Introduction
In China, laryngeal squamous cell carcinoma (SCC)
accounts for ~7.9–35% of all the head and neck malignant tumors,
and ~1.2–1.6% of all malignant tumors (1). The induction and development of
tumors involves multiple factors (2). As the tumor grows larger than 1 × 1 ×
2 mm, its further expansion relies on the growth of new inner blood
vessels, which is subject to the modulation of various cytokines.
Angiopoietin (Ang) and its receptor Tie-2 are the two key cytokines
regulating the development of tumor blood vessels (3). The positive rate of Ang-2 expression
in laryngeal SCC is significantly higher than in normal mucosa
tissue. As the differentiation decreased, the expression of Ang-2
increased The overexpression of Ang-2 contributes to the occurrence
of laryngeal SCC. It appears that laryngeal SCC is associated with
the expression of Ang-2. Previous studies show that cyclin D1 is
not or seldom expressed in the normal tissue, but it is highly
expressed in numerous malignant tumors. Cyclin D1 is associated
with the tumor classification and may be regarded as a potential
prognostic indicator. The aim of this study is to investigate the
expression of Ang-2 and cyclin D1 in the laryngeal SCC and
clinicopathological signification. It is likely that they play a
significant role in tumor induction, development and metastasis
(4).
Materials and methods
Materials
A total of 116 laryngeal SCC specimens were
collected between January 2003 and December 2011 from The
Otolaryngology Department of The Affiliated Hospital of Nantong
University (Nantong, China). The complete clinical and pathological
materials were well preserved. Of these patients, there were 107
males and 9 females aged from 42 to 83 years, with an average age
of 62.8 years. According to the standard classification of head and
neck neoplasm histopathology listed by the WHO (World Health
Organization) (5) in 2005, 60
cases were well differentiated, 45 moderately differentiated and 11
poorly differentiated. The patients studied were initial cases
without radiotherapy, chemotherapy or biotherapy before surgery. In
addition, five cases of vocal cord polyp and five cases of atypical
hyperplasia were used as control groups. We tracked the survival
condition of 116 cases following surgery and recorded the causes of
mortality in patients. For example, certain individuals succumbed
to tumor, accident or other causes. The calculation of overall
survival (OS) starts from the established diagnosis to the
mortality caused by the tumor or the termination of follow-up
study. Any mortality irrelevant to laryngeal SCC was recorded as
truncation. This study was approved by the Ethics Committee of
Affiliated Hospital of Nantong University, Nantong, China). Written
informed consent was obtained from all patients.
Specimen preparation
Fresh tissues were fixed with 4% paraformaldehyde,
embedded with paraffin, cut into serial sections with a thickness
of 4 μm and stained with hematoxylin and eosin and Ang-2 and
cyclin D1 immunohistochemical stains.
Immunohistochemical staining
Paraffin sections were deparaffinized, dehydrated
and antigen retrieval was conducted by microwave for 15 min within
citrate buffer solution (pH 6.0). Droplets of polyclonal rabbit
anti-human Ang-2 and cyclin D1 were distributed into the
deparaffinized sections for streptavidin-perosidase (S-P) staining.
The known positive section was used as a positive control, normal
goat serum as a negative control in place of primary antibody and
PBS as a blank control also in place of primary antibody.
Immunohistochemical scoring
Ang-2 was located in the cytoplasm of tumor cells.
Positive staining is observed as bright yellow, brown yellow or
brown granules focally or diffusively distributed. Cyclin D1 was
located in the tumor cell nucleus. Cyclin D1 protein expression is
indicated by bright yellow, brown yellow or brown granules focally
or diffusively distributed. Semi-quantitative results were obtained
under a microscope (Olympus BX51-P; Olympus, Tokyo, Japan) using
the method of Xu and Yang (6),
which is dependent on the staining intensity and number of positive
cells. Standard scores of staining intensity were as follows: no
staining, 0 points; bright yellow, 1 point; brown yellow, 2 points;
brown, 3 points. Number of positive cells under the same objective:
negative, 0; number of stained cells within one field of vision
≤10%, 1 point; 11–50%, 2 points; 51–75%, 3 points; ≥76%, 4 points.
