Introduction
Resistant starch (RS) is the sum of starch and
starch degradation products that are not absorbed in the small
intestine since they are resistant to enzymatic digestion (1). RS3 is retrograded starch, formed on
cooling in processed foods, including cooled cooked potato, bread
and cornflakes (2). RS appears to
have a number of physiological effects, including weight control,
prevention of diabetes, lipid reduction and promotion of inorganic
salt absorption (3). Stachyose is
a tetrasaccharide present in the tubers of the Chinese artichoke
(4). It is not decomposed by
digestive enzymes and it will be changed in specific conditions
when in direct contact with the intestinal tract (5). It increases probiotic activity;
intestinal flora become contained within the intestines, form a
thick protective film against bacteria and stop the proliferation
of toxins within the intestinal tract (6). Stachyose also promotes intestinal
peristalsis and accelerates the excretion of pathogens and toxins
(7).
Ulcerative colitis is a type of inflammatory bowel
disease that affects the lining of the large intestine and rectum
(8). The symptoms vary in severity
and may start slowly or suddenly. Approximately half of patients
have only mild symptoms. Others have more severe attacks that occur
more frequently. A number of factors lead to attacks, including
respiratory infections or physical stress. Ulcerative colitis is
one of the two main forms of chronic inflammatory disease of the
gastrointestinal tract (9); the
other form is Crohn’s disease. Normally, the large intestine
absorbs water from stools and changes them from a liquid to a
solid. In ulcerative colitis, inflammation causes loss of the
lining of the colon, leading to bleeding, diarrhea and abdominal
discomfort (10). Cytokines may be
centralized around this organ as it hosts cells that are highly
susceptible to the action of these proteins. Cytokines, such as
IL-6 and TNF-α, are small proteins that are produced and released
from a number of cells under physiological and pathological
conditions (11). IL-6 is
increasingly recognized as an almost ubiquitous participant in
numerous types of inflammatory processes (12). TNF-α is a macrophage-derived
cytokine with chemotactic potency, which has been implicated in the
acute phase reaction under various inflammatory conditions
(13).
In the present study, RS3 was used as a carrier for
stachyose and its preventative effect on colitis was examined. The
levels of the inflammation-related cytokines IL-6 and TNF-α were
used to determine the preventative effects on dextran sulfate
sodium (DSS)-induced colitis in mice. Colon tissue histology was
also used to determine the preventative effects in vivo.
Materials and methods
Chemicals
RS3 was supplied by National Starch (Sterling
Forest, NY, USA). RS3 (7 g) was added to 65 ml water, 0.4 g
emulsifier (sucrose esters of fatty acids, Liuzhou Gaotong Food
Chemicals Co., Ltd., Liuzhou, China), 100 ml soybean oil, 1.2 g
crosslinking agent (POCl3) and 0.6 g initiator (cerium
ammonium nitrate) at pH 8–9, 55°C. The products were left to
crosslink for 3 h, thereby producing the RS3 microspheres. Then,
0.05 g stachyose (Sigma, St Louis, MO, USA) was added at 37°C for 3
h static adsorption. Ethanol was used to wash and filter the
stachyose RS3 microspheres. Ordinary starch (COFCO Corporation
Chongqing Company, Chongqing, China) was transformed into
stachyose-containing starch using the same method. Mouse diets with
a 15% starch content were prepared from the two kinds of
stachyose.
Animals
Female C57BL/6 mice (n=40, 7 weeks old) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The mice were maintained in a
temperature-controlled (temperature, 25±2°C; relative humidity,
50±5%) facility with a 12-h light/dark cycle and free access to a
standard mouse chow diet and water.
DSS-induced colitis model
The mice were divided into four groups (n=10 each).
The normal group received a standard diet and water during the
experimental period. The mice in the RS3 + stachyose and starch +
stachyose groups were fed with a mouse diet containing 15%
stachyose-containing RS3 and starch microspheres, respectively, for
2 weeks. In the second week, ulcerative colitis was induced in the
control and sample groups by providing water containing 5% (wt/wt)
DSS (molecular weight, 36,000–50,000; MP Biomedicals, Solon, OH,
USA) ad libitum for 7 days, as described previously
(14). The body weight was
recorded daily and the colon length was measured. These experiments
followed a protocol approved by the Animal Ethics Committee of
Chongqing Medical University (Chongqing, China).
Analysis of inflammation-related
cytokines in serum by enzyme-linked immunosorbent assay
(ELISA)
For the serum cytokine assay, blood from the
inferior vena cava was collected in a tube and centrifuged at 1,100
× g, 4°C for 10 min. The serum was aspirated and assayed as
described below. Concentrations of inflammatory-related cytokines
IL-6 and TNF-α in serum were measured by ELISA according to the
manufacturer’s instructions (Biolegend, San Diego, CA, USA).
