Introduction
The development and progression of chronic kidney
disease (CKD) is associated with inflammatory responses of various
etiologies (1,2). Several previous studies have
demonstrated that a variety of inflammatory mediators are involved
in the pathophysiological processes of CKD (3,4).
Glomerular endothelial cells (GENCs), which are important
components of the glomerular filtration barrier, are the main
target cells for inflammatory mediators and are important in the
initiation and progression of CKD (5,6).
Several studies have shown that changes in the structure,
distribution and function of the endothelial cell skeleton are the
main mechanisms underlying the increased vascular permeability
during early inflammation (7).
F-actin, the main component of the cytoskeleton, is rearranged to
enable endothelial cell (EC) contraction, crack formation and
increases in permeability when regulated by various inflammatory
mediators (8). Angiotensin II (Ang
II) is the major bioactive substance in the renin-angiotensin
system (RAS). It is involved in the regulation of vascular tension
and blood flow, and the promotion of cell growth and proliferation,
and may also act as a proinflammatory factor. Studies have
confirmed that the activity of the RAS in the kidney tissues of
patients with CKD is elevated independently of the presence or
absence of hypertension, and that the concentration of Ang II is
significantly higher than that in plasma, indicating its importance
in inflammation-mediated EC injury (9,10).
The aim of the present study was to investigate the mechanism by
which Ang II causes inflammatory damage in GENCs by observing the
effects of Ang II on GENC monolayer permeability and F-actin
distribution.
Materials and methods
Reagents and animals
Dulbecco’s modified Eagle’s medium (DMEM) dried
powder and fetal bovine serum (FBS) were purchased from Hyclone
(Logan, UT, USA). Fluorescein isothiocyanate (FITC)-phalloidin and
trypsin (activity, 1:20) were purchased from Sigma (St. Louis, MO,
USA). Sumianxin was provided by the Veterinary Research Institute
of Changchun University (Changchun, China). The VIII R:Ag testing
kits (secondary antibodies tagged with biotin; DAKO, Carpinteria,
CA, USA), CD31 and CD34 were purchased from Dako (Carpinteria, CA,
USA). Heparin was purchased from the Nanjing biochemistry
pharmaceutical factory (Nanjing, China). The nitrate-mixed acetate
fibre membrane (0.45 μm) was provided by the Shanghai New
Asia Purification Devices Plant (Shanghai, China).
This study was performed at the Shanghai Ninth
People’s Hospital affiliated with Shanghai Jiao Tong University
School of Medicine (Shanghai, China) from October 2010 to November
2011. All animal experimental protocols were approved by the Animal
Care and Use Committee of Shanghai Ninth People’s Hospital
affiliated with Shanghai Jiao Tong University School of Medicine
and conformed to the Guide for the Care and Use of Laboratory
Animals (National Research Council, Chinese version, 1996)
(11). A total of 25 male Wistar
rats (weight, ~120 g) were supplied by the Shanghai Experimental
Animal Center of Chinese Science College (Shanghai, China) and
housed in a room (n=3 rats per cage) with a controlled temperature
and humidity. The rats were fed with standard rat chow and had
access to tap water ad libitum.
GENC isolation, culture and
identification
Following a previously published protocol (12,13),
the 25 male Wistar rats were anaesthetized by intraperitoneal (i.p)
injection of sumianxin (0.8 mg/kg) and received an i.p injection of
3,000 units heparin sodium. After being fixed in a supine position,
the chest and abdomen of each rat were disinfected and the thoracic
aorta was isolated for perfusion. Cold aseptic Hank’s solution was
used to wash away the blood while the renal vein was cut to be
conducive to the liquid outflow. After 1–2 min, when the color of
the kidneys appeared white, the kidneys were placed into an
ice-bath for preservation. Under sterile conditions, the renal
capsule was torn from the lavaged kidney and the renal cortex was
cut into 1–2-mm3 thick fragments. The kidney fragments
were ground with a 100 mesh steel sieve and then successively
filtered through 150 and 200 mesh steel sieves, respectively. The
resultant glomeruli were collected in the 200 mesh steel sieve.
