Introduction
Transmembrane protein 174 (TMEM174) was identified
among a large pool of genes by high-throughput cell screening
technology, which is used to isolate functional genes and to
provide insight into the mechanisms of gene function (1). Genes are investigated using a reverse
biological strategy in which gene expression profiling of tissues
and cell lines is performed. This approach revealed high expression
levels of TMEM174 in kidney tissue and the lymphadenoma-derived
Raji cell line (2). The TMEM174
gene is activated by the activator protein-1 (AP-1) pathway in HeLa
and 293T cells (~3- and 9-fold, respectively). Preliminary studies
of the mechanism underlying the promotion of cell proliferation
indicated that TMEM174 is linked with Ras and Raf in the
extracellular-signal-regulated kinase (ERK) pathway. Furthermore,
TMEM174 has a role in promoting the G2/S phase of the cell cycle
(2). However, the underlying
mechanism remains to be elucidated and other pathways and
transcription factors have been suggested to be involved in this
process.
The cyclic-AMP response element binding (CREB)
protein is a transcription factor that affects a spectrum of
cellular activities. including glucose homeostasis, growth
factor-dependent survival, proliferation, differentiation and
memory (3,4). CREB recruitment to the cyclin D1
promoter promotes cyclin D1 transcription and cell proliferation
(5). Transgenic animal studies
have shown that overexpression of CREB confers oncogenic
characteristics on cells in various tissues and that abnormal CREB
expression is associated with tumor development in humans (6). CREB plays an important role in the
development of brain tumors, leukemias and other types of cancer
(7). Evidence based on the high
level of homology of zebrafish CREB and its mammalian counterpart
suggests that activated (phosphorylated) CREB is localized in
proliferation zones (7).
Activation of CREB has been suggested to stimulate cellular
proliferation through the PI3-K/AKT pathway (8) and to act as a crucial transcription
factor for the regulation of TMEM174 expression.
AP-1 family members are important upstream
activators of the ERK signaling pathway (9), which are involved in cell
proliferation, transformation, differentiation and death (10–12).
AP-1 is a collective term referring to dimeric transcription
factors composed of Jun, Fos, activating transcription factor (ATF)
and musculoaponeurotic fibrosarcoma (MAF) protein subunits that
bind to a common AP-1 binding site (13,14).
c-Jun is a positive regulator of cell proliferation, while JunB
mediates the converse effect. Jun promotes apoptosis by
participating in cell stress-induced transcriptional activation of
apoptotic target genes (15).
Previous studies have indicated that TMEM174 overexpression
activates AP-1, since the upstream molecules ERK, ELK-1 and Fos are
markedly activated or stimulated by sequential blockade of these
upstream factors in the ERK pathway (2).
These studies contribute to the understanding of the
mechanism by which cell proliferation is promoted at the protein
level. However, details of the mechanism at the transcriptional
level remain to be elucidated. Identification of the core promoter
is a critical first step in this process, facilitating subsequent
determination of the important transcription factors.
In the present study, the mechanism by which TMEM174
promotes cell proliferation at the transcriptional level was
investigated. The promoter region and transcription factor binding
sites were predicted by bioinformatics analysis. Among the large
numbers of binding sites predicted, such as CREB, AP-1, nuclear
factor-κB (NF-κB) and Oct1, CREB and AP-1 were identified with
potential binding sites for interaction with the TMEM174 promoter
region by electrophoretic mobility shift assay (EMSA).
