Introduction
Diseases of the common bile duct (CBD) represent a
significant danger to the patient, since they may lead to
obstructive jaundice, biliary colic, cholangitis or pancreatitis
(1,2). Traditionally, surgeons explore the
CBD and then conduct T-tube drainage following the removal of CBD
stones or lesions. It is believed that a T-tube is necessary since
it allows spasm or edema of sphincter to settle following the
trauma of the exploration. Despite this potential advantage,
T-tube-associated complications, including CBD obstruction and bile
leakage, often occur after CBD surgery (3–5). An
indwelling T-tube requires prescription by a doctor, continuous
management and the restriction of patient activity. At the end of
treatment, the process of T-tube removal may cause pain and risk to
the patient (6–8). The avoidance of complications of the
T-tube and the need to support the bile duct are the major
challenges faced by surgeons and researchers.
Since biodegradable implant materials in the human
body may be gradually dissolved and absorbed, secondary surgery to
remove the implants is not required (9). To date, a great number of studies
have reported on biodegradable CBD stents in animal experiments
(10–12). However, there are certain
limitations for the animal experiments, since they are often
conducted in large animals such as pigs or dogs (usually with a
small sample size of 4–7 dogs or pigs). Large animals have a
relatively large CBD which is convenient for the CBD surgery.
However, large animals such as dogs and pigs have two
disadvantages. Firstly, they are expensive, which limits the number