Briefly, biotinylated antibody reagent was added to 96-well plates,
then supernatants of homogenized serum were added and the plates
were incubated at 37°C in CO2 for 2 h. After washing
with PBS, streptavidin-horseradish peroxidase (HRP) solution was
added and the plate was incubated for 30 min at room temperature.
The absorbance was measured at 450 nm using a microplate reader
(iMark; Bio-Rad, Hercules, CA, USA) (15).
Analysis of serum levels of superoxide
dismutase (SOD)
For the blood biochemical assay, blood from the
inferior vena cava was collected in a tube and centrifuged at 3,000
rpm, 4°C for 10 min. The serum levels of SOD were determined using
a superoxide dismutase assay kit (Asan Pharm. Co. Ltd., Seoul,
South Korea). The kit contained all reagents required for
determining SOD activity in an indirect assay method based on
xanthine oxidase (XO) and a color reagent that provides linearity
of test results over a broad range. Briefly, the biotin-antibody
reagent was added to 96-well plates, the supernatants of
homogenized colon tissue were added and the plates were incubated
at 37°C in CO2 for 1 h. After each well was aspirated
and washed, HRP-avidin reagent was added to each well and the
plates were incubated for 1 h at 37°C. The absorbance was measured
at 450 nm using a microplate reader.
Histological examination
At the end of the experimental period, the colon
tissues were removed and cleaned in saline to remove fecal residue.
