A total of 16 cases with different types of
serrated lesions were associated with invasive carcinoma/HIN, while
20 cases with colorectal adenocarcinoma served as controls. In
addition, clinical and endoscopic data were collected for all the
samples. All the subjects were informed of the study and provided
written informed consent. The study was approved by the ethics
committee of the hospital (The General Hospital Of Beijing PLA
Military Region).
Histological classification
According to the WHO Classification for the Tumours
of the Digestive System (3,4), 258
cases of serrated lesions were classified as HP, SSA, TSA, mixed
serrated polyp/adenoma and mixed serrated conventional adenoma. The
histopathological changes of the 16 cases with serrated lesions
associated with invasive carcinoma/HIN were then assessed. The
lesions were grouped into the serrated lesion region, the
corresponding cancer, carcinogenesis region and controls. The
clinicopathological features of the serrated lesions were analyzed
by combining clinical and endoscopic data.
Immunohistochemical examination
Immunohistochemical staining was performed in 14/16
cases of cancerous serrated lesions, 20 cases of colorectal
adenocarcinoma and 5 cases of normal colorectal mucosa based on
primary antibodies against MLH1, MutS homolog 2 (MSH2), K-ras and
O6-methylguanine-DNA methyltransferase (MGMT). All the
specimens were fixed with 10% neutral buffered form-aldehyde,
embedded in paraffin and cut into 4-μm sections.
Immunohistochemistry was performed using the MaxVision one-step
immunohistochemical staining reagent kit following the
manufacturer’s instructions (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China). The primary antibodies
used in the present study are shown in Table I.
 | Table I.Origin, concentration and expression
site of antibodies. |
Table I.
Origin, concentration and expression
site of antibodies.
| Antibody | Clone number | Manufacturer | Work
concentration | Expression site |
|---|
| MLH1 | G168–728 | Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. | Ready-to-use | Cytoblast |
| MSH2 | G219–1129 | Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. | Ready-to-use | Cytoblast |
| K-ras | Polyclonal | Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. | Ready-to-use | Cytoplasm |
| MGMT | MT23.2 | Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. | Ready-to-use | Cytoblast |
Following immunohistochemical staining, K-ras was
located on the cell membrane, while MLH1, MSH2 and MGMT were
located on the cell nucleus. The percentage of positively
immunostained cells was determined using a method previously
described (12). The region with
five clear well-structured positive cells was sampled from each
section, and the percentage of positively stained cells (not
including interstitial or non-tumor cells) in 100 cells was counted
in each region under a microscope at high magnification. The mean
percentage of positively stained cells was calculated based on 5
counts. Immunohistochemical staining was classified into 4 grades
based on the percentage of positively stained cells: grade I, score
0; II, score 1; III, score 2; and IV, score 3 with <25, 26–50,
51–75 and 76–100% positively stained cells, respectively.
The presence of clear brown-yellow granules was
evaluated as positive staining, and the intensity of
immunohistochemical staining was classified into five grades:
colorless as grade 0 (score 0); light yellow as grade I (score 1);
yellow as grade II (score 2); brown yellow as grade III (score 3);
and brown as grade IV (score 4). The multiplication of two types of
scores in each section was defined as the final score of the
expression intensity. Scores 0, 1–4, 6–8 and >8 were marked as
(−), (+), (++) and (+++), respectively; while (−) to (+) was
defined as a reduced expression or loss of expression.
Statistical analysis
All the statistical analyses were performed using
the statistical software SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Chi-square and Kruskal-Wallis H tests were used for statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histopathological observations
The 16 cases with serrated lesions associated with
invasive carcinoma/HIN showed the following characteristics when
observed under a microscope: i) there were three cases of HP that
appeared with serration along with a narrow and small basement
membrane. The nucleus of the cells in the HP was small and regular,
and was located close to the basement membrane. Differentiated and
mature goblet cells along with the columnar cells were abundant in
the HP without any evident atypism. The invasive carcinoma tissues
from the three patients were closely adjacent to the HP including
two cases with moderate- or low-differentiated adenocarcinoma
(Fig. 1A and B) and one case with
local serrated adenocarcinoma. HP was observed in the
cancer-adjacent regions of the three cases with invasive
adenocarcinoma, where a close association was detected. However,
the polyp-cancer sequence was not fully clear. ii) There were seven
cases of TSA associated with malignancy in which the crypt of the
TSA appeared as an evident serration along with ectopic crypts. The
serrated crypt was not adjacent to the mucosal muscularis and the
epithelial cells showed an apparent atypism, which was
characterized by different degrees of dysplasia and even HIN. The
cell nuclei appeared to undergo rod- or vacuole-like changes and
the cytoplasm of the cells was acidophilic. Out of the seven
patients, five cases were classified as filiform serrated adenomas,
a specific subtype of TSA characterized by long villiform
extensions and serration. Epithelial cells in the TSA appeared as
non-mucosal high-columnar-shaped cells with an acidophilic
cytoplasm along with some atypism. Among the seven cases of TSA,
two cases were associated with HIN (Fig. 1C), one with the sequence HP/TSA and
cancerated tissues without any distinct infiltration observed,
while four cases had cancerated tissues associated with
infiltration. iii) There were six patients with SSA associated with
malignancy (Fig. 1D and E). The
crypt of the SSA with serrated changes was closely adjacent to the
mucosal muscularis and was close to the cancer tissues. The crypt
of the adenoma was markedly expanded and distorted, while it was
broadened towards the basement membrane, appearing as inverted T-
or L-shaped branches. The majority of the cell nuclei were
elongated, appearing with a rod-like shape. Some cells on the
middle and upper layers of the crypt showed atypia and the
formation of pseudostratified layers, while certain other cells
only showed abnormalities in the crypt structure without an
apparent cell atypia. The 6 patients with SSA included one case
associated with serrated adenocarcinoma (Fig. 1F), one associated with mucinous
adenocarcinoma, three associated with common adenocarcinoma and one
associated with HP/SSA and cancerated tissues. iv) Features of
serrated adenocarcinomas were also observed. The 16 patients with
serrated lesions were associated with invasive carcinoma/HIN and
the cancer tissues from two additional cases had pathological
features of serrated adenocarcinoma (Fig. 1F). In addition to the common
characteristics of the cancer tissues, such as invasive growth and
atypia of glandular cells, serrated adenocarcinoma was
characterized by serrated cancer tissues, acidophilic cytoplasm,
abundant mucus in peri-cancer tissues, rod- or vacuole-shaped cell
nuclei and a relatively low percentage of karyoplasm.v) An
HP-SSA-Adenocanceration sequence was observed in one case, and an
HP-TSA-carcinogenesis sequence was observed in another case.