Materials and methods
PCR amplification and molecular
cloning
Primers were designed for the amplification of
fragments of various lengths corresponding to the 4.8 kb sequence
upstream of the TMEM174 gene (available in the NCBI mapview
database: http://www.ncbi.nlm.nih.gov/mapview) and using TFEARCH
online software (http://www.cbrc.jp/research/db/TFSEARCH.html) to
predict the candidate promoter region and transcription factor
binding sites. Fragments were amplified for cloning from whole
blood genomic DNA. The primers used are shown in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Primer sequences | Position (bp) |
|---|
| P1 | F: 5′-CGGGGTACCGTTTTGCAAGTCAATGACAAGCTGTCTC-3′ | −186 to +674 |
| R: 5′-CCCAAGCTTCTTTCACGGACGGTGGAAATCACAG-3′ |
| P2 | F: 5′-CGGGGTACCCAGCCAATTTTTAAAATTTTTTGTAGAGATAGG-3′ | −466 to +674 |
| R: 5′-CCCAAGCTTCTTTCACGGACGGTGGAAATCACAG-3′ |
| P3 | F: 5′-CGGGGTACCCAGGAGTCTAACCTGATTTACCTAGTGGTTC-3′ | −700 to +674 |
| R: 5′-CCCAAGCTTCTTTCACGGACGGTGGAAATCACAG-3′ |
| P4 | F: 5′-CGGGGTACCGTTTGGGGAGTAATTCCAGCTTTGGG-3′ | −890 to +674 |
| R: 5′-CCCAAGCTTCTTTCACGGACGGTGGAAATCACAG-3′ |
| P5 | F: 5′-CGGGGTACCGACACATGCTTCGGACCCTCCCTC-3′ | −1000 to +674 |
| R: 5′-CCCAAGCTTCTTTCACGGACGGTGGAAATCACAG-3′ |
| P6 | F: 5′-CGGGGTACCACAGGGAGACTTCAAGGTGGGAGAAAGGAG-3′ | −2500 to +1 |
| R: 5′-CCCAAGCTTCTTCTATAACTAATTTGGACCTGTGATTCCTTG-3′ |
The PCR amplification reaction system (Table II) conditions were as follows:
initial denaturation at 95°C for 5 min; 30 cycles of 95°C for 30
sec; 55–60°C (depending on the primer pair used) for 30 sec and
72°C for 1 min and final elongation at 72°C for 7 min. The PCR
products were purified by agarose gel electrophoresis
(Sigma-Aldrich, Schnelldorf, Germany) and Vigorous purification
kits (Vigorous Biotechnology, Beijng, China). The purified products
were cloned into the KpnI and HindIII restriction
enzyme sites of pGL3-basic (Promega, Madison, WI, USA). The
recombined vectors were sequenced by SinoGenoMax Co., Ltd.
(Beijing, China).
 | Table II.PCR amplification reaction system. |
Table II.
PCR amplification reaction system.
| Substance | Value
(μl) |
|---|
| 2.5 mM dNTP
mixture | 2 |
| 10X Pyrobest™
buffer | 2.5 |
| Template DNA | 0.1 |
| Forward primer | 2.5 |
| Reverse primer | 2.5 |
| Pyrobest™ DNA
polymerase | 0.3 |
| ddH2O | 15.1 |
| Total | 25 |
Transfection and dual luciferase reporter
assay
293T cells were seeded in 96-well plates
(1×104/well) in complete Dulbecco’s modified Eagle’s
medium (DMEM; HyClone, Logan, UT, USA) containing 10% fetal bovine
serum (FBS) and were transfected the next day with Vigofect
(Vigorous Biotechnology) according to the instructions provided by
the manufacturer. A total of 104 ng plasmid DNA/well was
transfected, including 100 ng recombinant pGL3 plasmid or
pGL3-basic (empty vector control) and 4 ng pRL-TK (Promega)
containing the Renilla luciferase gene as an internal
control. Transfections were performed in triplicate. The cells were
lysed in standard 1X lysis buffer (Promega) for 30 min at room
temperature (RT) and the cell lysates were assayed for both firefly
and Renilla luciferase activity using the Dual-Luciferase
Reporter assay kit (Promega) according to the instructions provided
by the manufacturer. Fluorescence was detected using a GENius Pro
microtiter plate reader (Tecan, Männedorf, Switzerland). Relative
luciferase activity was determined by normalizing the activity of
the firefly luciferase activity (F value) against the
Renilla luciferase activity (R value). Each experiment was
performed at least three times. The F/R value was calculated
respectively and an average value was obtained as the recombined
plasmid activity. pGL3-basic was used as the negative control and
its activity was defined as 1. The activities of all other
recombined plasmids were compared with the negative control.
EMSA
Nuclear extracts of HepG2 cells were prepared using
Nuclear Extract kits (Active Motif, Carlsbad, CA, USA). Protein
concentrations were determined using BCA protein assay kits
(Vigorous Biotechnology). The oligonucleotide probes (CREB, AP-1
and competitive probes) used in EMSA were designed according to a
computer-based search with the software Promoter Scan (ProScan
version 1.7 suite of programs developed by Dr Dan Prestridge,
http://www-bimas.cit.nih.gov/molbio/proscan/).