The colon tissues were fixed in 10% (v/v) buffered formalin and
embedded in paraffin. Then, 4-μm-thick slices from paraffin
sections were stained with hematoxylin and eosin (H&E) prior to
microscopic observation. Histological analysis was conducted by a
pathologist who was unaware of the experimental protocol.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Differences between the mean values for individual groups
were assessed with one-way analysis of variance (ANOVA) with
Duncan’s multiple range test. P<0.05 was considered to indicate
a statistically significant differences. SAS version 9.1 (SAS
Institute Inc., Cary, NC, USA) was used for the statistical
analyses.
Results
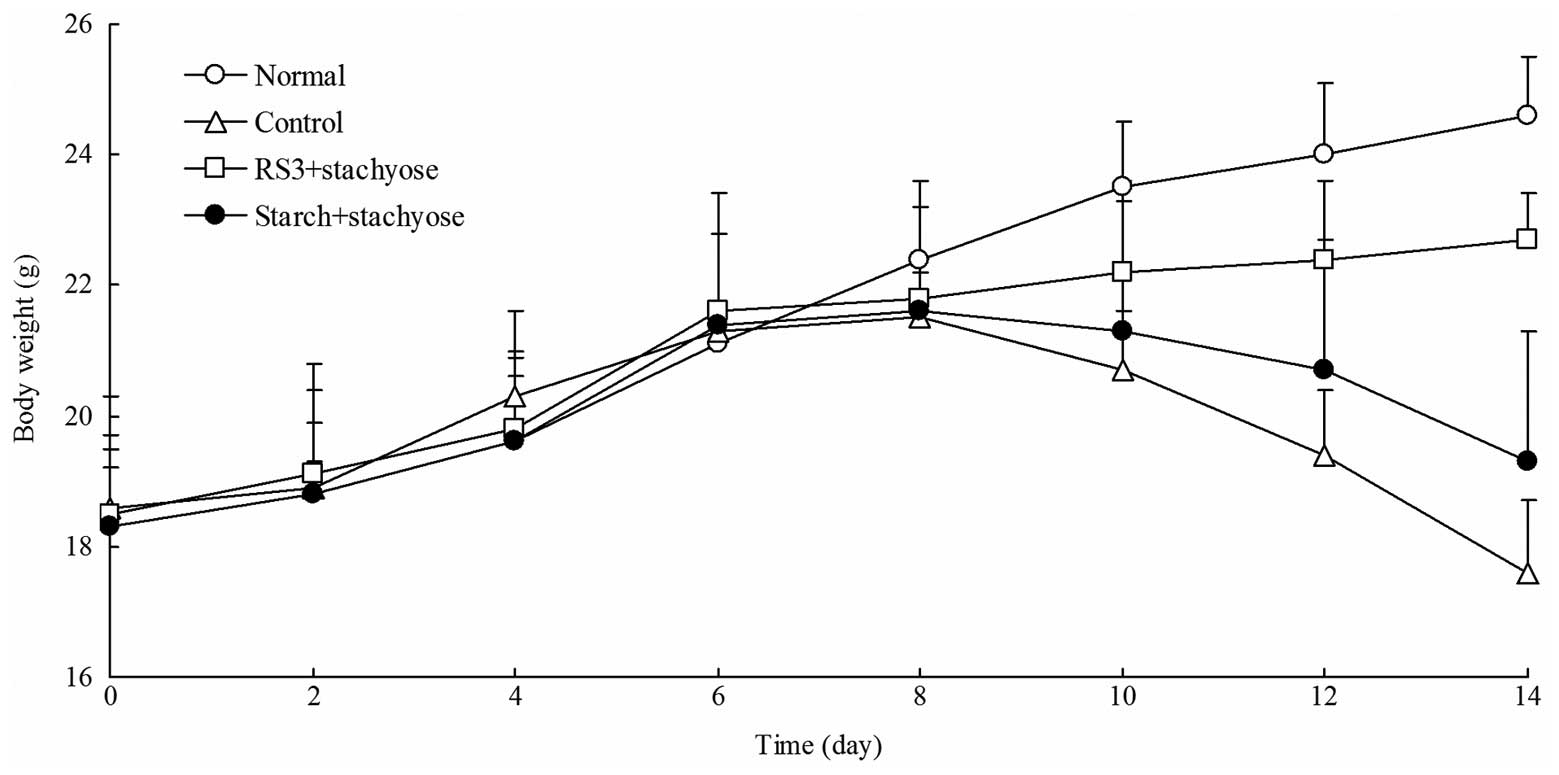
Changes in body weight
The normal mice had a normal diet and their body
weights increased in the whole process of experiment. The body
weights of the control mice with DSS-induced colitis were
significantly decreased after 7 days. As shown in Fig. 1, following the initiation of
DSS-induced colitis, the body weights of the mice in the RS3 +
stachyose and starch + stachyose groups were significantly lower
compared with those of the normal mice. The mice in the RS3
+stachyose group had higher body weights than the mice in the
starch + stachyose group (P<0.05). After 7 days, the body weight
of RS3 + stachyose group and starch + stachyose group mice were
higher than that of control group mice, and the body weight of the