The result of the two scores multiplied was graded: 0–2 as (-); 3
as (+); 4 as (++); ≥5 as (+++); results between + and +++ indicate
to presence of expression.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) was
employed to statistically process the relevant data. The
χ2 test was used to compare the expression of Ang-2 and
cyclin D1 in each group. The correlation comparison between Ang-2
and cyclin D1 was calculated using the Spearman’s rank correlation
analysis. P<0.05 was considered to indicate a statistically
significant result.
Results
Expression of Ang-2 in vocal cord polyp,
atypical hyperplasia and laryngeal SCC tissues
The positive rate of Ang-2 was 0% (0/5) in vocal
cord polyp and 0% (0/5) in atypical hyperplasia. The positive rate
in laryngeal SCC tissues was 40% for well differentiated, 66.7% for
moderately differentiated and 100% for poorly differentiated. The
positive staining located in the cytoplasm was as bright yellow,
brown yellow or brown granules focally or diffusively distributed
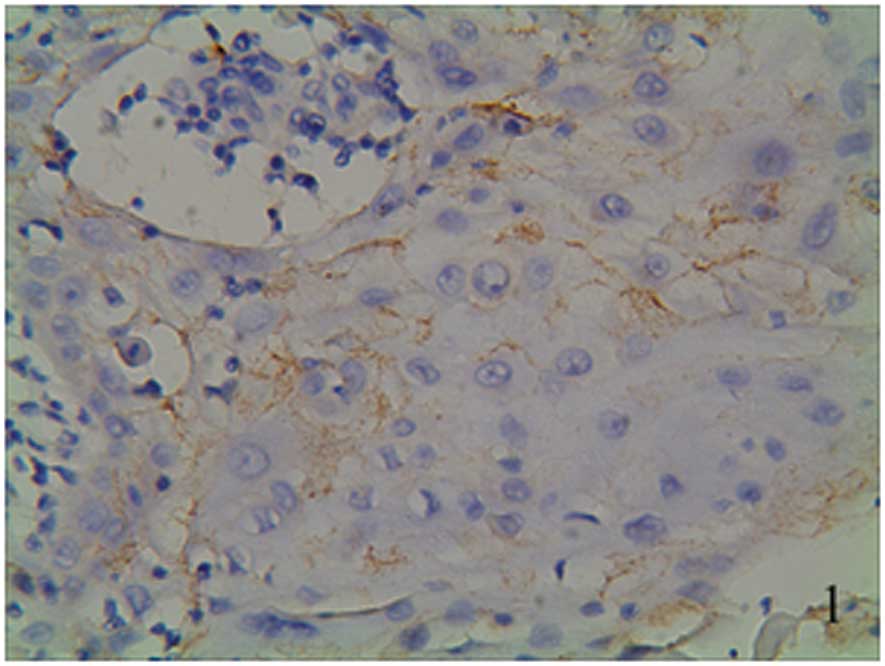
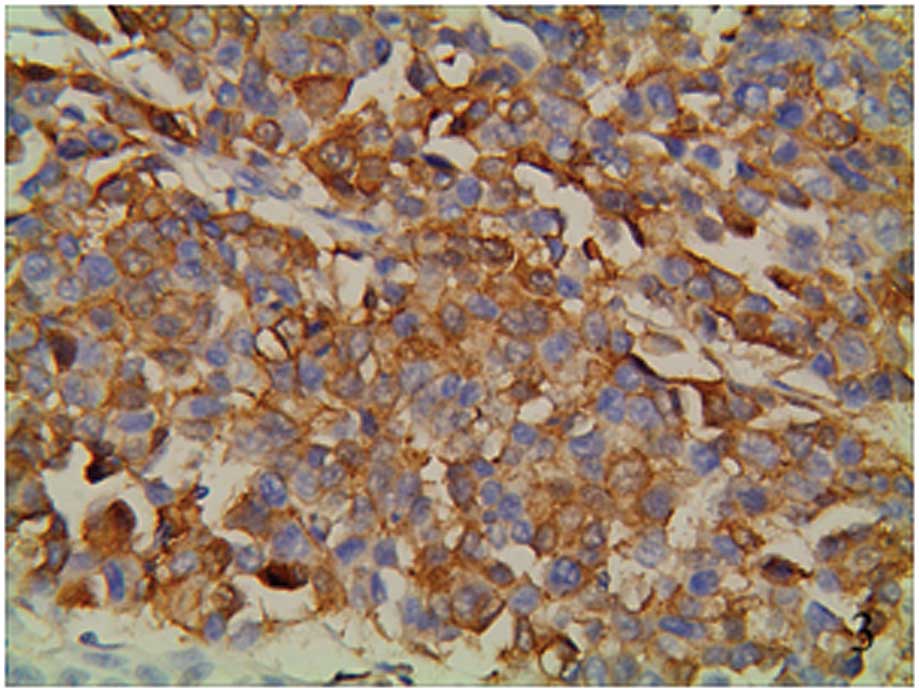
(Figs. 1–3). Ang-2 expression presented as brown
staining in the vascular endothelial cells (Fig. 4). one group from the groups of the
vocal cord polyp and atypical hyperplasia and one group from the
groups of well, moderately and poorly differentiated laryngeal SCC,
statistical significance was indicated (P<0.05). A pairwise
comparison was conducted among the well, moderately and poorly
differentiated laryngeal SCC groups (P<0.05; Table I).