Microscopic observations identified that the purity of the
glomeruli was 99%. Following centrifugation at 462 × g for 5 min,
the glomeruli were washed twice with serum-free DMEM. The separated
glomeruli were digested with type IV collagenase, mixed with DMEM
and then centrifuged at 462 × g for 5 min. The supernatant was
collected and centrifuged again at 462 × g for 5 min to provide a
precipitate containing the ECs. Approximately
102–103 primary cells were isolated from each
kidney resulting in a passage proportion of 1:2. The glomerular ECs
were seeded in a germ-free plastic bottle pre-coated with 1%
gelatin and the prepared EC cultured medium (DMEM medium with 20%
FBS, 5 U/ml heparin, 5 U/l insulin, 5 μg/ml transferrin and
5 μg/ml selenium) was added. The cells were cultured in an
incubator at 37°C with 5% CO2 and a humidity of 95–100%.
The cultured medium was replaced every 3–4 days until 80–90% of the
cells formed a confluent monolayer in the 2nd–3rd week. The cells
were digested with 0.25% trypsin and then subcloned in 96-well
plates at different densities as follows: 1 cell per well in the
1st row, 2 cells per well in the 2nd row, 4 cells per well in the
3rd row, 8 cells per well in the 4th row, 16 cells per well in the
5th row, 32 cells per well in the 6th row, 64 cells per well in the
7th row, and 128 cells per well in the 8th row. When cloning could
be observed after culturing for 3–4 days, the cells were collected
into culture bottles for further culturing. The cultured medium was
changed every 3–4 days and the cells were subcultured every 6–7
days. The cells were digested with trypsin, and digestion was
terminated by the addition of DMEM with 10% FBS. The cells were
cultured in separate bottles at a ratio of 1:2. Cells in the
2nd–3rd generations were identified by detecting factor
VIII-associated antigen, CD31 and CD34, and morphological
observations. The 2nd–3rd generation cells were used during this
experiment as they did not show cell senescence, differentiation or
cytometaplasia during repeated passages (14,15).
Detection of the effects of Ang II on
F-actin by flow cytometry
GENCs were cultured in 50 ml culture bottles; Ang II
(10 mg/l) and Ang II (10 mg/l) + dexamethasone (10 mg/l) were
separately added once 80–90% of the cells had formed a confluent
monolayer. In addition a normal control group (GENC with
FITC-phalloidin) and a negative control group (GENC without
FITC-phalloidin) were established. Cells were removed from the
culture bottles after 6 and 12 h and treated as follows: (i) The
cells were washed twice with DMEM containing 1% FBS; (ii) the cells
were digested with 0.5% trypsin and placed into a flow cytometry
detection tube; (iii) the cell suspension was centrifuged at 1,847
× g for 10 min and the supernatant was discarded; (iv) the
precipitate was washed twice with phosphate-buffered saline (PBS)
and centrifuged at 462 × g for 5 min to isolate the cells; (v) the
cells were fixed with 4% paraformaldehyde for 10 min at 4°C and 30
min at room temperature and the samples were then centrifuged at
462 × g for 5 min to remove the supernatant; (vi) the cells were
washed twice with PBS and centrifuged at 462 × g for 5 min to
discard the liquid; (vii) 1 ml of 0.5mg/l FITC-phalloidin (100
μg FITC-phalloidin was dissolved in 0.2 ml methanol and
diluted with 0.01 M PBS) was added, and the cells were reacted for
40 min in the dark; (viii) samples were washed with PBS three times
and centrifuged at 462 × g for 5 min to remove the supernatant and
eliminate the uncombined FITC-phalloidin; and (ix) cells were
gently blended after adding 0.8 ml PBS. The changes in F-actin were
detected by flow cytometry (FACScalibur™; BD Biosciences, Franklin
Lakes, NJ, USA), with the absence of FITC-phalloidin in the
negative control group (16,17).
Detection of EC growth on the filter
membrane
The filter membrane was treated with 0.5% acetic
acid for 20 min at 50°C, boiled for 60 min in 0.1% gelatin and then
parched with fire prior to use. GENCs were inoculated on the
membrane at a density of 5×105/per hole. The membrane
was removed from the nesting after 7–10 days, based on previous
studies (17,18), which showed that ECs reach a state
of cell fusion and covered the filter membrane within a period of
7–10 days. The membrane was then washed three times with PBS, fixed
with 95% alcohol for 10 min, washed again three times with PBS,
stained with hematoxylin and eosin for 5 min and rinsed with water
three times. Furthermore, the membrane was weathered with 1%
hydrochloric acid alcohol and dyed blue with 1% ammonia. The cells
were monitored by transmission light microscopy, and detection was
performed when 80–90% of the cells became a converged monolayer.