RS3 + stachyose group was higher than starch + stachyose group.
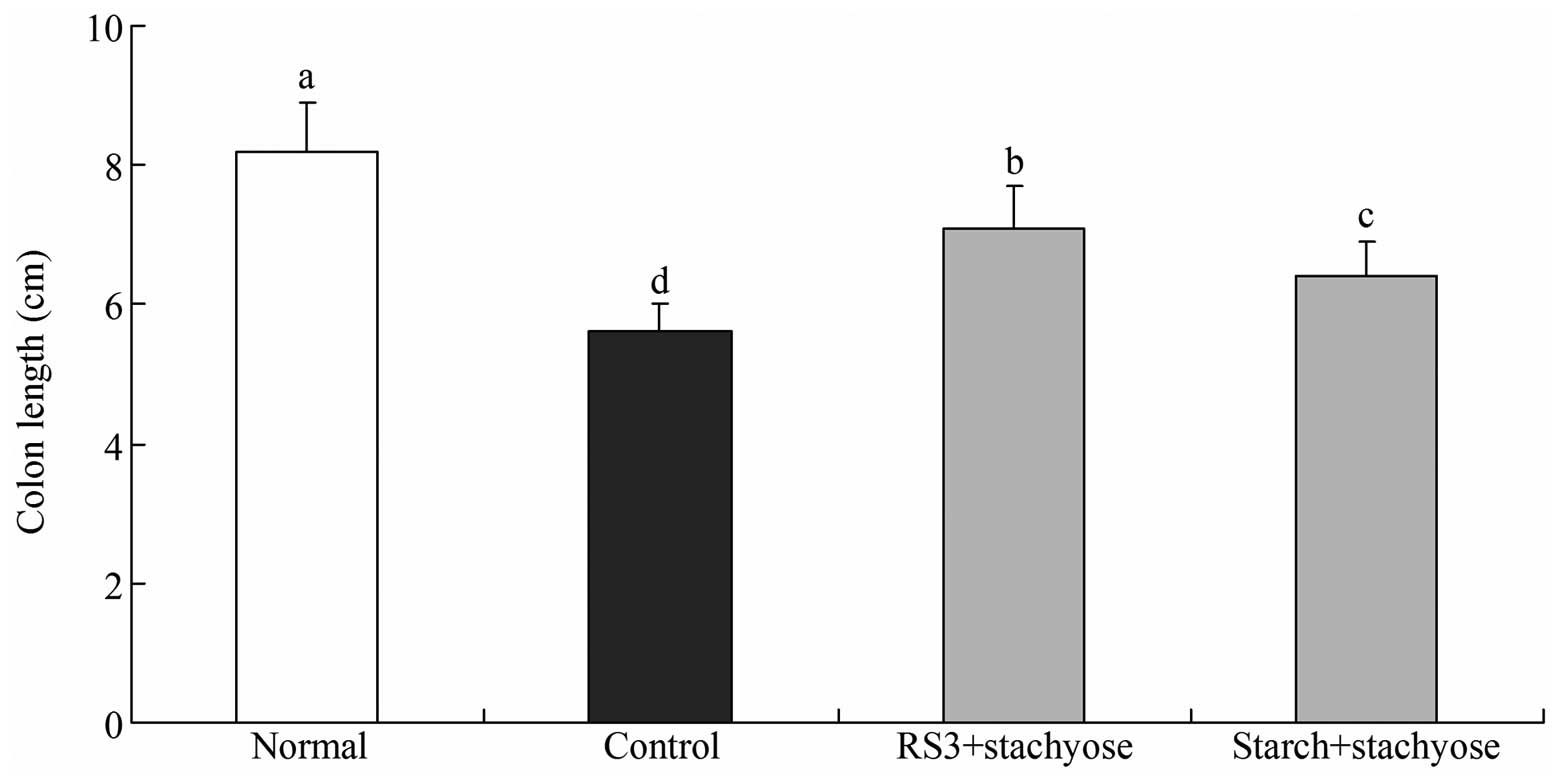
Changes in colon length
The total colonic length was significantly shorter
in the DSS-treated mice (control group and sample treated group)
compared with the normal mice as shown in Fig. 2 (P<0.05). The normal mice had
the longest colon length at 8.2±0.7 cm and the colon length of
control mice was the shortest (5.6±0.4 cm). The total colonic
length was longer in the RS3 + stachyose-treated mice (7.1±0.6 cm)
than in the starch + stachyose-treated mice (6.4±0.5 cm). The
significant shortening of the colonic length in DSS-treated mice
indicates that DSS contributed to the process of edematous changes
in the colon in DSS-induced colitis.
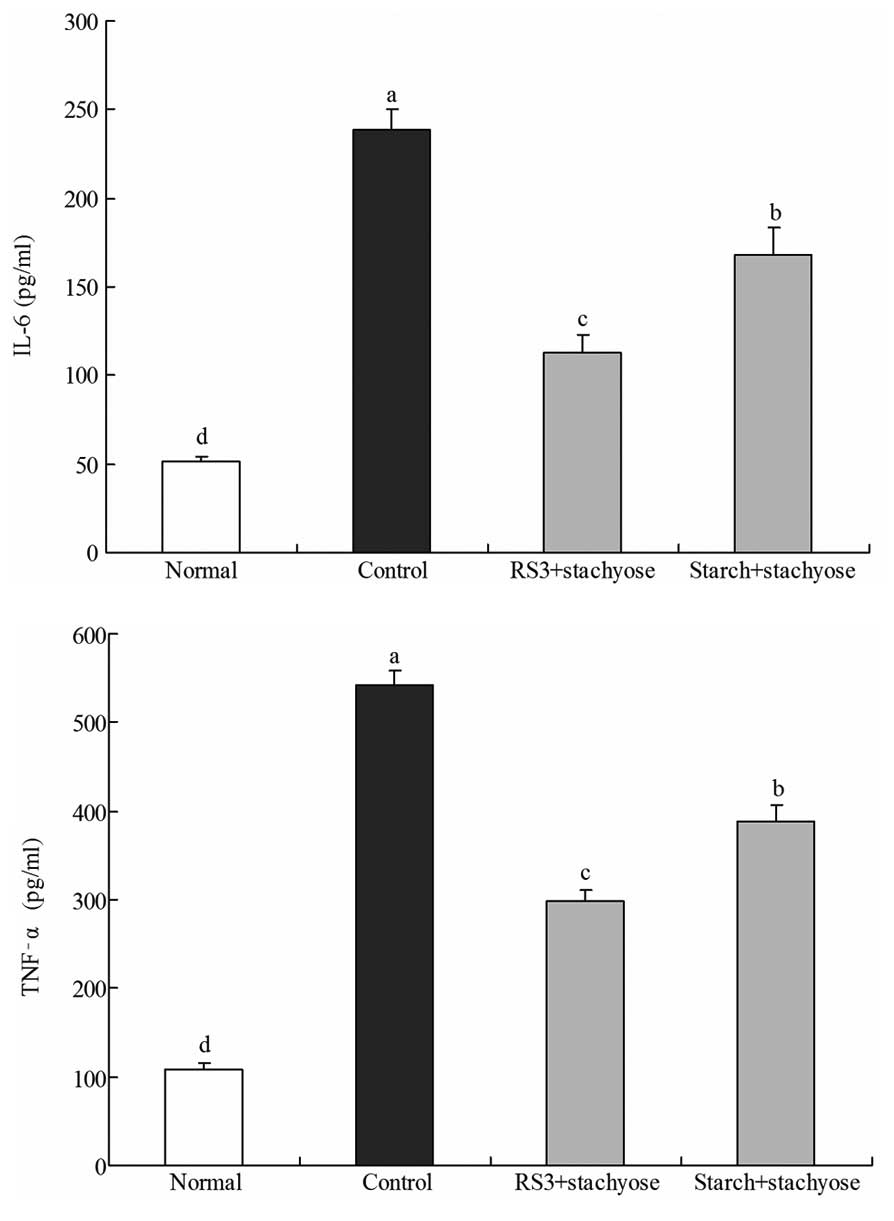
Effect of RS3 + stachyose on serum levels
of IL-6 and TNF-α
The IL-6 level of normal mice was 51.1±2.7 pg/ml;
however, in control mice the IL-6 level was significantly increased
to 238.6±11.6 pg/ml. The levels of IL-6 in mice fed with RS3 +
stachyose and starch + stachyose were 112.6±10.6 and 168.4±15.4
pg/ml, respectively (Fig. 3). The
TNF-α levels in the normal, control, RS3 + stachyose-treated and
starch + stachyose-treated mice were 108.6±7.8, 542.3±16.3,
298.4±12.5 and 388.3±17.7 pg/ml, respectively. The serum IL-6 and
TNF-α levels in the mice in the RS3 + stachyose-treated groups were
significantly lower compared with those in the control and starch +
stachyose groups.