of experimental animals that may be used. Secondly, there are few
antibodies against pigs or dogs, which add extra difficulties for
molecular experiments in the CBD.
The current study describes a new technique designed
for the placement of biodegradable stents into the CBDs of rabbits.
The purpose of this study was to provide a safe CBD surgical method
in order to enable the sample size of the animals to be expanded
and to increase the credibility of experiments concerning
biodegradable CBD stents in animals.
Materials and methods
Biodegradable CBD stent and stent
introducer system
The biodegradable stents made of Mg-6Zn alloy,
donated by Shanghai Origin Material and Medical Technology Co. Ltd.
(Shanghai, China), were made with high purity Mg (99.99%) and high
purity zinc (Zn; 99.999%) under a clean process. The chemical
composition of the Mg-6Zn alloy is shown in Table I. Materials were prepared as
previously described (13). The
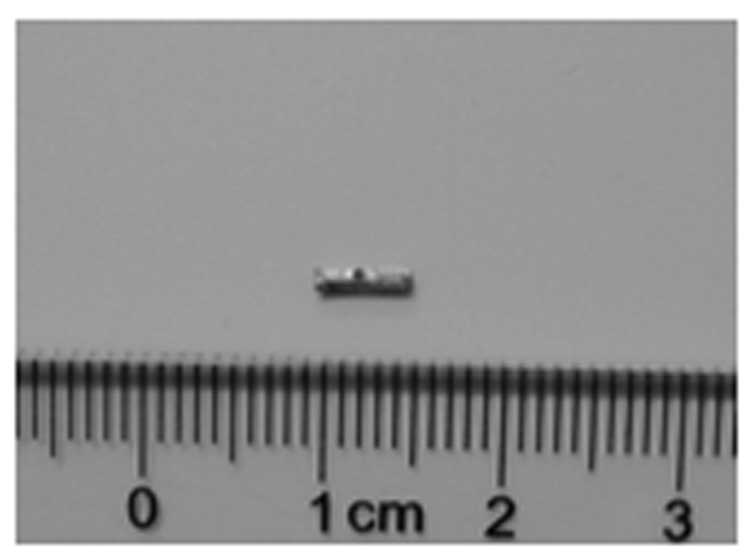
biodegradable biliary stents were U-shaped and possessed a luminal
diameter of 1.0 mm and a length of 5 mm (Fig. 1). A small hole was drilled in the
middle of the stent to enable the stent to be sutured to the CBD
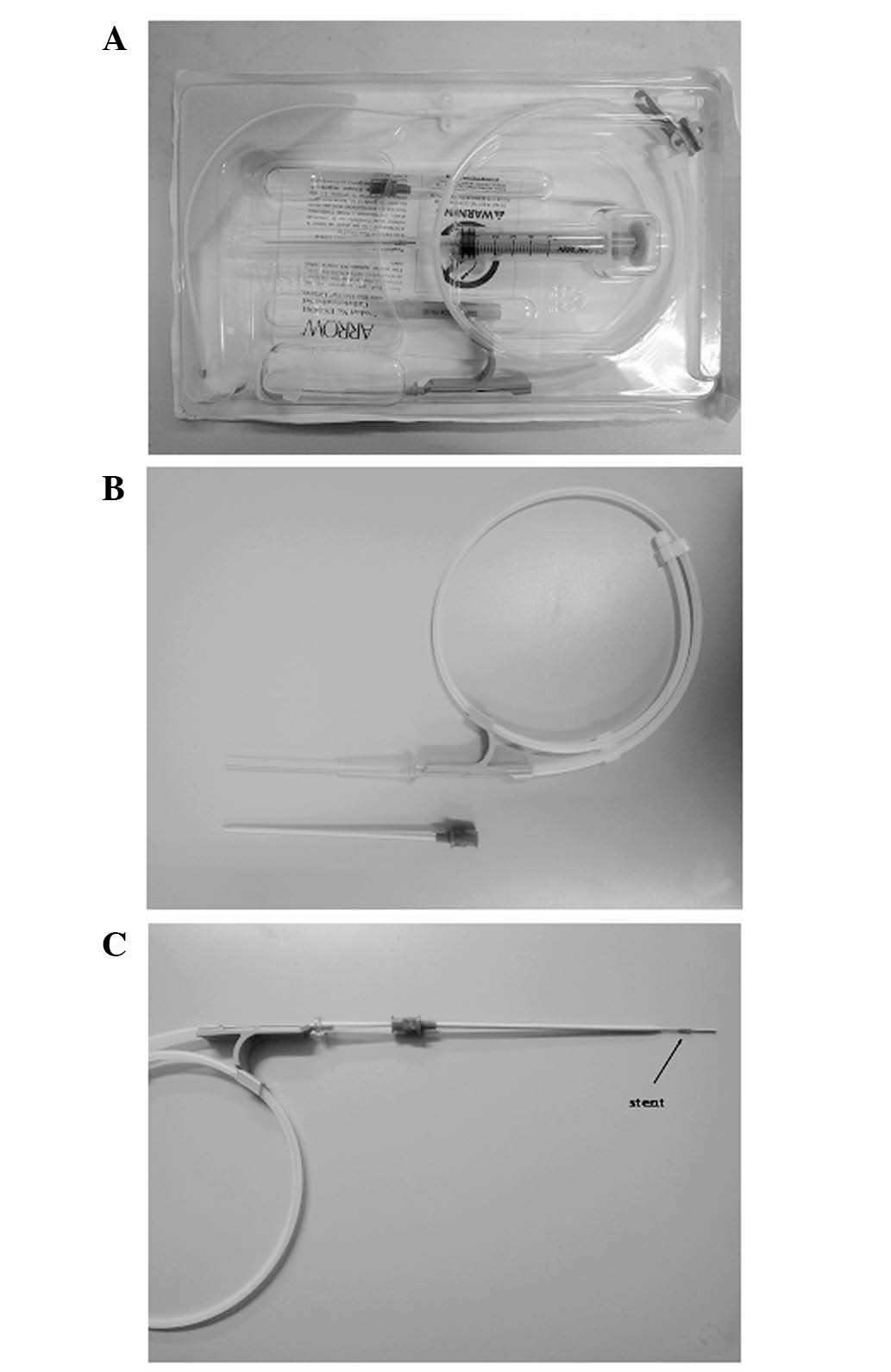
wall. The system used in endoscopic retrograde
cholangiopancreatography (ERCP) prompted the use a central venous
catheterization set (REF CS-24301-E; Arrow International, Inc.,
Reading, PA, USA) as a special stent introducer system (Fig. 2). The parts used included a plastic
jacket tube and a metal guide wire, which formed the CBD introducer
system.
 | Table I.Chemical composition of Mg-6Zn
alloy. |
Table I.