Experimental groups were as follows: CREB probe/nuclear extract,
AP-1 probe/nuclear extracts, CREB probe/nuclear extract +
competitive probe and AP-1 probe/nuclear extract + competitive
probe. Free probe was used as a negative control. Nuclear extracts
(5 μg) were incubated with 2 μl (200 nM)
biotin-labeled DNA probe and mixed with 10X binding buffer (2
μl), 50% glycerol (1 μl), MgCl2 (1
μl), Poly (dI.dC) (1 μl) and 1% NP-40 (1 μl)
for 10 min at RT. Bound DNA complexes were separated by
non-denaturing polyacrylamide gel (6.5%) electrophoresis and
transferred to a positively charged nylon membrane. Cross-linking
was performed under ultraviolet (UV) light for 20 min and the
membranes were probed with a streptavidin-horseradish peroxidase
(HRP) conjugate and incubated with the chemiluminescent substrate
for band detection. For competition experiments, unlabeled
competitor oligonucleotides were pre-incubated (200-fold excess)
with the labeled probe.
Statistical analysis
Differences between the levels of expression were
analyzed by one-way ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
TMEM174 promoter cloning
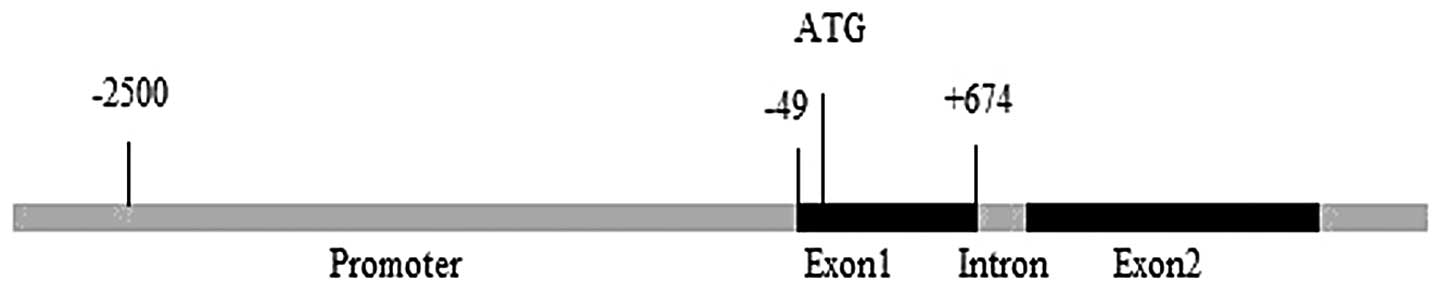
The promoter region of TMEM174 was amplified from
whole blood genomic DNA as described in Materials and methods. Five
fragments of various lengths corresponding to the predicted
promoter region and the first exon of TMEM174 were amplified: −186
to +674, −466 to +674, −700 to +674, −890 to +674 and −1,000 to
+674 bp. Amplified products of 860, 1,140, 1,347, 1,564 and 1,674
bp were cloned into pGL3-basic. The cloned sequences were identical
to the original genomic sequences shown in Fig. 1A. Numerous transcription factor
binding sites were predicted within the promoter region by
TFSEARCH, including CREB, AP-1, P300, NF-κB and Oct1 (Fig. 1B).
Characterization of TMEM174 promoter
activity
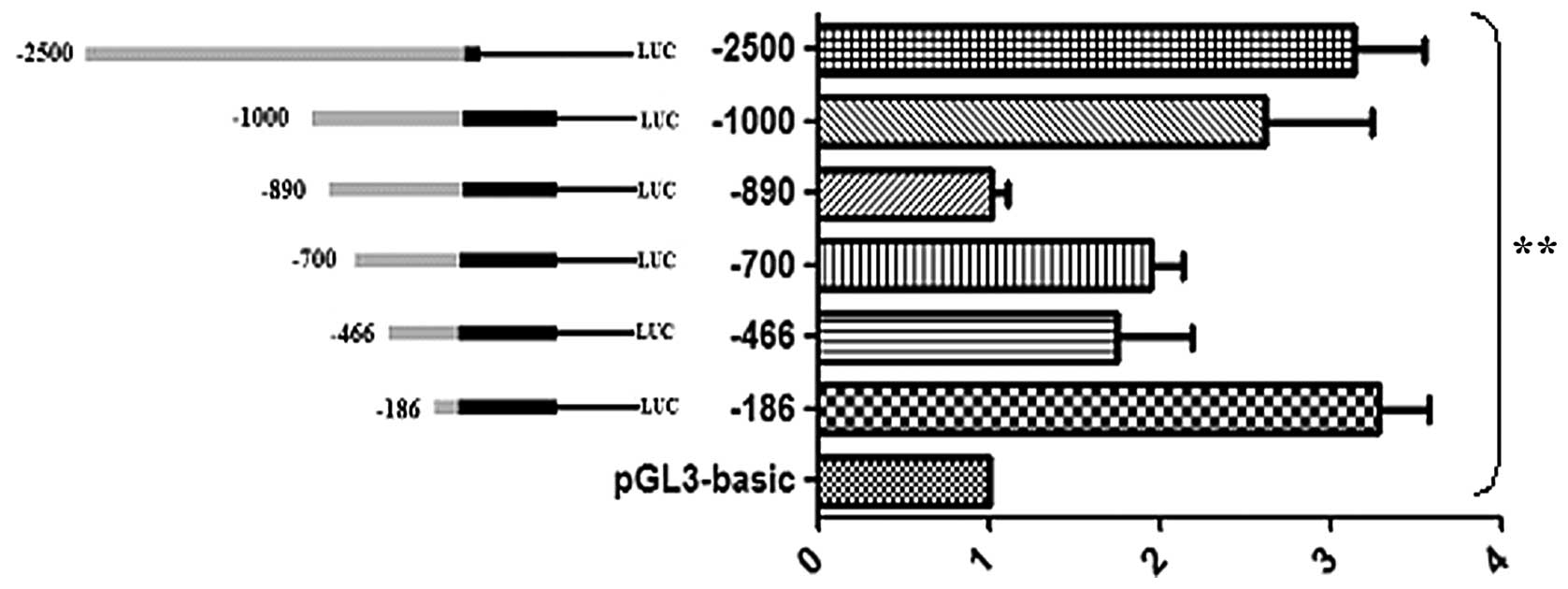
Dual luciferase reporter assays were performed to
detect promoter activity. Analysis of the recombined plasmids
following transfection into 293T cells indicated that fragments
−186 to +674, −700 to +674, −1,000 to +674 bp and −2,500 to +1 bp
exhibited higher levels of activity compared with fragments −466 to
+674 and −890 to +674 bp, with the two highest levels of activity
detected for fragments −186 to +674 and −2,500 to +1 bp.