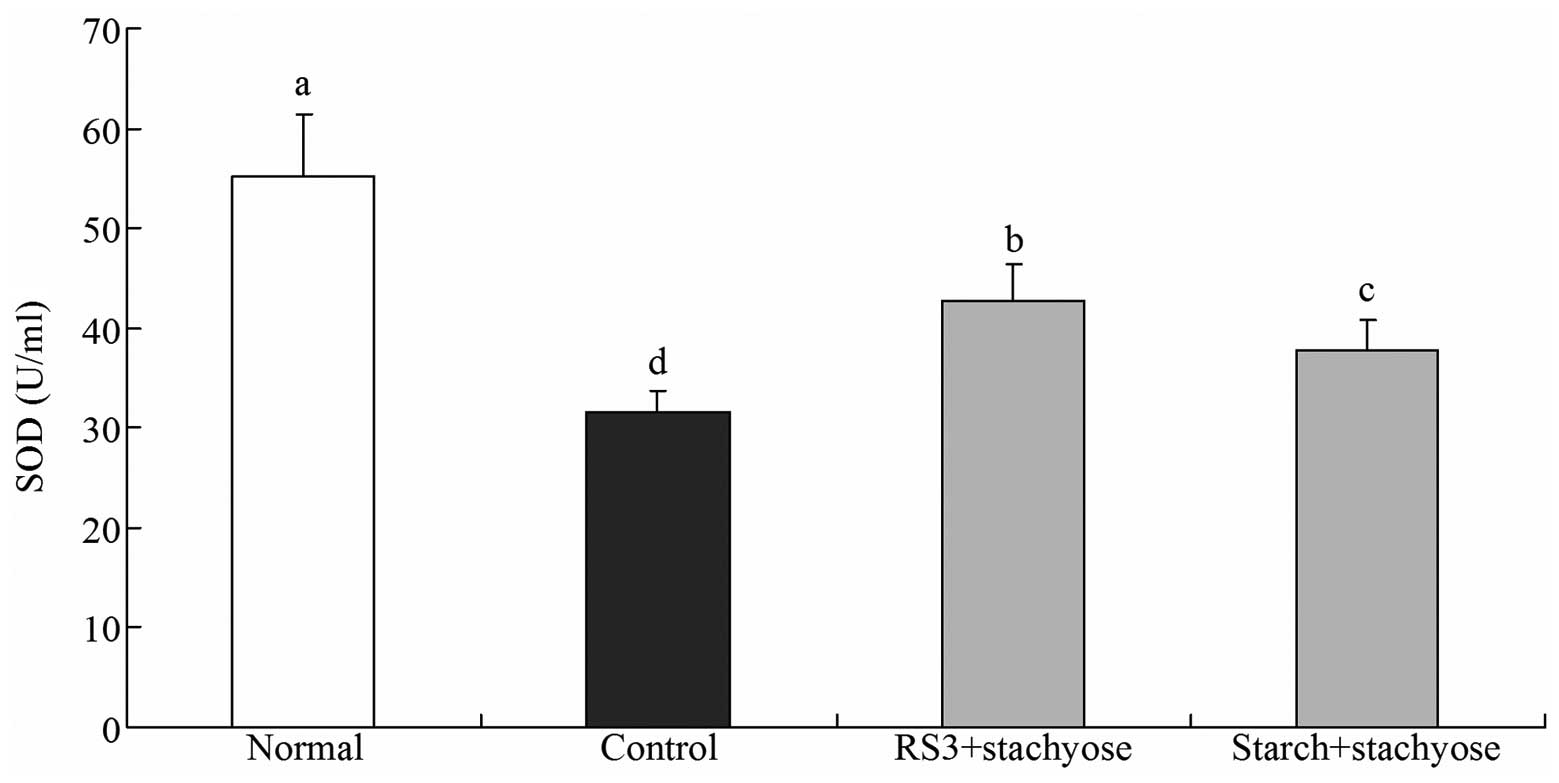
Effect of RS3+stachyose on serum levels
of SOD
The SOD level of normal mice was 55.2±6.3 U/ml and
control mice demonstrated the lowest level at 31.6±2.1 U/ml. The
SOD levels in the RS3 + stachyose- and starch + stachyose-treated
mice increased to 42.8±3.6 and 37.7±3.2 U/ml, respectively
(Fig. 4).