Chemical composition of Mg-6Zn
alloy.
| Material | Chemical composition
(weight %)
|
|---|
| Fe | Si | Ni | Cu | Al | Mn | Zn | Mg |
|---|
| Mg-6Zn | 0.0038 | 0.0016 | 0.0005 | 0.0005 | 0.0085 | 0.0004 | 5.6210 | 94.3637 |
Animal model and study design
The animal experiment was conducted according to the
Guidance Suggestions for the Care and Use of Laboratory Animals
(issued by the Ministry of Science and Technology of the People’s
Republic of China), and was approved by the Ethics Committee of the
Sixth People’s Hospital of Shanghai Jiao Tong University (Shanghai,
China). The animals were supplied by the Sino-British Sippr/BK Lab
Animal Ltd., Co. [license no. SCXK(hu)2008-0016; Shanghai, China].
Twelve adult New Zealand rabbits with a mean body weight of 2.5±0.5
kg were used in the experiments.
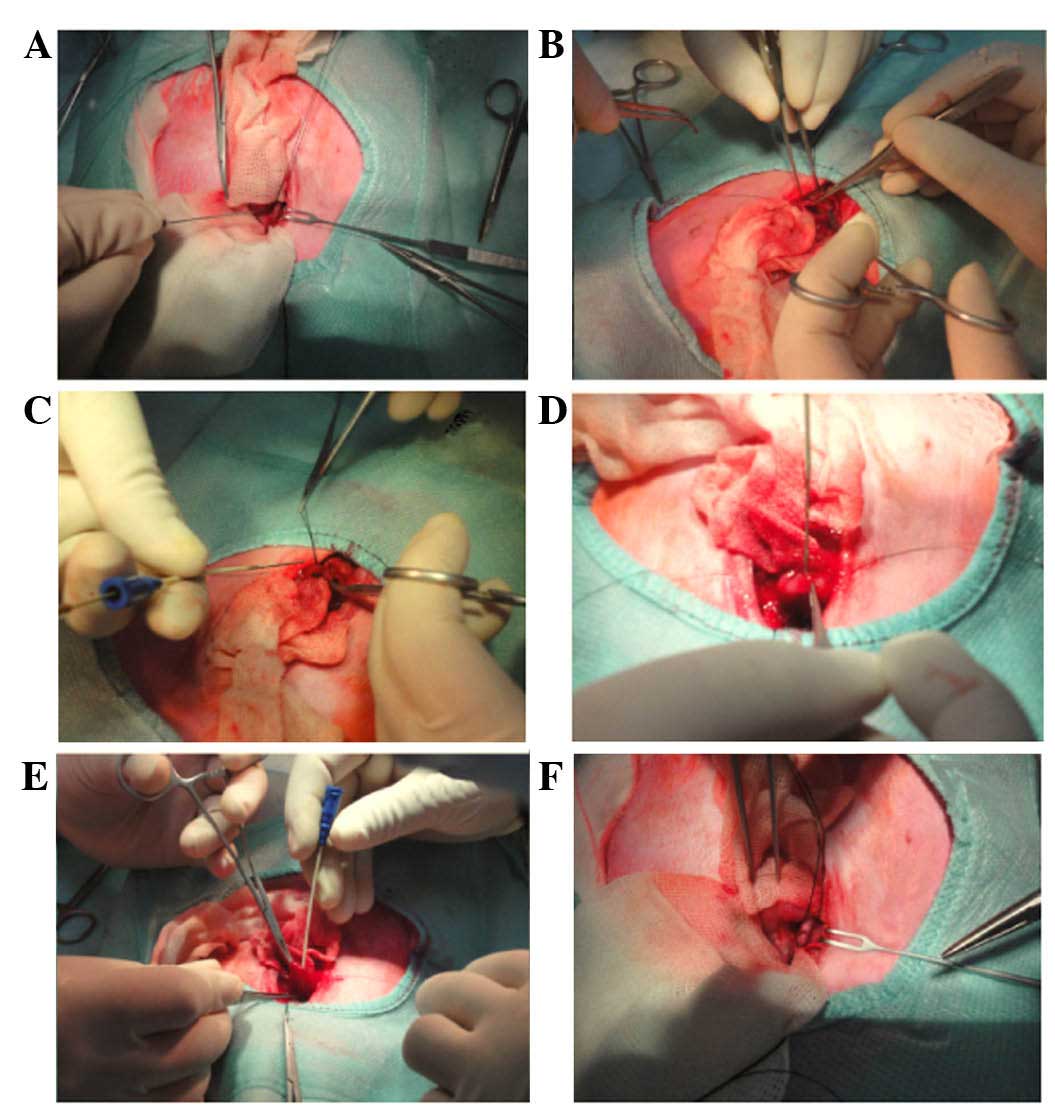
Rabbits were placed under general anesthesia by the
intravenous administration of sodium pentobarbital at a dose of 30
mg/kg body weight. All surgical procedures were carried out under
sterile conditions. Through a midline laparotomy, the CBD was
exposed and dissected along the CBD to 10 mm from the duodenal
wall. The mean diameter of the CBD was 1.5 mm and the mean length
was 12 mm in 10 rabbits. A ligature was loosely placed on the CBD
as a marker of the location of desired stent placement. A 15-mm
longitudinal incision was made in the duodenum, 5 mm away from the
duodenal papilla. The stents were mounted onto the special stent
introducer system. When the metal guide wire arrived at the
ligature marker on the CBD, the plastic jacket tube was pushed
back. Then, the stent was advanced along the duodenal papilla and
the metal guide wire was withdrawn. As the CBDs of rabbits are
translucent, it was possible to view the stent in the CBD under
direct vision and to suture the stent to the CBD wall through the