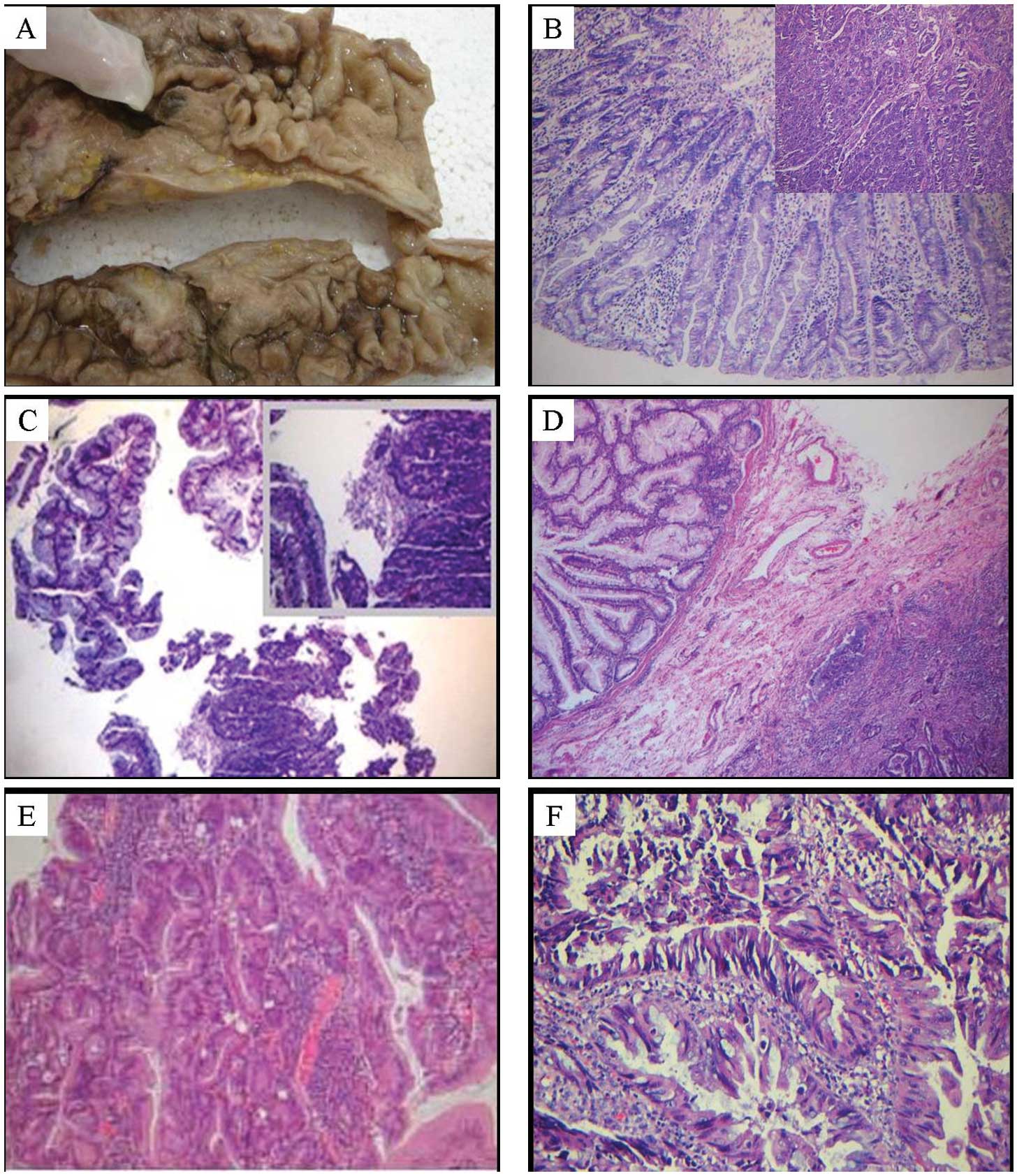 | Figure 1.(A) General view of HP associated with
low-differentiated adenocarcinoma. The black arrow indicates the
tangent plane of colon cancer, and the red arrow indicates HP. (B)
Microscopic observation of the HP shown in (A). The lesion
conformed to GCHP and appeared as serrated changes. There were many
goblet cells in the gland with the nucleus mildly stained and
closely adjacent to the basement, without any atypia. The small
figure in the right upper corner is a microscopic view of the
moderately differentiated adenocarcinoma (Hematoxylin and eosin,
magnification ×100). (C) Filiform TSA associated with
carcinogenesis. The left upper half indicates the filiform serrated
lesions, and the lower half and the right upper corner indicate the
carcinogenesis area (Hematoxylin and eosin, magnification ×100).