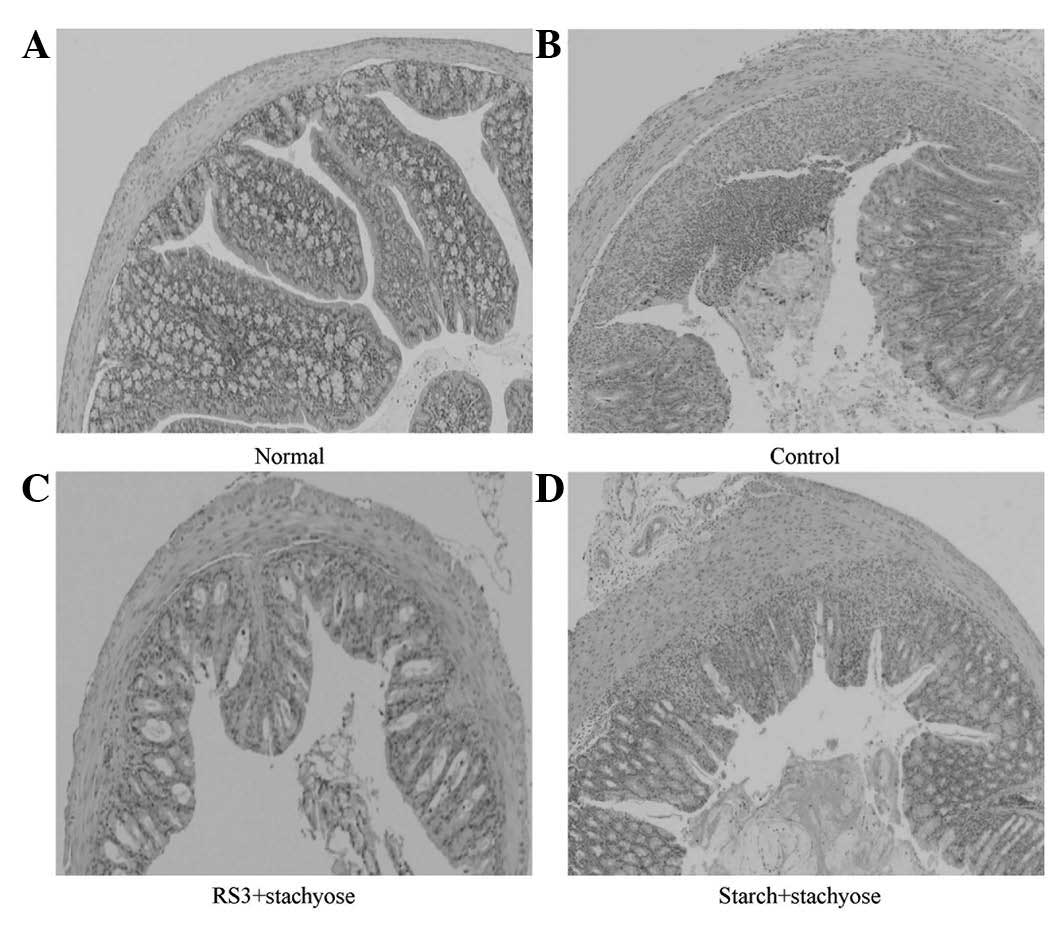
Effect of RS3 + stachyose on histological
damage in the colon tissue of mice with DSS-induced colitis
After collecting colonic tissue samples, the
severity of colitis was characterized by macroscopic examination of
the colon and histological analysis of H&E stained colonic
sections. The inhibitory effects of the stachyose-containing
microspheres on the DSS-induced damage of the colon tissue are
shown in Fig. 5. H&E staining
analysis indicated that the administration of DSS markedly
increased the severity of the colitis compared with that in the
normal mice (Fig. 5B). A typical
lesion of the colon in the DSS-treated group manifested multifocal
areas, mucosal erosion, loss of epithelial and goblet cells,
shortening and collapse of crypts, and submucosal edema. RS3 +
stachyose and starch + stachyose administration reduced the lesions
of the colon in DSS-induced colitis in the starch carrier
technique-dependent manner, as shown in Fig. 5C and D. The protective and healing
effects of RS3 + stachyose on colon damage were more prominent
(Fig. 5C).