small hole on the stent. The plastic jacket tube was then
withdrawn, and the duodenum and abdomen was closed (Fig. 3).
Computed tomography (CT) scanning of the CBD stent
in vivo was conducted after the surgical procedures
immediately and postoperatively for 1–3 weeks. To investigate
changes in the pancreatic function of the rabbits, the serum lipase
(LPS) values of each rabbit were determined. In brief, a 1-ml blood
sample was collected prior to the surgical procedures and
postoperatively for 1–3 weeks from the ear vein of the rabbit using
a vein puncture into a heparinized syringe. The samples were then
immediately analyzed using an automatic blood biochemistry analyzer
(Hitachi 7600-020; Hitachi High-Technologies, Tokyo, Japan; LPS kit
was provided by Koch Industries, Inc., Wichita, KS, USA). Eleven
rabbits were sacrificed at 3 weeks after surgery.
Statistical analysis
Statistical analysis was performed with SPSS 18.0
software package (SPSS Inc., Chicago, IL, USA). The experimental
values were analyzed using the paired-samples t-test and expressed
as the mean values ± standard deviation (SD). One-way ANOVA
analysis was calculated to determine differences between groups for
each evaluated parameter at each time point. Non-parametric tests
[κ independent samples tests (Kruskal-Wallis Test)] were calculated
when equal variances were not assumed in one-way ANOVA. P<0.05
was considered to indicate a statistically significant result.
Results
Twelve rabbits underwent CBD stent insertion using
the stent introducer system. One animal died due to an anesthetic
accident. The remaining 11 rabbits that were included in the final
analysis grew well and their activities, diet and drinking were
normal. When these 11 rabbits were sacrificed after 3 weeks, no
jaundice or bile leakage was observed.