 | Table I.Ang-2 expression in vocal cord polyp,
atypical hyperplasia and laryngeal SCC tissues. |
Table I.
Ang-2 expression in vocal cord polyp,
atypical hyperplasia and laryngeal SCC tissues.
| Histopathological
type | n | Ang-2 expression
|
|---|
| Negative | Positive | Positive rate
(%) |
|---|
| Vocal cord polyp | 5 | 5 | 0 | 0.0 |
| Atypical
hyperplasia | 5 | 5 | 0 | 0.0 |
| Laryngeal SCC | | | | |
| Well
differentiated | 60 | 36 | 24 | 40.0 |
| Moderately
differentiated | 45 | 15 | 30 | 66.7 |
| Poorly
differentiated | 11 | 0 | 11 | 100.0 |
Expression of cyclin D1 in vocal cord
polyp, atypical hyperplasia and laryngeal SCC tissues
The incidence of cyclin D1 expression was 0% (0/5)
in vocal cord polyp and 0% (0/5) in atypical hyperplasia. As for
its positive rate in laryngeal SCC tissues, it was 50% for well
differentiated, 66.7% for moderately differentiated and 100% for
poorly differentiated laryngeal SCC. The positive staining was
located in the nucleus as bright yellow, brown yellow or brown
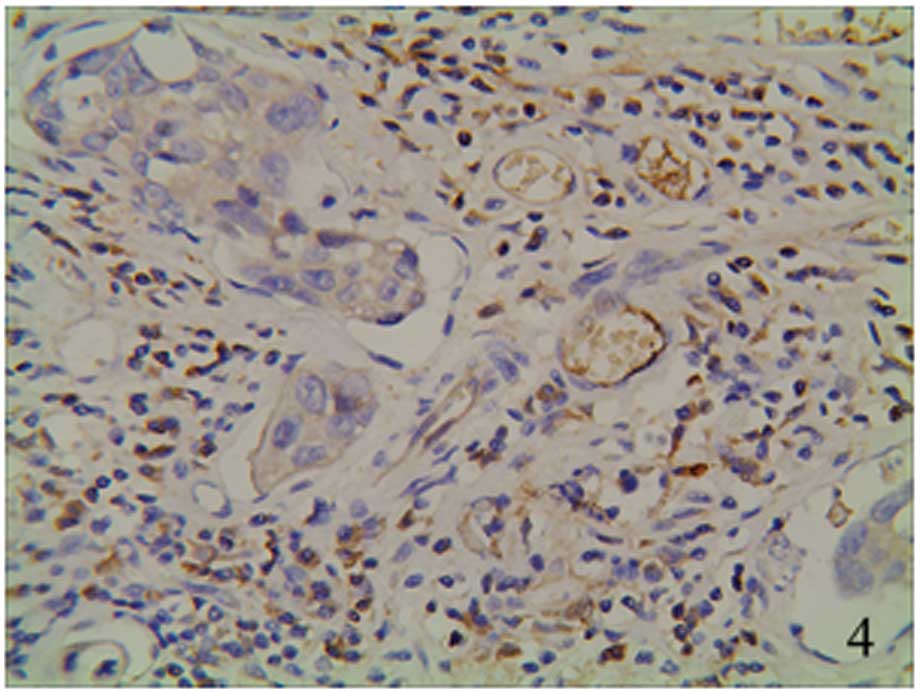
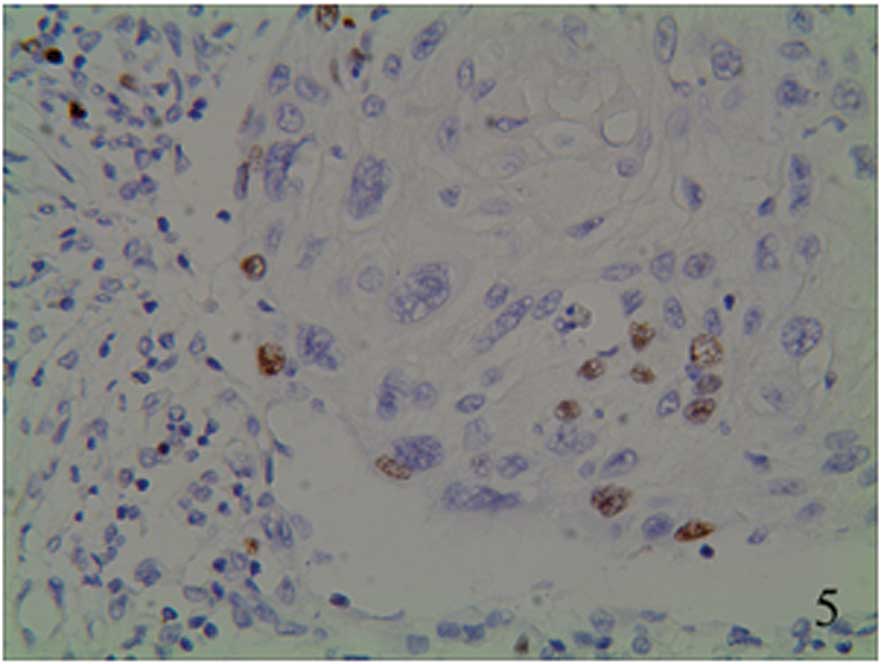
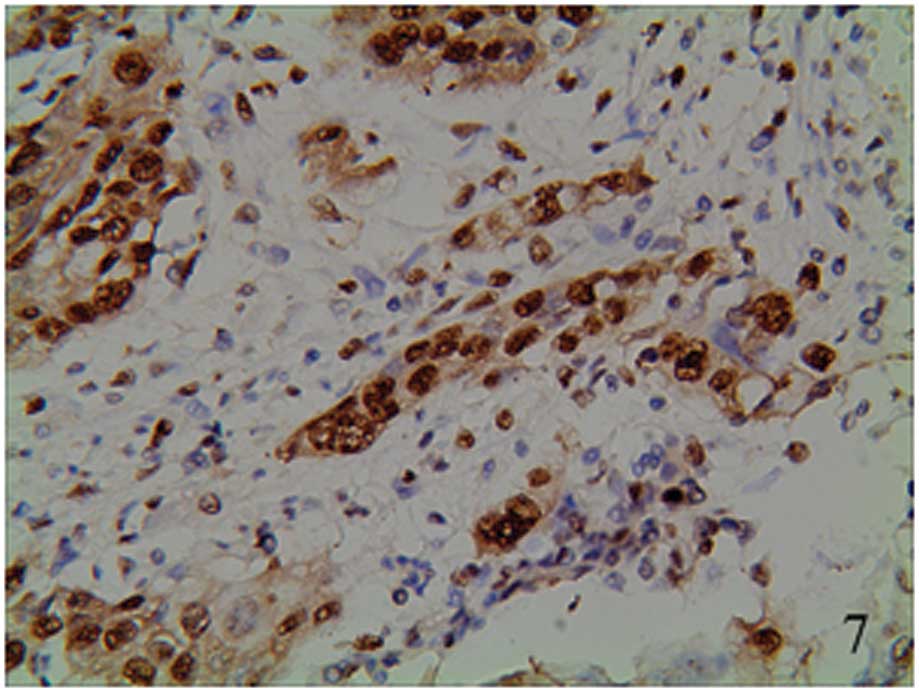
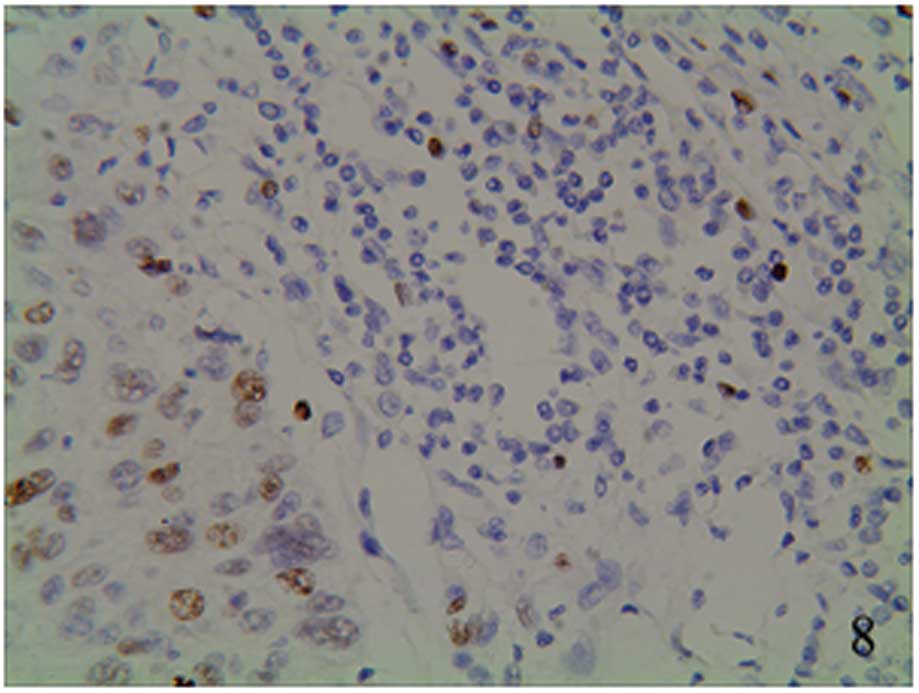