(D) SSA associated with moderately differentiated adenocarcinoma,
and the crypt of the SSA exhibited an inverted L shape, which was
closely adjacent to the mucosal muscularis. Arrows indicate
invasive cancer tissues adjacent to the SSA (Hematoxylin and eosin,
magnification ×100). (E) SSA associated with HIN. Glands exhibited
marked atypia (Hematoxylin and eosin, magnification ×100). (F) SSA
associated with SAC. Disorder of the serrated structures was
observed in SAC, where cell nuclei were vacuole-like shaped and the
cytoplasm was acidophilic (Hematoxylin and eosin, magnification
×200). HP, hyperplastic polyp; GCHP, goblet-cell rich HP; TSA,
traditional serrated adenoma; SSA, sessile serrated adenoma; HIN,
high-grade intraepithelial neoplasm; SAC, serrated
adenocarcinoma. |
Clinical features of the patients with
colorectal polyps/adenomas
Out of the 5,347 patients with colorectal
polyps/adenomas sampled, a total of 258 cases had serrated lesions
and 16 cases had serrated lesions associated with invasive
carcinoma/HIN, which consisted of 0.297% of the total number of
patients with polyps or adenomas and 6.2% of the patients with
serrated lesions, respectively. Among the 16 patients with serrated
lesions associated with invasive carcinoma/HIN, seven cases had TSA
associated with invasive carcinoma/HIN, which comprised 2.71% of
the total number of patients with serrated lesions; six cases had
SSA associated with invasive carcinoma/HIN, which comprised 2.33%
of the total number of patients with serrated lesions; and three
cases had HP associated with invasive carcinoma, which comprised
1.16% of the total number of patients with serrated lesions.
The 16 patients with serrated lesions associated
with invasive carcinoma/HIN included four males and 12 females; Two
males and five females with TSA associated with invasive
carcinoma/HIN, one male and five females with SSA associated with
invasive carcinoma/HIN, and one male and two females with HP
associated with invasive carcinoma. This indicated that the
serrated lesions associated with invasive carcinoma/HIN
predominantly occurred in females. All three cases of HP were
associated with invasive carcinoma in the rectum. In the seven
patients with TSA, the site of the associated invasive
carcinoma/HIN was the rectum in five cases, the descending colon in
one case and the ascending colon in one case. In the six cases of
SSA, the associated invasive carcinoma/HIN was located in the
rectum in three cases, in the ileocecal junction in two cases, and
in the sigmoid colon in one case. The onset of HP associated with
the development of invasive carcinoma occurred at the age of 44–48
years with a mean age of 46.3 years; the onset of TSA associated
with invasive carcinoma occurred at the age of 38–77 years with a
mean age of 63.1 years, while the onset of SSA associated with
invasive carcinoma occurred at the age of 42–60 years with a mean
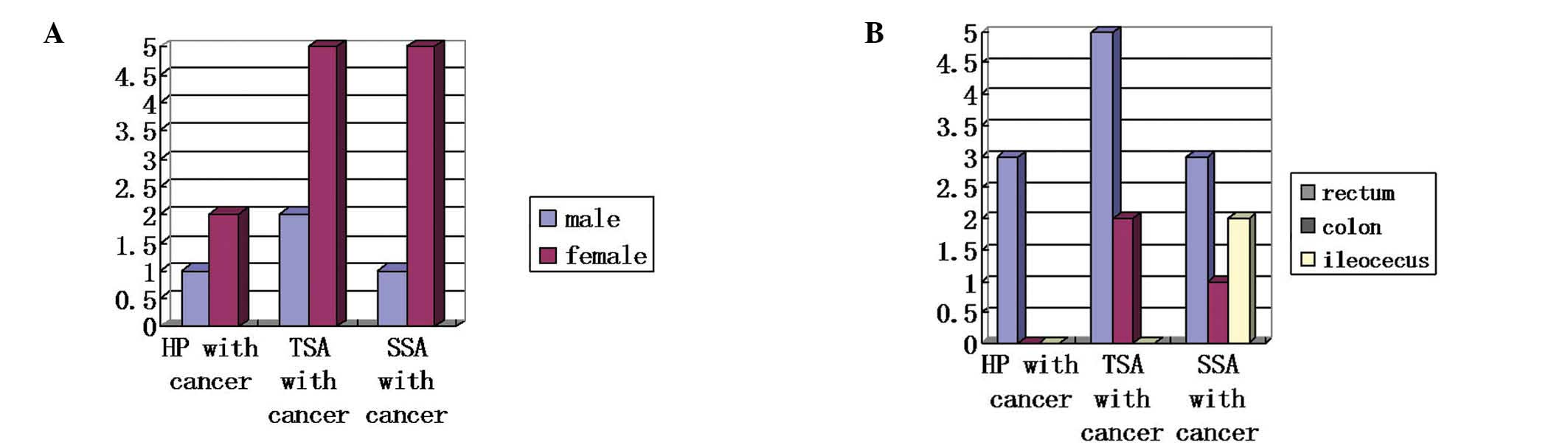
age of 51 years (Table II and
Fig. 2).