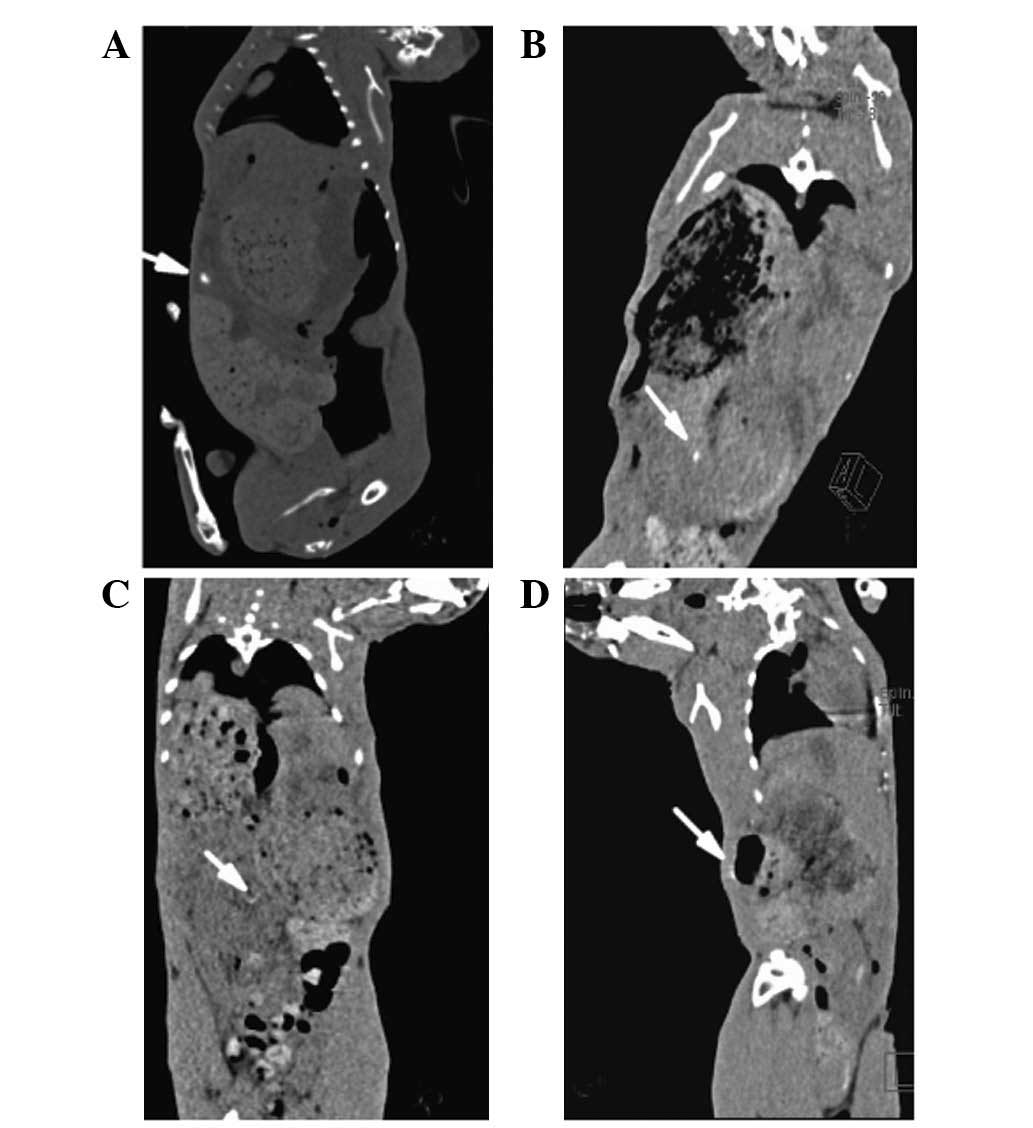
CT scanning for these 11 rabbits suggested that the
biodegradable Mg-6Zn stent was successfully placed into the CBD.
Since the stents degraded gradually, after 3 weeks, the stent was
rarely identified (Fig. 4).