granules (Figs. 5–7). The staining was focally positive and
infiltrated into the lymph gland (Fig.
8). The interclass differences between the well, moderately and
poorly differentiated laryngeal SCC groups and the vocal cord polyp
and atypical hyperplasia groups was statistically significant
(P<0.05). Interclass expressions for the well and moderately
differentiated groups and the well and poorly differentiated groups
were statistically significant (P<0.05). No statistical
significance was identified between the moderately and poorly
differentiated groups (Table
II).
 | Table II.Cyclin D1 expression in vocal cord
polyp, atypical hyperplasia and laryngeal SCC tissues. |
Table II.
Cyclin D1 expression in vocal cord
polyp, atypical hyperplasia and laryngeal SCC tissues.
| Histopathological
type | n | Cyclin D1 expression
|
|---|
| Negative | Positive | Positive rate
(%) |
|---|
| Vocal cord polyp | 5 | 5 | 0 | 0.0 |
| Atypical
hyperplasia | 5 | 5 | 0 | 0.0 |
| Laryngeal SCC | | | | |
| Well
differentiated | 60 | 30 | 30 | 50.0 |
| Moderately
differentiated | 45 | 15 | 30 | 66.7 |
| Poorly
differentiated | 11 | 0 | 11 | 100.0 |
Association between Ang-2 expression in
laryngeal SCC and clinically pathological factors
The proportions of positive staining of Ang-2 in the
well, moderately and poorly differentiated laryngeal SCC groups
were 40, 66.7 and 100%, respectively. Differences exist among the
three groups, for example, the poorly differentiated group showed
an expression rate higher than those of the well and moderately
differentiated groups. The interclass difference was statistically
significant (Z=4.020; P<0.05). The proportions of positive
staining of laryngeal SCC in TNM grades T1+T2 and T3+T4 were 46.7
and 73.2%, respectively. The positive rate of Ang-2 increases at
later pathological stages. The Ang-2 expression of advanced
laryngeal SCC is higher than that of early-stage cancer (Z=−3.006,
P<0.05). The positive rate of lymph-metastasis group and that of
non-lymph-metastasis group was 82.1 and 47.7%, respectively, the
expression difference was statistically significant (Z=−4.129,
P<0.05). The proportions of positive staining of the smoking
group and that of the non-smoking group were 67.6 and 39.6%,
respectively, the expression difference was significantly different
(Z=−3.190, P<0.05).