Significant differences between groups are indicated in Fig. 2.
Identification of transcription factors
binding to the human TMEM174 promoter region
The identification of transcription factor binding
sites within the predicted promoter region is important in
determining the mechanism of transcriptional activation of this
gene. Numerous transcription factor binding sites were identified
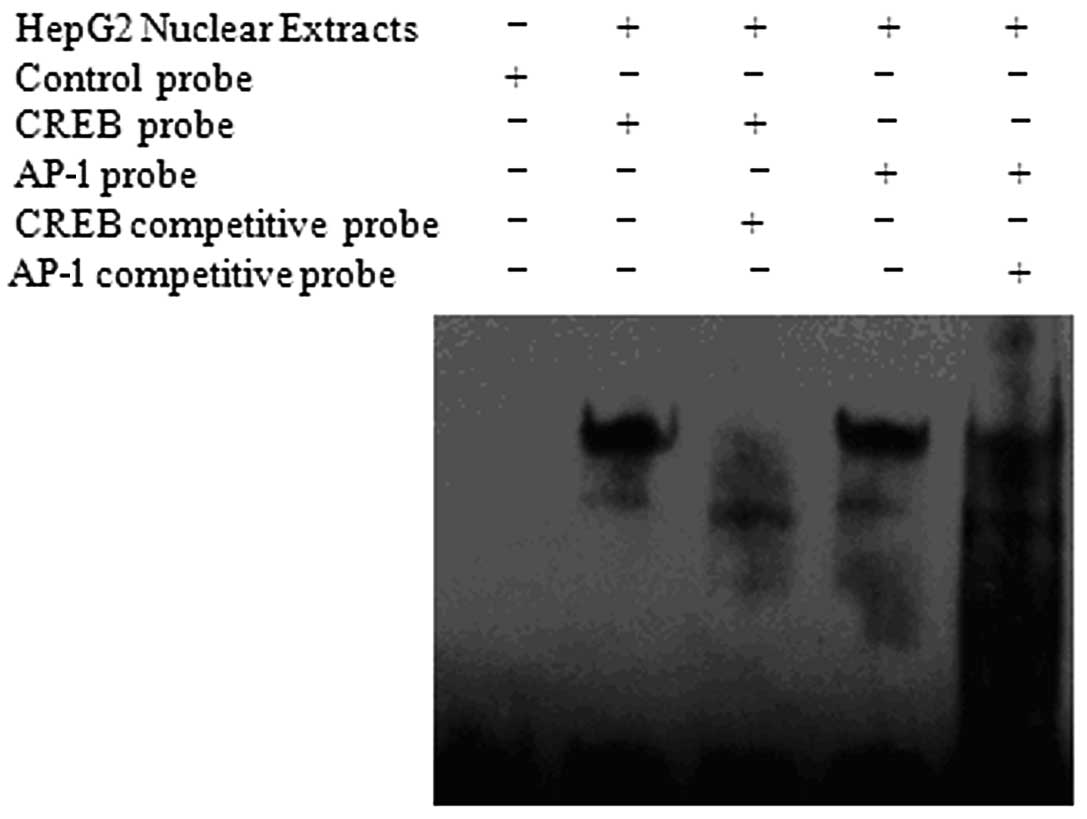
within the predicted promoter region. Among these, CREB and AP-1
were related to cell proliferation. EMSA was performed as described
in Materials and methods to investigate transcription factor
binding. These experiments demonstrated specific binding of CREB
with the TMEM174 promoter. The AP-1 probe designed according to the
predicted TMEM174 promoter AP-1 binding site bound to HepG2 nuclear
extract; this binding was partially inhibited by the addition of a
competitive probe, indicating that AP-1 binds non-specifically
within the TMEM174 promoter region (Fig. 3).
Discussion
A previous study focused on the mechanism by which
TMEM174 promotes cell proliferation at the protein level have
indicated potential signal transduction pathways involved in this
process (2). The present study
aimed to further elucidate this mechanism at the transcriptional
level. Investigations of the activity of the predicted promoter
showed that fragments −186 to +674, −700 to +674, −1,000 to +674
and −2,500 to +1 bp exhibited higher levels of activity compared
with fragments −466 to +674 and −890 to +674 bp. The fragment −186
to +674 bp exhibited the highest activity indicating that the core
promoter region might be located within this region of the TMEM174
promoter. Furthermore, the variation detected in the levels of
activity demonstrated the regulatory complexity of this promoter.
Based on the results of these experiments, the regions spanning
−186 to −466 and −700 to −890 bp are suggested to contain strong
negative regulatory elements. These data indicate that the apparent
changes in promoter activity are the result of complex interactions
between different regulatory elements.
TMEM174 has been identified as a potential regulator
of cell proliferation using high-throughput cell screening
technology (data not shown). This method allows the rapid