 | Table II.Clinicopathological features of 16
patients with serrated lesions associated with invasive
cancer/HIN. |
Table II.
Clinicopathological features of 16
patients with serrated lesions associated with invasive
cancer/HIN.
| Case no. | Age (years) | Gender | Site of lesion | Pathological
diagnosis and classification | Presence of
infiltration |
|---|
| 1 | 48 | Male | Rectum | HP associated with
moderate- or high-differentiated adenocarcinoma (some are SAC) | Yes |
| 2 | 47 | Female | Rectum | GCHP in the
low-differentiated adenocarcinoma-adjacent region | Yes |
| 3 | 44 | Female | Rectum |
Moderate-differentiated adenocarcinoma, HP
located on the cancer-adjacent region | Yes |
| 4 | 68 | Female | Rectum | TSA associated with
moderate-differentiated adenocarcinoma | Yes |
| 5 | 53 | Female | Rectum | HP and TSA associated
with HIN | Yes |
| 6 | 38 | Female | Descending colon | Filiform TSA
associated with moderate-differentiated adenocarcinoma | Yes |
| 7 | 77 | Female | Ascending colon | Filiform TSA
associated with HIN | Not found |
| 8 | 75 | Female | Rectum | Filiform TSA
associated with HIN | Not found |
| 9 | 59 | Male | Sigmoid
colon/rectum | Filiform TSA of the
sigmoid colon associated with rectal adenocarcinoma | Yes |
| 10 | 72 | Male | Rectum/sigmoid
colon | HP of the sigmoid
colon, and filiform TSA associated with invasive cancer in the
rectum | Yes |
| 11 | 52 | Male | Rectum | SSA associated with
carcinogenesis, and some developed mucosal carcinoma | Yes |
| 12 | 47 | Female | Ileocecal
junction | SSA associated with
SAC | Local |
| 13 | 56 | Female | Ileocecal
junction | SSA associated with
HIN | Local |
| 14 | 42 | Female | Rectum/sigmoid
colon | SSA of the sigmoid
colon associated with HIN | Not found |
| 15 | 49 | Female | Rectum | SSA associated with
moderate- or high-differentiated adenocarcinoma | Yes |
| 16 | 60 | Female | Rectum | SSA associated with
HIN | Not found |
Immunohistochemical observations
Immunohistochemistry was performed to determine the
14 patients with serrated lesions associated with invasive
carcinoma/HIN. The determination of MLH1, MSH2, K-ras and MGMT
expression was conducted in the serrated lesions and their
complicating cancer tissues (experimental group) in the 20 patients