Differences in Ang-2 expression according to gender,
age, primary tumor site or drinking habits were not statistically
significant (Table III).
 | Table III.Expression of Ang-2 and
clinicopathologic characteristics of the patients with laryngeal
SCC. |
Table III.
Expression of Ang-2 and
clinicopathologic characteristics of the patients with laryngeal
SCC.
| Characteristics | n | Ang-2 expression
| Z | P-value |
|---|
| − | + | ++ | +++ |
|---|
| Gender | | | | | | | |
| Male | 107 | 47 | 22 | 20 | 18 | −0.038 | 0.9695 |
| Female | 9 | 4 | 2 | 1 | 2 | | |
| Age (years) | | | | | | | |
| ≤60 | 43 | 18 | 10 | 8 | 7 | 0.127 | 0.8990 |
| >60 | 73 | 33 | 14 | 13 | 13 | | |
| Smoking | | | | | | | |
| Yes | 68 | 22 | 16 | 13 | 17 | −3.190 | 0.0014 |
| No | 48 | 29 | 8 | 8 | 3 | | |
| Drinking | | | | | | | |
| Yes | 49 | 26 | 8 | 7 | 8 | 1.350 | 0.1771 |
| No | 67 | 25 | 16 | 14 | 12 | | |
| TNM staging | | | | | | | |
| T1+T2 | 75 | 40 | 16 | 9 | 10 | −3.006 | 0.0026 |
| T3+T4 | 41 | 11 | 8 | 12 | 10 | | |
| Lymph node
metastasis | | | | | | | |
| Yes | 28 | 5 | 5 | 7 | 11 | −4.129 | 0.0000 |
| No | 88 | 46 | 19 | 14 | 9 | | |
| Tumor
differentiation | | | | | | | |
| Well | 60 | 36 | 14 | 5 | 5 | −3.258 | 0.0011 |
| Moderately | 45 | 15 | 8 | 12 | 10 | −4.360 | 0.0000 |
| Poorly | 11 | 0 | 2 | 4 | 5 | −2.274 | 0.0230 |
| Primary tumor
site | | | | | | | |
| Supraglottic | 28 | 12 | 7 | 2 | 7 | 0.860 | 0.390 |
| Glottic | 73 | 36 | 11 | 14 | 12 | | |
| Subglottic | 15 | 3 | 6 | 5 | 1 | | |
Association between cyclin D1 expression
and laryngeal SCC clinical pathological factors
The proportions of positive staining of cyclin D1 in
the well, moderately and poorly differentiated laryngeal SCC group
tissues were 50, 66.7 and 100%, respectively. Interclass
comparisons of the expression in the well and moderately
differentiated groups and well and poorly differentiated groups
were statistically significant (Z=−2.814, P<0.05 and Z=−4.087,
P<0.05). No statistically significant difference of expression
was observed between the moderately and poorly differentiated
groups. The proportions of positive staining of laryngeal SCC in
the T1+T2 and T3+T4 TNM stages were 52.0 and 78.0%, respectively.
The positive rate of cyclin D1 increased with later pathological
stage. The expression of advanced laryngeal SCC was higher than
that of early-stage laryngeal SCC (Z=−3.182, P<0.05). The
proportions of positive staining of the lymph-metastasis and
non-lymph-metastasis groups were 82.1 and 54.5%, respectively; the
expression difference was statistically significant (Z=−4.211,
P<0.05). The proportions of positive staining of the smoking and
non-smoking groups were 70.6 and 47.9%, respectively; the
expression difference was statistically significant (Z=−2.518,
P<0.05).
The expression of cyclin D1 was not significantly
associated with gender, age, primary tumor site or drinking habits
(Table IV).
 | Table IV.Expression of cyclin D1 and
clinicopathologic characteristics of the patients with laryngeal
SCC. |
Table IV.