identification of functional genes from a large pool and provides
insights into to the function of these genes. However, this method
is limited in its application for subsequent studies of the
mechanism of gene function due to the potential omission of the
role of such proteins in other pathways.
RNA in situ hybridization analysis showed
that TMEM174 is highly expressed in some types of renal cancer,
such as squamous cell carcinoma with necrosis, papillary renal cell
carcinoma and transitional cell carcinoma, and that TMEM174 is
expressed in the majority of renal cancers and pyelonephritis (data
not shown). These data indicate that TMEM174 plays a role in the
development of renal cancer.
NF-κB, STAT3, AP-1, CREB and nuclear factor
erythroid 2-related factor (Nrf2) are transcription factors that
regulate tumor cell proliferation, transformation, survival,
invasion, angiogenesis, metastasis, chemoresistance and
radioresistance (16). In the
current study, sequence analysis identified numerous transcription
factor binding sites within the predicted promoter sequence of
TMEM174. Among these, CREB and AP-1 are involved in cell
proliferation. EMSA indicated specific binding of CREB within the
TMEM174 gene promoter region, while binding of the AP-1 was shown
to be non-specific. Thus, CREB is suggested to be an important
transcription factor involved in the regulation of TMEM174 gene
expression, and the inhibition of CREB, and possibly AP-1, is
suggested to inhibit the migration and invasion of cancer cells
(17). Furthermore, cell line
expression profiling showed that TMEM174 was expressed in the
lymphadenoma-derived Raji cell line, indicating a role for TMEM174
in the development of lymphadenoma. CREB is overexpressed in the
bone marrow of the majority of patients with acute myeloid leukemia
(AML) and is associated with a poor initial outcome of clinical
disease in AML patients. Moreover, CREB plays an important role in
hematopoiesis (18–20). Thus, TMEM174 is suggested to play a
role in leukemogenesis by activating CREB. Among the numerous
transcription factors identified in the promoter sequence, CEBP has
also been shown to be involved in inflammation (21). Therefore, TMEM174 is suggested to
be involved in the regulation of pyelonephritis via CREB. However,
these hypotheses require further investigation.