One random nested filter membrane was dyed prior to detection to
determine whether the ECs on the filter membrane reached a
condition of cell fusion. In addition, all nested filter membranes
were dyed at the end of the experiment to test the EC
integrity.
Permeability test
The permeability test was performed using a
modification of a previously published method (17,18).
Briefly, GENCs were digested with 0.5% trypsin to prepare a cell
suspension and the cell count was adjusted to
3.3×105/ml. The cell suspension (1.5 ml) was inoculated
on the handled membrane in the micropore nest for culturing the
cells, which was placed into a small well of a 6-well culture plate
with 2.5 ml culture medium in each well. The plate was placed in an
incubator with 5% CO2 at 37°C and 95–100% humidity and
the culture medium was replaced every 2 days. The nest and the
small well of the 6-well plate consisted of two relatively isolated
chambers (the inner and outer chambers). The exchange of substances
between the inner and outer chambers was mediated by the filter
membrane and the EC mono-layer. When the ECs reached a certain
level of confluency, 10 mg/l Ang II diluted in DMEM containing 1%
FBS (2 ml per nest) was added in one group, and 10 mg/l Ang II + 10
mg/l dexamethasone (2 ml) was added to the remaining group; the
control group was treated with 2 ml DMEM containing 1% FBS.
Simultaneously, 100 mg/l biotin-BSA was added as a permeability
indicator. The cultured media of the inner and outer chambers were
collected separately after 6 and 12 h to detect the concentration
of biotin-BSA (n=4 per group). The albumin clearance of the EC
monolayer was calculated with the following formula: Clearance (%)
= biotin-BSA density of outside chamber/biotin-BSA density of inner
chamber ×100.
Biotin-BSA density test
The capture enzyme-linked immunosorbent assay
(ELISA) was used to detect the concentration of biotin-BSA in the
inner and outer chambers (Vector Laboratories, Burlingame, CA,
USA). A micropore plate was coated overnight with 5 mg/l
streptavidin at 4°C. The liquid coating was removed, 300
μl/well blocking buffer was added and the plate was
incubated at room temperature for 2 h and then washed three times
with PBS. After diluting a 1-ml sample at a ratio of 1:100, the
sample was added to the ELISA plate to react for 1 h at room
temperature. The plate was washed three times with PBS and 100
μl streptavidin conjugated with 5 mg/l horseradish peroxide
enzyme was added to react for 1 h at room temperature. Again, the
plate was washed six times with PBS. Tetramethyl-benzidine was
added as the bottom material and coloration was allowed to develop
for 20 min at room temperature. Finally, 1.8 mol/l
H2SO4 (80 μl) was added into each well
to inhibit the reaction and the absorbance at 450 nm was measured
in the microplate.
Statistical analysis
Data were analyzed with SPSS software, version 11.0
(SPSS, Inc., Chicago, IL, USA). The results are expressed as the
mean ± standard deviation (19).
Differences between groups were assessed using the t-test and
one-way analysis of variance, and the Spearman grade correlativity
was used to verify the correlation between the GENC monolayer
permeability and distribution of F-actin. P<0.05 was considered
to indicate a statistically significant difference.