Expression of cyclin D1 and
clinicopathologic characteristics of the patients with laryngeal
SCC.
|
Characteristics | (n) | cyclin D1
expression
| Z | P-value |
|---|
| − | + | ++ | +++ |
|---|
| Gender | | | | | | | |
| Male | 107 | 42 | 33 | 17 | 15 | −0.796 | 0.4260 |
| Female | 9 | 3 | 1 | 4 | 1 | | |
| Age (years) | | | | | | | |
| ≤60 | 43 | 15 | 10 | 11 | 7 | −0.045 | 0.9641 |
| >60 | 73 | 30 | 19 | 11 | 13 | | |
| Smoking | | | | | | | |
| Yes | 68 | 20 | 21 | 16 | 11 | −2.518 | 0.0118 |
| No | 48 | 25 | 13 | 5 | 5 | | |
| Drinking | | | | | | | |
| Yes | 49 | 18 | 17 | 6 | 8 | −0.106 | 0.9159 |
| No | 67 | 27 | 17 | 15 | 8 | | |
| TNM staging | | | | | | | |
| T1+T2 | 75 | 36 | 22 | 10 | 7 | −3.182 | 0.0015 |
| T3+T4 | 41 | 9 | 12 | 11 | 9 | | |
| Lymph node
metastasis | | | | | | | |
| Yes | 28 | 5 | 5 | 9 | 9 | −4.211 | 0.0000 |
| No | 88 | 40 | 29 | 12 | 7 | | |
| Tumor
differentiation | | | | | | | |
| Well | 60 | 30 | 22 | 4 | 4 | −2.814 | 0.0049 |
| Moderately | 45 | 15 | 9 | 13 | 8 | −4.087 | 0.0000 |
| Poorly | 11 | 0 | 3 | 4 | 4 | −2.071 | 0.0384 |
| Primary tumor
site | | | | | | | |
| Supraglottic | 28 | 8 | 9 | 6 | 5 | 0.460 | 0.640 |
| Glottic | 73 | 33 | 21 | 13 | 6 | | |
| Subglottic | 15 | 4 | 4 | 2 | 5 | | |
Correlation analysis of Ang-2 and cyclin
D1 expression in laryngeal SCC
In this study, all cases were stained for cyclin D1
and Ang-2 simultaneously. There were 28 cases negative for both and
48 cases positive for both Ang-2 and cyclin D1. Spearman’s rank
correlation analysis results demonstrated a positive correlation
between cyclin D1 and Ang-2 expression in laryngeal SCC (r=0.5042;
P<0.05; Table V).
 | Table V.Correlation analysis of Ang-2 and
cyclin D1 expression in laryngeal SCC. |
Table V.
Correlation analysis of Ang-2 and
cyclin D1 expression in laryngeal SCC.
| Cyclin D1
expression | n | Ang-2 expression
|
|---|
| − | + | ++ | +++ |
|---|
| − | 45 | 28 | 8 | 8 | 1 |
| + | 34 | 18 | 10 | 4 | 2 |
| ++ | 21 | 4 | 4 | 5 | 8 |
| +++ | 16 | 1 | 2 | 4 | 9 |
| Total | 116 | 51 | 24 | 21 | 20 |
One-way analysis of clinically
pathological parameters and laryngeal SCC patient survival
time
Using log-rank one-way analysis, we demonstrated
that laryngeal SCC prognosis is associated with smoking, TNM stage,
lymph metastasis, tumor classification, tumor location, cyclin D1
and Ang-2, but is not correlated with age, gender or drinking habit
(Table VI).
 | Table VI.One-way analysis of clinically
pathological parameters and laryngeal SCC patient survival
time. |
Table VI.
One-way analysis of clinically
pathological parameters and laryngeal SCC patient survival
time.