Discussion
The most significant symptom in DSS-induced colitis
in mice is body weight loss (16).
Colon length may be measured to determine the severity of colitis.
Changes in colon weight and colon length reflect the inflammatory
status in mice with DSS-induced colitis and also demonstrate which
treatment has preventative effects against DSS-induced colitis in
mice (17).
The serum IL-6 and TNF-α levels in patients with
inflammatory diseases are higher compared with those in healthy
individuals (18). Lower levels of
IL-6 and TNF-α are indicative of improved anti-inflammatory
effects. IL-6 acts as a pro-inflammatory and anti-inflammatory
cytokine. IL-6 is secreted by T cells and macrophages to stimulate
the immune response, particularly in tissue damage leading to
inflammation. IL-6 also plays a role in fighting infection
(19). TNF-α is a cytokine
involved in systemic inflammation and is a member of a group of
cytokines that stimulate the acute phase reaction (20). IL-6 and TNF-α are key mediators in
a number of experimental colitis models (21).
SOD is a protein that neutralizes free radicals.
This protein is an enzyme that inhibits or regulates a specific
chemical or signaling action (22); specifically, it is one of the
important anti-oxidative enzymes. It catalyzes the dismutation of
the superoxide anion into hydrogen peroxide and molecular oxygen
(23). The rate of the reduction
of a superoxide anion is linearly related to the XO activity and is
inhibited by SOD. Therefore, the inhibitory activity of SOD may be
determined by a colorimetric method. In normal body cells, SOD
protects the cytoplasmic structures from damage caused by free
radicals (24).
RS is not digested in the stomach. It resists
enzymatic decomposition and enters into the colon as a nutrient
source for colonic bacteria. Through fermentation, these microbes
metabolize carbohydrates and generate short-chain fatty acids,
including butyric acid. Butyric acid lowers the pH value of the
colon and feces to promote colon health and prevent colitis
(25).
Under the activation of stachyose, bacteria produce
a large amount of short-chain fatty acids, which reduce intestinal
pH and Eh values, prevent the growth of harmful bacteria and
promote intestinal peristalsis in order to accelerate the excretion
of pathogenic bacteria and toxins. Stachyose itself decomposes
immunity factors, including manninotriose and melibiose. These
factors induce the body to produce more endocrine immunoglobulin
(IgA) and thereby neutralize bioactive antigens such as viruses,
toxins and pathogenic bacteria. These factors have an extensive
protective function (26) and
improve immunity. Stachyose has a positive effect on reducing the
intestinal pH value, adjusting the intestinal micro-ecological
balance, inhibiting the metabolism of pathogenic and spoilage
organisms, promoting intestinal peristalsis and immunity, and
curing enteritis (5).
In the present study, mice consumed a mouse diet
that included RS3 and stachyose. Stachyose combined with RS3 is not
absorbed in the stomach. After it enters into the colon, RS3 is
broken down by the intestinal bacteria and stachyose is released.
This is advantageous to the proliferation of intestinal probiotics
and has the effect of preventing colitis. By contrast, large
amounts of ordinary starch are decomposed by enzymes prior to
entering the colon; therefore, stachyose is only partly absorbed in
the colon, which reduces its efficacy. As indicated by the
experimental results, combining RS3 with stachyose enables the full
efficacy of stachyose to be utilized. Compared with the direct use
of stachyose, this method produces better preventative effects
against colitis.
Acknowledgements
This study was supported by Chongqing
Science Technology Commission (No. cstc2011jjA80001), China.
References
|
1.
|
No authors listed:. Report of a Joint
FAO/WHO Expert Consultation: Carbohydrates in human nutrition. FAO
Food Nutr Pap. 66:1–140. 1998.
|
|
2.
|
Englyst HN, Kingman SM and Cummings JH:
Classification and measurement of nutritionally important starch
fractions. Eur J Clin Nutr. 46(Suppl 2): S33–S50. 1992.PubMed/NCBI
|
|
3.
|
Baghurst PA, Baghurst KI and Record SJ:
Dietary fiber, non-starch polysaccharides and resistant starch - a
review. Food Australia. 48(Suppl): S1–S36. 1996.
|
|
4.
|
Yin J, Yang G, Wang S and Chen Y:
Purification and determination of stachyose in Chinese artichoke
(Stachys Sieboldii Miq.) by high-performance liquid
chromatography with evaporative light scattering detection.
Talanta. 70:208–212. 2006.PubMed/NCBI
|
|
5.
|
Liying Z, Li D, Qiao S, Johnson EW, Li B,
Thacker PA and Han IK: Effects of stachyose on performance,
diarrhoea incidence and intestinal bacteria in weanling pigs. Arch
Tierernahr. 57:1–10. 2003.PubMed/NCBI
|
|
6.
|
Macfarlane GT and Cummings JH: Probiotics
and prebiotics: can regulating the activities of intestinal
bacteria benefit health? West J Med. 171:187–191. 1999.
|
|
7.
|
Xu Q, Chao YL and Wan QB: Health benefit
application of functional oligosaccharides. Carbohydrate Polymers.