Results
GENC morphology, growth characteristics
and identification
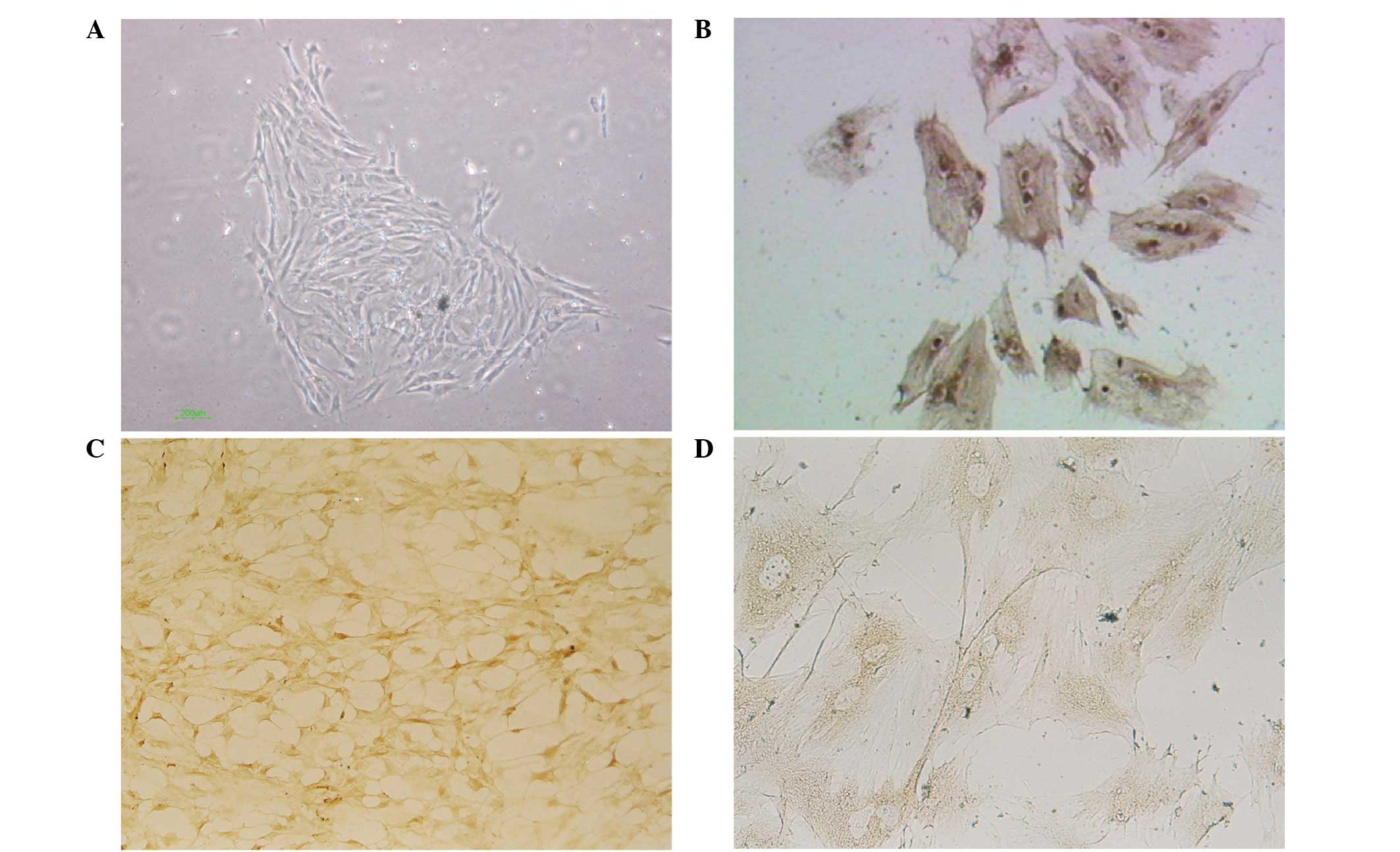
When observed under an inverted microscope, the
GENCs appeared adherent following primary culture for 48 h and
began to grow and divide after 72 h. The majority of the cells were
round and polygonal and showed a tendency to form a glomus. Evident
nuclei and a small number of cells were interconnected with each
other as observed under a high magnification (inverted microscope;
magnification, ×100). Monolayer convergence was identified at 2–3
weeks, at which time the majority of cells showed a polygonal and
short fusiform morphology with an appearance similar to that of
paving stones. Cells in the split-phase appeared quasi-circular and
marginally stained showing single-layer adherent growth. Contact
inhibition occurred among the cells as detected by the arrest of
cell division when the single-layer was completely convergent
(Fig. 1A). The GENCs were positive
for factor VIII-associated antigen, CD31 and CD34 reflecting the
endothelial growth characteristics of these cells (Fig. 1B–D).
Effects of Ang II on GENC monolayer
permeability
The albumin permeability of GENCs treated with 10
mg/l Ang II increased significantly following 6 and 12 h of culture
compared with that of the untreated control group (P<0.05 and
P<0.01, respectively). The albumin permeability of GENCs treated
with 10 mg/l Ang II and dexamethasone was significantly lower than
that of cells treated with Ang II alone (P<0.01) indicating that
dexamethasone inhibited the Ang II-induced increase of albumin
permeability and played a protective role (Table I).
 | Table I.Effect of Ang II on GENC monolayer
permeability. |
Table I.