In conclusion, the results of the present study
indicate that the core promoter region of the human TMEM174 gene is
located in the region spanning −186 to +674 bp and that the
transcription factors CREB and AP-1 are involved in the
transcriptional regulation of this gene. These findings provide
further insight into the mechanism of TMEM174 gene regulation, with
the identification of CREB representing a basis for further studies
concerning this mechanism.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (nos. 81072093 and
30671092) and the Natural Science Foundation of Hebei Province
(nos. C2009001260 and C2013209024).
References
|
1.
|
Wan D, Gong Y, Qin W, et al: Large-scale
cDNA transfection screening for genes related to cancer development
and progression. Proc Natl Acad Sci USA. 101:15724–15729. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wang P, Sun B, Hao D, Zhang X, Shi T and
Ma D: Human TMEM174 that is highly expressed in kidney tissue
activates AP-1 and promotes cell proliferation. Biochem Biophys Res
Commun. 394:993–999. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kinjo K, Sandoval S, Sakamoto KM and
Shankar DB: The role of CREB as a proto-oncogene in hematopoiesis.
Cell Cycle. 4:1134–1135. 2005. View Article : Google Scholar
|
|
4.
|
Cho EC, Mitton B and Sakamoto KM: CREB and
leukemogenesis. Crit Rev Oncog. 16:37–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
De Falco V, Tamburrino A, Ventre S, et al:
CD44 proteolysis increases CREB phosphorylation and sustains
proliferation of thyroid cancer cells. Cancer Res. 72:1449–1458.
2012.PubMed/NCBI
|
|
6.
|
Mantamadiotis T, Papalexis N and Dworkin
S: CREB signalling in neural stem/progenitor cells: recent
developments and the implications for brain tumour biology.
Bioessays. 34:293–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dworkin S, Heath JK, deJong-Curtain TA, et
al: CREB activity modulates neural cell proliferation,
midbrain-hindbrain organization and patterning in zebrafish. Dev
Biol. 307:127–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through PI3-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogen-activated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
10.
|
Shen G, Jeong WS, Hu R and Kong AN:
Regulation of Nrf2, NF-kappaB, and AP-1 signaling pathways by
chemopreventive agents. Antioxid Redox Signal. 7:1648–1663. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li G, Gustafson-Brown C, Hanks SK, et al:
c-Jun is essential for organization of the epidermal leading edge.
Dev Cell. 4:865–877. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Andrecht S, Kolbus A, Hartenstein B, Angel
P and Schorpp-Kistner M: Cell cycle promoting activity of JunB
through cyclin A activation. J Biol Chem. 277:35961–35968. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shaulian E and Karin M: AP-1 in cell
proliferation and survival. Oncogene. 20:2390–2400. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Eferl R and Wagner EF: AP-1: a
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Weitzman JB, Fiette L, Matsuo K and Yaniv
M: JunD protects cells from p53-dependent senescence and apoptosis.
Mol Cell. 6:1109–1119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shanmugam MK, Nguyen AH, Kumar AP, Tan BK
and Sethi G: Targeted inhibition of tumor proliferation, survival,
and metastasis by pentacyclic triterpenoids: Potential role in
prevention and therapy of cancer. Cancer Lett. 320:158–170. 2012.
View Article : Google Scholar
|
|
17.
|
Chien MH, Ying TH, Hsieh YS, et al:
Dioscorea nipponica Makino inhibits migration and invasion
of human oral cancer HSC-3 cells by transcriptional inhibition of
matrix metalloproteinase-2 through modulation of CREB and AP-1
activity. Food Chem Toxicol. 50:558–566. 2012. View Article : Google Scholar
|
|
18.
|
Shankar DB, Cheng JC and Sakamoto KM: Role
of cyclic AMP response element binding protein in human leukemias.
Cancer. 104:1819–1824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cheng JC, Esparza S, Sandoval S, Shankar
D, Fu C and Sakamoto KM: Potential role of CREB as a prognostic
marker in acute myeloid leukemia. Future Oncol. 3:475–480. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Shankar DB and Sakamoto KM: The role of
cyclic-AMP binding protein (CREB) in leukemia cell proliferation
and acute leukemias. Leuk Lymphoma. 45:265–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Söhle J, Machuy N, Smailbegovic E, et al:
Identification of new genes involved in human adipogenesis and fat
storage. PLoS One. 7:e311932012.PubMed/NCBI
|