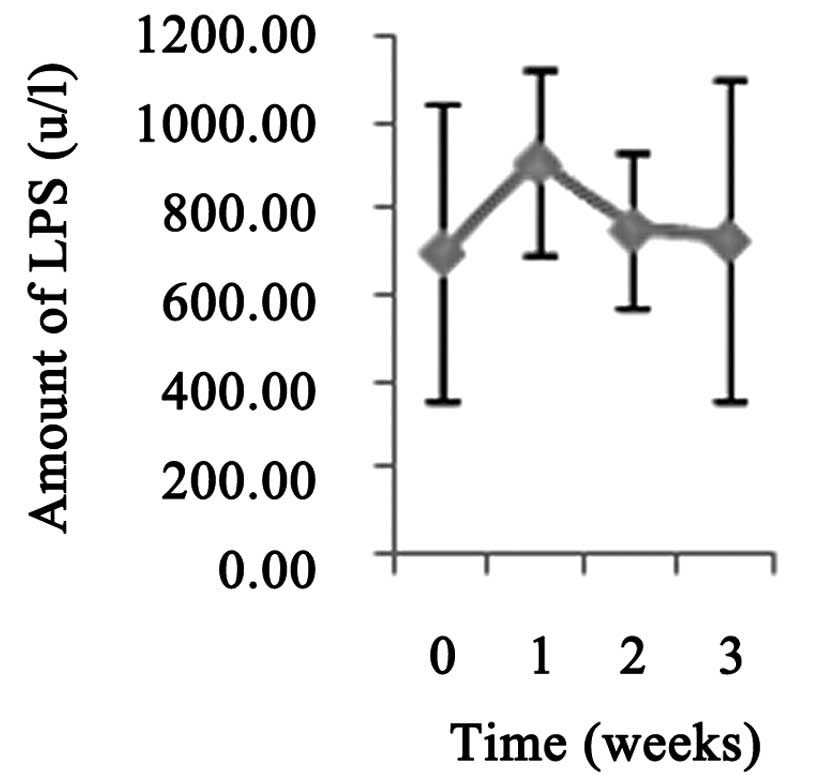
Although the levels of LPS 1 week postoperatively were slightly
higher than the preoperative levels, no statistically significant
differences were observed (P>0.05; Fig. 5).
Discussion
Following CBD exploration, it is important to insert
a T-tube in the CBD to provide a support and to maintain an open
CBD (14). However, T-tube
drainage is controversial since it appears to prolong the hospital
stay and is associated with an increased cost of care (15). There are risks for T-tube
placement, including increased morbidity or mortality secondary to
biliary infection, migration of the tube causing bile duct
obstruction, or bile leaks or peritonitis following the removal of
the tube (1,16,17).
Therefore, there is a great clinical requirement for biodegradable
CBD stents. Animal experiments for new surgical biliary stents made
of novel materials are being conducted more frequently than ever
before. However, in practice, it is difficult to perform a safe
choledochotomy for small animals, such as rabbits.
In the present study, a biodegradable Mg-6Zn alloy
CBD stent was inserted into the CBDs of rabbits via a central
venous catheterization set. At present, there are several materials
that may be used to create a biodegradable stent and in which it is
easy to drill a hole in the surface. Since the CBD of rabbits is
translucent, it is possible to observe these stents in the CBD, and
the hole in the middle of the stent under direct vision. The stent
was fixed to the CBD wall by a suture through the hole. Due to the
stent compression of the sutured wall of CBD, no bile leakage was
observed following suture completion. Fixing the stent to the CBD
wall has certain advantages. Firstly, it demonstrates whether a
stent may induce histological changes where it contacts with the
CBD. Secondly, a fixed CBD stent is required for study of further
stent biodegradation behavior .
One main problem to be overcome is the requirement
that the progression of the stent through the duodenal papilla
should be completed within a short time, which may reduce the
adverse effects on the duodenal papilla. In order to clearly
explore the duodenal papilla and the relationship between the
papilla and the direction of the CBD, a relatively large
longitudinal incision (15 mm) was cut in the duodenum, 5 mm away
from the duodenal papilla. The results of serum LPS assays showed
that this method for CBD stent placement had no significant impact
on the function of the pancreas.