Effect of Ang II on GENC monolayer
permeability.
| Group (n=3) | Monolayer clearance
(%) |
|---|
|
|---|
| 6 h | 12 h |
|---|
| Filter without
ECs | 94.65±0.42 | 99.62±0.71 |
| Normal control | 27.71±0.21 | 27.78±0.48 |
| Ang II | 32.97±0.91a | 34.83±1.2b |
| Ang II +
dexamethasone | 29.16±0.36c | 28.03±0.46c |
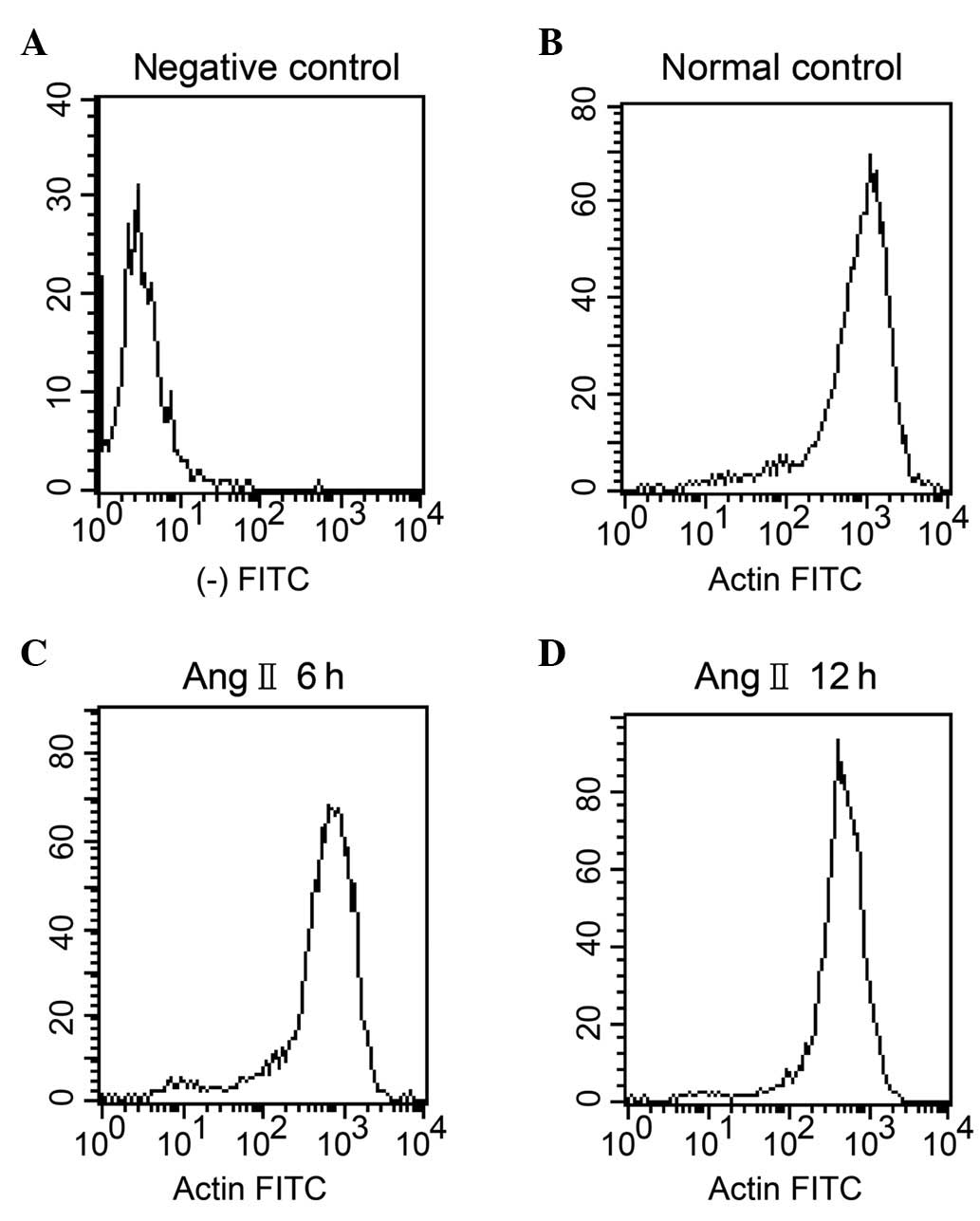
Effects of Ang II on GENC F-actin
detected by flow cytometry
The fluorescence intensity of F-actin in the GENCs
treated with 10 mg/l Ang II decreased significantly after 6 and 12
h of culture compared with that of the normal control cells at the
corresponding times (P<0.01). The fluorescence intensity of
F-actin in GENCs treated with Ang II and dexamethasone was
significantly higher than that of the cells treated with Ang II
alone (P<0.01) indicating that dexamethasone inhibited the
depolymerisation of F-actin induced by Ang II and played a
protective role (Table II,
Fig. 2). FITC-phalloidin was not
detected in the negative control group.