with common tubular adenocarcinoma and five cases with colorectal
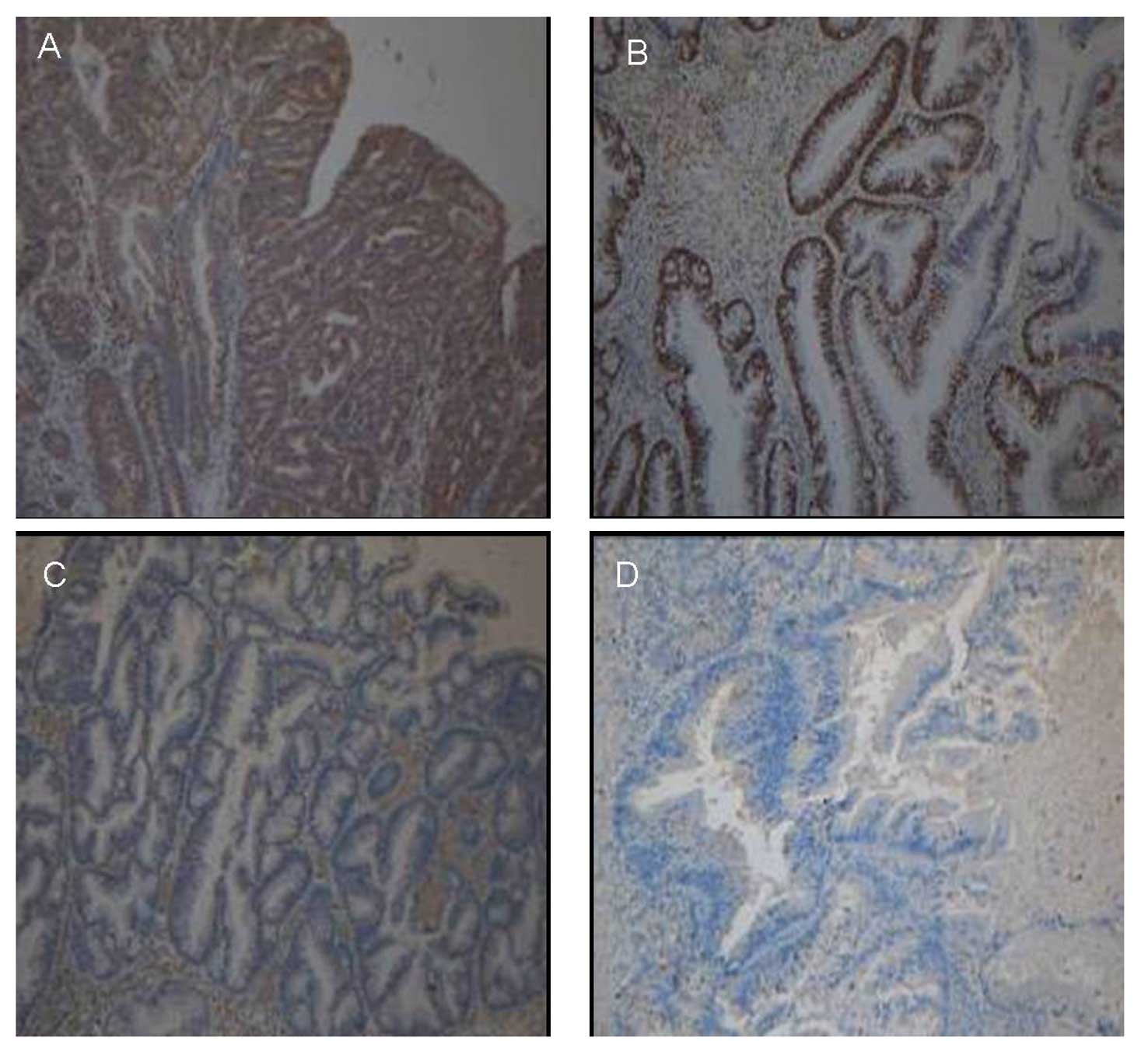
mucosal tissues (control group) as shown in Table III and Fig. 3. No significant differences were
observed in the expression of K-ras, MSH2, MLH1 and MGMT in the
cancer tissues from the patients in the experimental group compared
with those in the control group (P=0.954, 0.809, 0.447 and 0.500,
respectively). Furthermore, the expression of K-ras, MSH2 and MLH1
in the serrated lesions from the experimental group was not
significantly different compared with that of the cancer tissues
from the control group (P=0.954, 0.500 and 0.861, respectively);
however, a significant difference was detected in MGMT expression
(P=0.002). The expression of K-ras, MSH2 and MLH1 in the serrated
lesions from the experimental group was not observed to be
significantly different compared with that of the cancer tissues
from the experimental group (P<1.000, 0.403 and 0.357,
respectively), while a significant difference in the expression of
MGMT was found between the groups (P=0.0022). The expression of
K-ras, MSH2 and MLH1 in the serrated lesions from the experimental
group was not significantly different compared with that of the
normal colorectal mucosal tissues (P=0.384, 0.145 and 0.190,
respectively), while a significant difference was observed between
MGMT expression in the two groups (P=0.003). Furthermore, no
significant differences in the expression of K-ras, MSH2, MLH1 and
MGMT among the three types of serrated lesions including HP, SSA
and TSA were observed (P<0.736, 0.969, 0.255 and 0.373,
respectively). MGMT expression was reduced in the serrated lesions
compared with the expression in the control group, while no
significant difference was observed in the MGMT expression among
the three types of serrated lesions. A reduced expression of MLH1
was detected in the four patients with serrated lesions, including
two cases with reduced MLH1 expression in the complicating cancer
tissues.
 | Table III.Immunohistochemical detection of
serrated lesions associated with invasive cancer/HIN. |
Table III.
Immunohistochemical detection of
serrated lesions associated with invasive cancer/HIN.