77:435–441. 2009. View Article : Google Scholar
|
|
8.
|
Jian R, Besterman HS, Sarson DL, et al:
Colonic inhibition of gastric secretion in man. Dig Dis Sci.
26:195–201. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Seo GS and Choi SC: Pathophysiology of
ulcerative colitis - relationship with genetics and immunity. Korea
J Med. 76:643–648. 2009.(In Korean).
|
|
10.
|
Stange EF, Travis SP, Vermeire S,
Beglinger C, Kupcinkas L, Geboes K, et al European Crohn’s and
Colitis Organisation: European evidence based consensus on the
diagnosis and management of Crohn’s disease: definitions and
diagnosis. Gut. 55(Suppl 1): 1–15. 2006.
|
|
11.
|
Ramadori G and Armbrust T: Cytokines in
the liver. Eur J Gastroenterol Hepatol. 13:777–784. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
McCurry KR, Campbell DA Jr, Scales WE,
Warren JS and Remick DG: Tumor necrosis factor, interleukin 6, and
the acute phase response following hepatic ischemia/reperfusion. J
Surg Res. 55:49–54. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ming WJ, Bersani L and Mantovani A: Tumor
necrosis factor is chemotactic for monocytes and polymorphonuclear
leukocytes. J Immunol. 138:1469–1472. 1987.PubMed/NCBI
|
|
14.
|
Fayyaz M and Lackner JM: Serotonin
receptor modulators in the treatment of irritable bowel syndrome.
Ther Clin Risk Manag. 4:41–48. 2008.PubMed/NCBI
|
|
15.
|
Melgar S, Karlsson L, Rehnström E,
Karlsson A, Utkovic H, Jansson L and Michaëlsson E: Validation of
murine dextran sulfate sodium-induced colitis using four
therapeutic agents for human inflammatory bowel disease. Int
immunopharmacol. 8:836–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ogawa A, Andoh A, Araki Y, Bamba T and
Fujiyama Y: Neutralization of interleukin-17 aggravates dextran
sulfate sodium-induced colitis in mice. Clin Immunol. 110:55–62.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Soriano A, Salas A, Salas A, Sans M,
Gironella M, Elena M, Anderson DC, Piqué JM and Panés J: VCAM-1,
but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced
colitis in mice. Lab Invest. 80:1541–1551. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ferguson-Smith AC, Chen YF, Newman MS, May
LT, Sehgal PB and Ruddle FH: Regional localization of the
interferon-beta 2/B-cell stimulatory factor 2/hepatocyte
stimulating factor gene to human chromosome 7p15–p21. Genomics.
2:203–208. 1988.PubMed/NCBI
|
|
19.
|
van der Poll T, Keogh CV, Guirao X,
Buurman WA, Kopf M and Lowry SF: Interleukin-6 gene-deficient mice
show impaired defense against pneumococcal pneumonia. J Infect Dis.
176:439–444. 1997.PubMed/NCBI
|
|
20.
|
Gosselin D and Rivest S: Role of IL-1 and
TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr Hyde
duality of the innate immune system. Brain Behav Immun. 21:281–289.
2007.PubMed/NCBI
|
|
21.
|
Wan GS, Zhao ZZ, Qian YL, Xie ZH, Wang ZH
and Zhu JJ: Changes in serum levels of SOCS-3, IL-6 and TNF-α and
their correlation in patients with ulcerative colitis. World
Chinese J Digestol. 19:3370–3373. 2011.(In Chinese).
|
|
22.
|
Barondeau DP, Kassmann CJ, Bruns CK,
Tainer JA and Getzoff ED: Nickel superoxide dismutase structure and
mechanism. Biochemistry. 43:8038–8047. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gaze DC: The role of existing and novel
cardiac biomarkers for cardioprotection. Curr Opin Investig Drugs.
8:711–717. 2007.PubMed/NCBI
|
|
24.
|
Alscher RG, Erturk N and Heath LS: Role of
superoxide dismutases (SODs) in controlling oxidative stress in
plants. J Exp Bot. 53:1331–1341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Topping DL and Clifton PM: Short-chain
fatty acids and human colonic function: roles of resistant starch
and nonstarch polysaccharides. Physiol Rev. 81:1031–1064.
2001.PubMed/NCBI
|
|
26.
|
Berggren AM, Bjorck IM, Nyman EM and Eggum
BO: Short-chain fatty acid content and pH in caecum of rats given
various sources of carbohydrates. J Sci Food Agri. 63:397–406.
1993.
|