 | Table II.Effects of 10 mg/l Ang II on GENC
F-actin levels. |
Table II.
Effects of 10 mg/l Ang II on GENC
F-actin levels.
| Group (n=4) | Fluorescence
intensity of F-actin in GENCs
|
|---|
| Negative control | Normal control | Ang II 6 h | Ang II 12 h | Ang II 6 h +
dexamethasone | Ang II 12 h +
dexamethasone |
|---|
| Fluorescence
intensity | 3.095±0.320 | 965.40±17.74 | 573.69±23.99a | 326.95±2.53a | 770.12±10.95b | 650.36±23.78b |
Correlation between GENC monolayer
permeability and F-actin
The Spearman grade correlation coefficient was used
to examine the correlation between the GENC monolayer permeability
and F-actin distribution. The results showed a negative correlation
indicating that monolayer permeability increased with F-actin
depolymerisation (r=−0.901, P<0.01).
Discussion
The vascular endothelium, including the EC monolayer
and basement membrane, is a semi-selective permeability barrier
that lines the luminal surface of blood vessels. The exchange of
solutes and liquids between the inner and outer surfaces of blood
vessels is regulated by the vascular endothelium. Permeability is
an objective measurable indicator of endothelial barrier function.
Glomerular endothelial cells are important for the integrity of the
glomerular vascular structure and are also an active organ with
autocrine and paracrine secretion functions. Endothelial cell
dysfunction and decreased endothelial cell concentration are
significant in the development of progressive renal disease and
chronic renal failure (20).
Increased glomerular filtration barrier permeability is a
pathological feature and a critical factor in the etiology of CKD.
Therefore, cultured GENCs are critical for studying the regulatory
mechanisms of glomerular filtration barrier permeability, which is
of great significance for the identification of effective
treatments to prevent and cure CKD.
The mechanisms underlying the increased permeability
of ECs caused by various damaging factors may be broadly classified
into two categories (21): (i) In
cases of severe injury or delayed response time, EC dissolution or
detachment from the basement membrane occurs, which affects the
integrity of the EC monolayer; and (ii) damaging factors cause EC
contraction or retraction, resulting in the formation of cracks
between cells. In recent years, the latter mechanism has received
increased attention. In a previous study the enhanced microvascular
permeability observed was mainly due to the retraction of
microvesicular ECs causing an increase in the space between cells
and EC injury (21). In response
to inflammatory mediators, such as lipopolysaccharides and platelet
activating factor, F-actin, an essential component of the
endothelial cytoskeleton, is depolymerised and rearranged to
increase tension, resulting in an intense cell contraction.
Furthermore, F-actin may affect the function of tight and adherent
junctions, damaging the integrity of the ECs, which results in the
formation of EC gaps that increase permeability (22,23).
In the present study, we observed that normal rat
GENCs showed a polygonal and short fusiform morphology in
vitro, in a pattern similar to that of paving stones. The cells
in split-phase were quasi-circular with a light color and showed
single-layer adherent growth. The GENCs were positive for factor
VIII-associated antigen, CD31 and CD34, which confirmed their
endothelial cell growth characteristics. Ang II is the major
bioactive substance in the RAS, and is associated with the
regulation of vascular tension and blood flow, promotes cell growth
and proliferation, and acts as a proinflammatory factor. Ang II
binds to a receptor of the G protein-coupled receptor (GPCR) family
and regulates a variety of cytokines and inflammatory mediators to
induce inflammatory cell activation, which triggers the
inflammatory cascade (22,24). Inflammatory cells may also activate
the RAS, which enhances the effects of Ang II on local inflammation
and in the process of inflammation (21). Studies have confirmed that RAS
activity in the kidney tissues of patients with CKD increases
regardless of the presence or absence of hypertension, and its
concentration is significantly higher than that in the plasma
(10). In the present study, we
showed that F-actin was depolymerised in GENCs treated with 10 mg/l
Ang II for 6 and 12 h. Furthermore, we examined the changes in the
monolayer permeability of GENCs in response to Ang II treatment at
different time points and identified that monolayer permeability
increased at 6 and 12 h. These results indicated that Ang II
induced F-actin depolymerisation and increased monolayer
permeability of GENCs at a certain density. This increase in
monolayer permeability may be negatively correlated with F-actin
depolymerisation. The increase in monolayer permeability of Ang
II-treated GENCs at 6 and 12 h was followed by fracture at 24 h.