In conclusion, although the advancement of the stent
through the duodenal papilla produces extra risks, our methods
appear to be feasible and safe for the placement of stents into the
CBDs of small animals. This study increases the number of animals
available for CBD studies and may improve the quality of
experiments concerning CBD stents in animals.
Abbreviations:
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 30901422),
Shanghai Jiao Tong University Interdisciplinary (Biomedical
Engineering) Research Fund (no. YG2010MS45) and Shanghai Jiao Tong
University School of Medical and Technology Fund (no.
09XJ21005).
References
|
1.
|
Verbesey JE and Birkett DH: Common bile
duct exploration for choledocholithiasis. Surg Clin North Am.
88:1315–1328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hungness ES and Soper NJ: Management of
common bile duct stones. J Gastrointest Surg. 10:612–619. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
El-Geidie AA: Is the use of T-tube
necessary after laparoscopic choledochotomy? J Gastrointest Surg.
14:844–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Leida Z, Ping B, Shuguang W and Yu H: A
randomized comparison of primary closure and T-tube drainage of the
common bile duct after laparoscopic choledochotomy. Surg Endosc.
22:1595–1600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ahmed I, Pradhan C, Beckingham IJ, Brooks
AJ, Rowlands BJ and Lobo DN: Is a T-tube necessary after common
bile duct exploration? World J Surg. 32:1485–1488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Daldoul S, Moussi A and Zaouche A: T-tube
drainage of the common bile duct choleperitoneum: etiology and
management. J Visc Surg. 149:e172–e178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lygidakis NJ: Hazards following T-tube
removal after choledochotomy. Surg Gynecol Obstet. 163:153–155.
1986.PubMed/NCBI
|
|
8.
|
Horgan PG, Campbell AC, Gray GR and
Gillespie G: Biliary leakage and peritonitis following removal of T
tubes after bile duct exploration. Br J Surg. 76:1296–1297. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Song G: Control of biodegradation of
biocompatable magnesium alloys. Corros Sci. 49:1696–1701. 2007.
View Article : Google Scholar
|
|
10.
|
Xu X, Liu T, Liu S, Zhang K, Shen Z, Li Y
and Jing X: Feasibility of biodegradable PLGA common bile duct
stents: an in vitro and in vivo study. J Mater Sci Mater Med.
20:1167–1173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zografakis JG, Jones BT, Ravichardran P,
Evancho-Chapman MM, et al: Endoluminal reconstruction of the canine
common biliary duct. Curr Surg. 60:437–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Laukkarinen J, Nordback I, Mikkonen J,
Kärkkäinen P and Sand J: A novel biodegradable biliary stent in the
endoscopic treatment of cystic-duct leakage after cholecystectomy.
Gastrointest Endosc. 65:1063–1068. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang E, Yin D, Xu L, Yang L and Yang K:
Microstructure, mechanical and corrosion properties and
biocompatibility of Mg-Zn-Mn alloys for biomedical application.
Mater Sci Eng C Mater Biol Appl. 3:987–993. 2009. View Article : Google Scholar
|
|
14.
|
Wu JS and Soper NJ: Comparison of
laparoscopic choledochotomy closure techniques. Surg Endosc.
16:1309–1313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Seale AK and Ledet WP Jr: Primary common
bile duct closure. Arch Surg. 134:22–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Isla AM, Griniatsos J and Wan A: A
technique for safe placement of a biliary endoprosthesis after
laparoscopic choledochotomy. J Laparoendosc Adv Surg Tech A.
12:207–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wu X, Yang Y, Dong P, et al: Primary
closure versus T-tube drainage in laparoscopic common bile duct
exploration: a meta-analysis of randomized clinical trials.
Langenbecks Arch Surg. 397:909–916. 2012. View Article : Google Scholar : PubMed/NCBI
|