| Group | K-ras
| MSH2
| MLH1
| MGMT
|
|---|
| −/+ | ++ to +++ | Positive rate
(%) | −/+ | ++ to +++ | Positive rate
(%) | −/+ | ++ to +++ | Positive rate
(%) | −/+ | ++ to +++ | Positive rate
(%) | n |
|---|
| Experimental | | | | | | | | | | | | | |
|
Carcinogenesis | 2 | 12 | 85.7 | 3 | 11 | 84.6 | 2 | 12 | 85.7 | 5 | 9 | 64.3 | 14 |
| Serrated
lesions | | | | | | | | | | | | | |
| HP | 0 | 3 | 100 | 1 | 2 | 66.7 | 2 | 1 | 33.3 | 2 | 1 | 33.3 | 3 |
| SSA | 1 | 5 | 83.3 | 2 | 4 | 66.7 | 1 | 5 | 83.3 | 4 | 2 | 33.3 | 6 |
| TSA | 1 | 4 | 80 | 2 | 3 | 60 | 1 | 4 | 80 | 5 | 0 | 0 | 5 |
| Control | | | | | | | | | | | | | |
|
Carcinogenesis | 3 | 17 | 85 | 5 | 15 | 75 | 5 | 15 | 75 | 5 | 15 | 75 | 20 |
| Normal | 0 | 5 | 100 | 3 | 2 | 40 | 0 | 5 | 100 | 0 | 5 | 100 | 5 |
Discussion
SSA and TSA have been reported to be neoplastic
polyps, which are precancerous lesions, while HP is a type of
non-neoplastic lesion that has no malignant potential (13). However, polyps (including HP) with
a serrated architecture have been reported to possess a malignant
potential (14). A HP with a
diameter of ≥10 mm has been shown to increase the risk of
development of colorectal carcinoma since the molecular background
of these polyps is similar to that of the corresponding subtypes of
colorectal carcinoma (15). In the
present study, two patients with HP associated with SSA/TSA and
cancerous tissues were identified; their histological transition
process was observed and immunohistochemical analysis revealed
similar results. MGMT expression was reduced in HP and SSA/TSA,
while the normal expression of MGMT was detected in the correlated
cancer tissue, suggesting that HP may be transformed into TSA or
SSA, followed by carcinogenesis. Our findings suggest that HP has a
malignant potential, and that follow-up should be strictly
practiced in patients with large HPs. In addition, three cases of
invasive adenocarcinoma had structures of HP in their
cancer-adjacent region. Immunohistochemical investigation showed a
positive expression of MGMT in cancer tissues with a reduced
expression of MGMT in HPs. Another case had negative expression of
MGMT in cancer tissues and HP. Due to the limited number of the
cases included in the present study, further studies are needed to
investigate the expression of MGMT in cancer tissues and HPs.
SSA and TSA are precancerous lesions of colorectal
carcinoma, which may be followed by cancer development. It has been
indicated that 5.3% of serrated adenomas and 2.2% of conventional
adenomas developed into cancer. Serrated adenomas with evident
dysplasia are suggested to have a higher malignant tendency
compared with conventional adenomas (16). However, the malignant potential of
serrated adenomas is considered to be lower compared with that of
conventional adenomas (3.2 vs. 9.3%) (17). Serrated adenomas have a clear
malignant potential; however, there are fewer cases of serrated
adenomas associated with high-grade intraepithelial neoplasm and
invasive adenocarcinoma than of cases with conventional adenomas
(18). The progression of
malignancy of serrated lesions is faster compared with that of
conventional adenomas (19). Based
on the example of the sessile serrated pathway, the time frame of
development from HP to SSA is unknown; however, SSA rapidly
develops into cancer, where <10 years are required for the
detection of a clear dysplasia. It has been estimated that ~5% of
SSAs develop into cancer within 20 years, while only 2.2% of
conventional adenomas develop into cancer (20). Currently, there are two major
serrated neoplasia pathways. The sessile serrated adenoma pathway
usually has high microsatellite instability (MSI-H). The mechanism
of carcinogenesis is mainly explained by BRAF mutation-induced
methylation of the mismatch repair gene MLH1 and a high level of
CpG island methylation, resulting in different degrees of dysplasia
and finally carcinogenesis, which is known as the sessile serrated
pathway. While the traditional serrated adenoma-induced colorectal
carcinoma usually has a low microsatellite instability (MSI-L) and
its mechanism of carcinogenesis is mainly K-ras mutation-induced
methylation of the MGMT DNA repair gene and a low level of CpG
island methylation, which is known as the traditional serrated
pathway (1,21).
The present study determined the expression of the
four antibodies K-ras, MSH2, MLH1 and MGMT in serrated lesions
associated with invasive cancer/HIN, while common colorectal
carcinoma and normal colorectal mucosal tissues served as controls.
MLH1 and MSH2 are mismatching repair genes, which are associated
with MSI. Immunohistochemical staining of SSA with MSI-H and
SSA-related cancer tissues indicated a significant loss of MLH1
expression in the SSA-associated high-grade dysplasia region or
intra-mucosal carcinoma regions (22). The expression of the mismatch
repair gene was lost in SSA adjacent to the cancer tissues or
dysplasia regions (23). MGMT is a
DNA repair gene, and its methylation and loss of expression have
been detected in sessile and traditional serrated pathways,
particularly in traditional serrated pathway-associated lesions,
such as TSA (24). TSA with
high-grade dysplasia has been shown to have a significantly higher
frequency of K-ras mutation and MGMT methylation (25). In the present study,
immunohistochemical analysis showed a positive expression of K-ras
and MSH2 in serrated lesions, cancer tissues and controls, and no
significant difference was observed among them (Fig. 3A and B). MGMT expression was
significantly lower in serrated lesion tissues compared with that
of complicating cancer tissues (P=0.022), control cancer tissues
(P=0.002) and normal colorectal mucosal tissues (P=0.003). However,
MGMT expression in cancer tissues from patients in the experimental
group was not significantly different from that of the controls
(P=0.500). Although no significant differences in the MLH1
expression were identified among the groups, a reduced expression
of MLH1 was detected in one case with SSA, one case with HP and the
complicating cancer tissues (serrated adenocarcinoma). Furthermore,
a reduction in the MLH1 expression was also observed in one case
with TSA and one case with HP, while no changes in MLH1 expression
were identified in the complicating cancer tissues.