These results indicated that Ang II may damage the barrier function
of ECs by combining with a specific receptor, such as a GPCR, to
activate a G protein function and further activate phospholipases
(PL)-D, PLC, PLA2 and the Ca2+ channel. PLC
produces DAG and activates protein kinase C (PKC). PKC activation,
Ca2+ and Ca2+/CaM induce the myosin light
chain kinase, which mediates the phosphorylation of the myosin
light chain, promotes F-actin depolymerisation, induces the
interaction between actin and myosin, and affects cell adhesion
connections and tense connections, resulting in the formation of EC
gaps and increased permeability (25,26).
The mechanisms of Ang II may be as follows: (i) Ang II may exert
direct effects on GENCs and a low concentration of Ang II may alter
the cellular morphology and induce actin depolymerisation,
rearrangement and fibrin loss, thereby increasing the mono-layer
permeability; and (ii) Ang II may stimulate GENCs to produce
inflammatory mediators, such as IL-6, IL-8 and NF-κB, to result in
cell injury (27).
Hormones are a major drug type in the treatment of
kidney diseases and their anti-inflammatory, anti-immunisation and
antitoxin effects have been demonstrated previously (28,29).
In the present study, we observed inhibition of the Ang II-induced
increase in monolayer permeability and F-actin depolymerisation
when dexamethasone was used in combination with Ang II. This result
indicated that dexamethasone may have inhibited the inflammatory
factors, such as IL-6, IL-8 and NF-κB, that were induced by Ang II
and thereby protect the GENCs. Our results partly clarified the
mechanisms underlying the inflammatory injury of the GENCs caused
by Ang II, which may provide a theoretical basis for the design of
therapeutic strategies against CKD development and progression.
Acknowledgements
The authors would like to thank
Shengfang Ge from the Shanghai Jiaotong University School of
Medicine for his helpful comments on the manuscript and
International Science Editing Compuscript Ltd. for reviewing the
revised manuscript prior to submission. The authors also thank the
Shanghai Key Laboratory of Tissue Engineering, Tissue Engineering
Center of Shanghai Jiao Tong University for technical assistance.
This study was supported by the Shanghai Natural Science Foundation
(grant no. 09ZR1417400).
References
|
1.
|
Vianna HR, Soares CM, Tavares MS, Teixeira
MM and Silva AC: Inflammation in chronic kidney disease: the role
of cytokines. J Bras Nefrol. 33:351–364. 2011.(In Portuguese).
|
|
2.
|
Cheung WW, Paik KH and Mak RH:
Inflammation and cachexia in chronic kidney disease. Pediatr
Nephrol. 25:711–724. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sun YB, Qu X, Zhang X, Caruana G, Bertram
JF and Li J: Glomerular endothelial cell injury and damage precedes
that of podocytes in adriamycin-induced nephropathy. PLoS One.
8:e550272013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Stehouwer CD: Endothelial dysfunction in
diabetic nephropathy: state of the art and potential significance
for non-diabetic renal disease. Nephrol Dial Transplant.
18:778–781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Birukova AA, Birukov KG, Adyshev D, et al:
Involvement of microtubules and Rho pathway in TGF-beta1-induced
lung vascular barrier dysfunction. J Cell Physiol. 204:934–947.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dudek SM and Garcia JG: Cytoskeletal
regulation of pulmonary vascular permeability. J Appl Physiol.
91:1487–1500. 2001.PubMed/NCBI
|
|
7.
|
Waschke J, Curry FE, Adamson RH and
Drenckhahn D: Regulation of actin dynamics is critical for
endothelial barrier functions. Am J Physiol Heart Circ Physiol.
288:H1296–H1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
You QH, Sun GY, Wang N, Chen S and Luo QL:
Role of src-suppressed C kinase substrate in rat pulmonary
microvascular endothelial hyperpermeability stimulated by
inflammatory cytokines. Inflamm Res. 59:949–958. 2010. View Article : Google Scholar
|
|
9.
|
Woolf AS, Gnudi L and Long DA: Roles of
angiopoietins in kidney development and disease. J Am Soc Nephrol.
20:239–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Navar LG and Nishiyama A: Why are
angiotensin concentrations so high in the kidney? Curr Opin Nephrol
Hypertens. 13:107–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yao Y, Wang L, Zhang H, et al: A novel
anticancer therapy that simultaneously targets aberrant p53 and
Notch activities in tumors. PLoS One. 7:e466272012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Akis N and Madaio MP: Isolation, culture,
and characterization of endothelial cells from mouse glomeruli.
Kidney Int. 65:2223–2227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Rops AL, van der Vlag J, Jacobs CW, et al:
Isolation and characterization of conditionally immortalized mouse
glomerular endothelial cell lines. Kidney Int. 66:2193–2201. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zhao JH, Huang L, Wang JP, et al: Culture
of endothelial cells from rat glomeruli and the effects of high
glucose concentration on its nitric oxide secretion. Immunol J.
23:13–15. 2007.(In Chinese).
|
|
15.
|
Green DF, Hwang KH, Ryan US and
Bourgoignie JJ: Culture of endothelial cells from baboon and human
glomeruli. Kidney Int. 41:1506–1516. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Johnston AP, Baker J, Bellamy LM, et al:
Regulation of muscle satellite cell activation and chemotaxis by
angiotensin II. PLoS One. 5:e152122010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Li Y, Wu Y, Gong X, et al: Low molecular
weight heparin decreases the permeability of glomerular endothelial
cells when exposed to pre-eclampsia serum in vitro. Nephrology
(Carlton). 17:754–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cooper JA, Del Vecchio PJ, Minnear FL, et
al: Measurement of albumin permeability across endothelial
monolayers in vitro. J Appl Physiol. 62:1076–1083. 1987.PubMed/NCBI
|
|
19.
|
Xu X, Jia R, Zhou Y, et al:
Microarray-based analysis: identification of hypoxia-regulated
microRNAs in retinoblastoma cells. Int J Oncol. 38:1385–1393.
2011.PubMed/NCBI
|
|
20.
|
Annuk M, Zilmer M, Lind L, Linde T and
Fellström B: Oxidative stress and endothelial function in chronic
renal failure. J Am Soc Nephrol. 12:2747–2752. 2001.PubMed/NCBI
|
|
21.
|
Garcia JG and Schaphorst KL: Regulation of
endothelial cell gap formation and paracellular permeability. J
Investig Med. 43:117–126. 1995.PubMed/NCBI
|
|
22.
|
Miura S, Saku K and Karnik SS: Molecular
analysis of the structure and function of the angiotensin II type 1
receptor. Hypertens Res. 26:937–943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Davis B, Dei Cas A, Long DA, et al:
Podocyte-specific expression of angiopoietin-2 causes proteinuria
and apoptosis of glomerular endothelia. J Am Soc Nephrol.
18:2320–2329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Cheng ZJ, Vapaatalo H and Mervaala E:
Angiotensin II and vascular inflammation. Med Sci Monit.
11:RA194–R205. 2005.PubMed/NCBI
|
|
25.
|
Kim J, Ahn S, Ren XR, et al: Functional
antagonism of different G protein-coupled receptor kinases for
beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl
Acad Sci USA. 102:1442–1447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yan M, Cheng C, Jiang J, et al: Essential
role of SRC suppressed C kinase substrates in Schwann cells
adhesion, spreading and migration. Neurochem Res. 34:1002–1010.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
28.
|
Lv J, Xu D, Perkovic V, Ma X, et al:
Corticosteroid therapy in IgA nephropathy. J Am Soc Nephrol.
23:1108–1116. 2012. View Article : Google Scholar
|
|
29.
|
Hodson EM, Willis NS and Craig J:
Corticosteroid therapy for nephrotic syndrome in children. Cochrane
Database Syst Rev. 4:CD0015332007.
|