Immunohistochemical analysis of 14 patients with serrated lesions
associated with invasive cancer/HIN showed different expression
levels of MGMT in the majority of the serrated lesions and their
complicating cancer tissues, while similar expression was detected
in cancer tissues from patients in the experimental and the control
groups. Consistent MGMT and MLH1 expression was observed in
serrated lesions and their complicating cancer tissues from certain
patients, suggesting that the mechanism underlying the
carcinogenesis of serrated lesions is more complex than expected.
Furthermore, serrated lesions are suggested to transform into
common or serrated adenocarcinomas.
It has been reported that ~17% of the serrated
adenocarcinomas are adjacent to the SSA with different degrees of
dysplasia (26). According to WHO
and Makinen’s criteria (21),
serrated adenocarcinoma is diagnosed based on the following
features: i) crypt epithelium with serration; ii) acidophilic or
neutrophilic cytoplasm; iii) abundant cytoplasm with a clear
vacuole-like nuclei; iv) no necrosis on the tumor surface, or an
area of necrosis of <10% of the cell surface area; v) presence
of the mucus components; and vi) spherical cells with a long
mastoid rod-like process in mucins of the tumors. In the present
study, in 2/16 cases, the cancerated tissues from the colorectal
serrated lesions associated with invasive carcinoma/HIN conformed
to the diagnosis of serrated adenocarcinoma, including one case of
sessile serrated adenoma associated with serrated adenocarcinoma
and one case of HP associated with the components of serrated
adenocarcinoma. Immunohistochemical analysis showed that the
negative expression of MLH1 in sessile serrated adenomas was
associated with serrated adenocarcinoma. Our findings showed that
not all the cancerated serrated lesions were serrated
adenocarcinomas; however, some might develop into tubular
adenocarcinomas. Serrated adenocarcinoma is defined as a subtype of
colorectal carcinoma in the WHO Classification of Tumors of the
Digestive System (2010 version). However, further studies are
required for the investigation of the clinicopathological
significance of serrated adenocarcinomas.
The present study demonstrated that TSA or HP
associated with invasive carcinoma/HIN occurred predominantly in
the rectum, while SSA associated with invasive carcinoma/HIN mainly
occurred in the ileocecal junction (Fig. 2B). This was in agreement with the
majority of previous studies (18). In addition, the age of the patients
at the onset of TSA and HP associated with invasive carcinoma/HIN
in this study was similar to the age of onset in previous studies.
Also, the age of the patients was lower at the onset of HP
associated with invasive carcinoma/HIN compared with the age of
onset of TSA-associated with invasive carcinoma/HIN. The 16 cases
of colorectal serrated lesions associated with invasive
carcinoma/HIN were screened among 5,347 patients with polyps or
adenomas, and included 4 males and 12 females. The male:female
ratios of the patients with TSA, SSA and HP associated with
invasive carcinoma/HIN were 1:2, 1:4 and 1:4, respectively. This
indicated that the number of female patients was significantly
greater compared with that of males (fig. 2A). This is significantly different
the findings of previous studies, which reported that more male
than female patients presented with serrated lesions (27).
In conclusion, TSA and SSA/P may develop into
invasive adenocarcinoma directly, and filiform serrated adenoma
(FSA) tend to evolve into HIN easily. HP may be adjacent to
invasive adenocarcinoma tissues, however, further observation is
required to investigate whether it may directly develop into
cancer. The results of immunohistochemistry showed that there was
no expression of MGMT in serrated lesions and 2 cases of SAC. There
was no expression of MLH1 in SSA/P. The results indicated that
there were different molecular pathways in different types of
serrated lesions.
References
|
1.
|
Snover DC: Update on the serrated pathway
to colorectal carcinoma. Hum Pathol. 42:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Longacre TA and Fenoglio-Preiser CM: Mixed
hyperplastic adenomatous polyps/serrated adenomas. A distinct form
of colorectal neoplasia. Am J Surg Pathol. 14:524–537. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Snover DC, Ahnen DJ, Burt RW, et al:
Serrated polyps of the colon and rectum and serrated polyposis. WHO
Classification of Tumours of the Digestive System. Bosman FT,
Carneiro F, Hruban RH and Theise ND: 4th edition. IARC Press; Lyon:
pp. 160–165. 2010
|
|
4.
|
Hamilton SR, Bosman FT and Boffetta P:
Carcinoma of the colon and rectum. WHO Classification of Tumours of
the Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise
ND: 4th edition. IARC Press; Lyon: pp. 134–146. 2010
|
|
5.
|
Lieberman DA, Prindiville S and Weiss DG:
Risk factors for advanced colonic neoplasia and hyperplastic polyps
in asymptomatic individuals. JAMA. 290:2959–2967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
O’Brien MJ, Yang S, Clebanoff JL, Mulcahy
E, Farraye FA, Amorosino M and Swan N: Hyperplastic (serrated)
polyps of the colorectum: relationship of CpG island methylator
phenotype and K-ras mutation to location and histologic subtype. Am
J Surg Pathol. 28:423–434. 2004.PubMed/NCBI
|
|
7.
|
Snover DC, Jass JR, Fenoglio-Preiser C and
Batts KP: Serrated polyps of the large intestine: a morphologic and
molecular review of an evolving concept. Am J Clin Pathol.
124:380–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hawkins NJ and Ward RJ: Sporadic
colorectal cancers with microsatellite instability and their
possible origin in hyperplastic polyps and serrated adenomas. J
Natl Cancer Inst. 93:1307–1313. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Torlakovic EE, Gomez JD, Driman DK,
Parfitt JR, Wang C, Benerjee T and Snover DC: Sessile serrated
adenoma (SSA) vs. tradition serrated adenoma (TSA). Am J Surg
Pathol. 32:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wang LP and Chen J: Concept and
pathological diagnosis of colorectal sessile serrated adenoma
closely associated with cancer. Chin J Diagn Pathol. 15:84–87.
2008.(In Chinese).
|
|
11.
|
Kudo Se, Lambert R, Allen JI, Fujii H,
Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka
S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O’Brien MJ, Lieberman
DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A,
Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M,
Vieth M, Jass JR and Hurlstone PD: Nonpolypoid neoplastic lesions
of the colorectal mucosa. Gastrointest Endosc. 68:S3–S47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Xu LZ and Yang WT: Criteria for
identification of immunohistochemical reaction results. Chin Oncol.
6:229–231. 1996.(In Chinese).
|
|
13.
|
Mäkinen MJ, George SM, Jernvall P, Mäkelä
J, Vihko P and Karttunen TJ: Colorectal carcinoma associated with
serrated adenoma - prevalence, histological features, and
prognosis. J Pathol. 193:286–294. 2001.PubMed/NCBI
|
|
14.
|
Winawer SJ, Fletcher RH, Miller L, Godlee
F, Stolar MH, Mulrow CD, Woolf SH, Glick SN, Ganiats TG, Bond JH,
Rosen L, Zapka JG, Olsen SJ, Giardiello FM, Sisk JE, Van Antwerp R,
Brown-Davis C, Marciniak DA and Mayer RJ: Colorectal cancer
screening: clincial guidelines and rationale. Gastroenterology.
112:594–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hiraoka S, Kato J, Fujiki S, Kaji E,
Morikawa T, Murakami T, Nawa T, Kuriyama M, Uraoka T, Ohara N and
Yamamoto K: The presence of large serrated polyps increases risk
for colorectal cancer. Gastroenterology. 139:1503–1510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lazarus R, Junttila OE, Karttunen TJ and
Mäkinen MJ: The risk of metachronous neoplasia in patients with
serrated adenoma. Am J Clin Pathol. 123:349–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Song SY, Kim YH, Yu MK, Kim JH, Lee JM,
Son HJ, Rhee PL, Kim JJ, Paik SW and Rhee JC: Comparison of
malignant potential between serrated adenomas and traditional
adenomas. J Gastroenterol Hepatol. 22:1786–1790. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang LP, Chen J, Ning HY, Zhang XZ, Cheng
J, Li L, Wang B, Dai XJ, Zhu HY, Miao JH and Wang L: Serrated
lesions of colon and their malignant potential. Zhonghua Bing Li
Xue Za Zhi. 39:447–451. 2010.(In Chinese).
|
|
19.
|
Snover DC, Jass JR, Fenoglio-Preiser C and
Batts KP: Serrated polyps of the large intestine: a morphologic and
molecular review of an evolving concept. Am J Clin Pathol.
124:380–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lu FI, van Niekerk de W, Owen D, Tha SP,
Turbin DA and Webber DL: Longitudinal outcome study of sessile
serrated adenomas of the colorectum: an increased risk for
subsequent right-sided colorectal carcinoma. Am J Surg Pathol.
34:927–931. 2010. View Article : Google Scholar
|
|
21.
|
Mäkinen M: Colorectal serrated
adenocarcinoma. Histopathology. 50:131–150. 2007.
|
|
22.
|
Sheridan TB, Fenton H, Lewin MR, Burkart
AL, Iacobuzio-Donahue CA, Frankel WL and Montgomery E: Sessile
serrated adenomas with low- and high-grade dysplasia and early
carcinomas: an immunohistochemical study of serrated lesions
‘caught in the act’. Am J Clin Pathol. 126:564–571. 2006.PubMed/NCBI
|
|
23.
|
Goldstein NS: Small colonic microsatellite
unstable adenocarcinomas and high-grade epithelial dysplasias in
sessile serrated adenoma polypectomy specimens: a study of eight
cases. Am J Clin Pathol. 125:132–145. 2006. View Article : Google Scholar
|
|
24.
|
Leggett B and Whitehall V: Role of the
serrated pathway in colorectal cancer pathogenesis.
Gastroenterology. 138:2088–2100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kim KM, Lee EJ, Kim YH, Chang DK and Odze
RD: KRAS mutations in traditional serrated adenomas from Korea
herald an aggressive phenotype. Am J Surg Pathol. 34:667–675.
2010.PubMed/NCBI
|
|
26.
|
Tuppurainen K, Mäkinen JM, Junttila O,
Liakka A, Kyllönen AP, Tuominen H, Karttunen TJ and Mäkinen MJ:
Morphology and microsatellite instability in sporadic serrated and
non-serrated colorectal cancer. J Pathol. 207:285–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Huang CS, O’brien MJ, Yang S and Farraye
FA: Hyperplastic polyps, serrated adenomas, and the serrated polyp
neoplasia pathway. Am J Gastroenterol. 99:2242–2255. 2004.
View Article : Google Scholar : PubMed/NCBI
|