| Parameter | Group | χ2 | P-value |
|---|
| Gender | Male/female | 0.005 | 0.945 |
| Age (years) | ≤60/>60 | 2.072 | 0.150 |
| Smoking | Yes/no | 4.598 | 0.032 |
| Drinking | Yes/no | 0.333 | 0.564 |
| TNM staging | T1+T2/T3+T4 | 6.026 | 0.014 |
| Lymph node
metastasis | Yes/no | 16.744 | 0.000 |
| Tumor
differentiation |
Well/moderately/poorly | 34.527 | 0.000 |
| Primary tumor
site |
Supraglottic/glottic/subglottic | 8.456 | 0.015 |
| Cyclin D1 |
(−)/(+)/(++)/(+++) | 42.220 | 0.000 |
| Ang-2 |
(−)/(+)/(++)/(+++) | 22.393 | 0.000 |
Survival analysis
Using multi-factor Cox proportional hazards
regression models to analyze TNM stage, lymph metastasis, tumor
classification, tumor location, cyclin D1 and Ang-2, the tumor
classification and cyclinD1 are associated with the survival rate
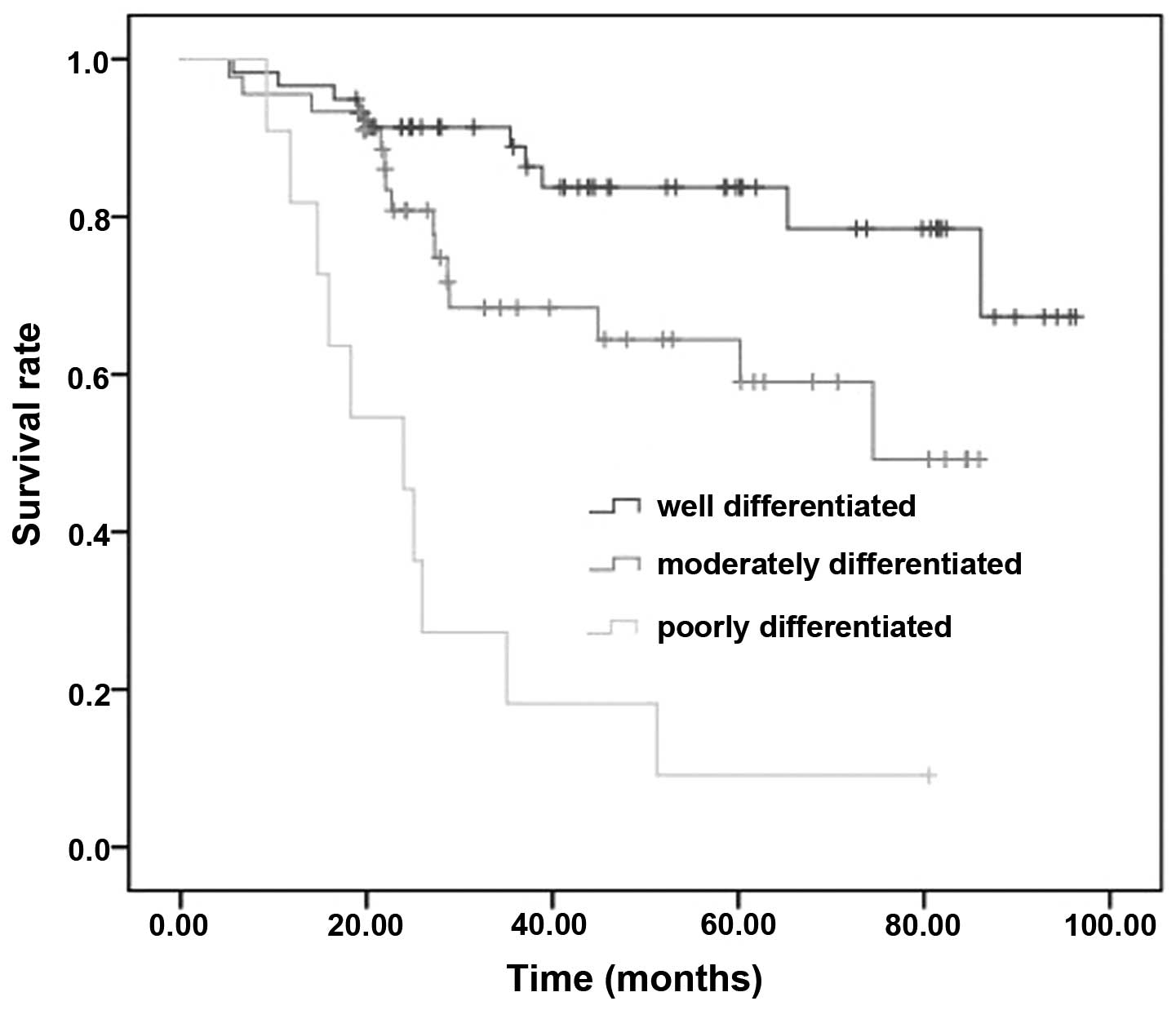
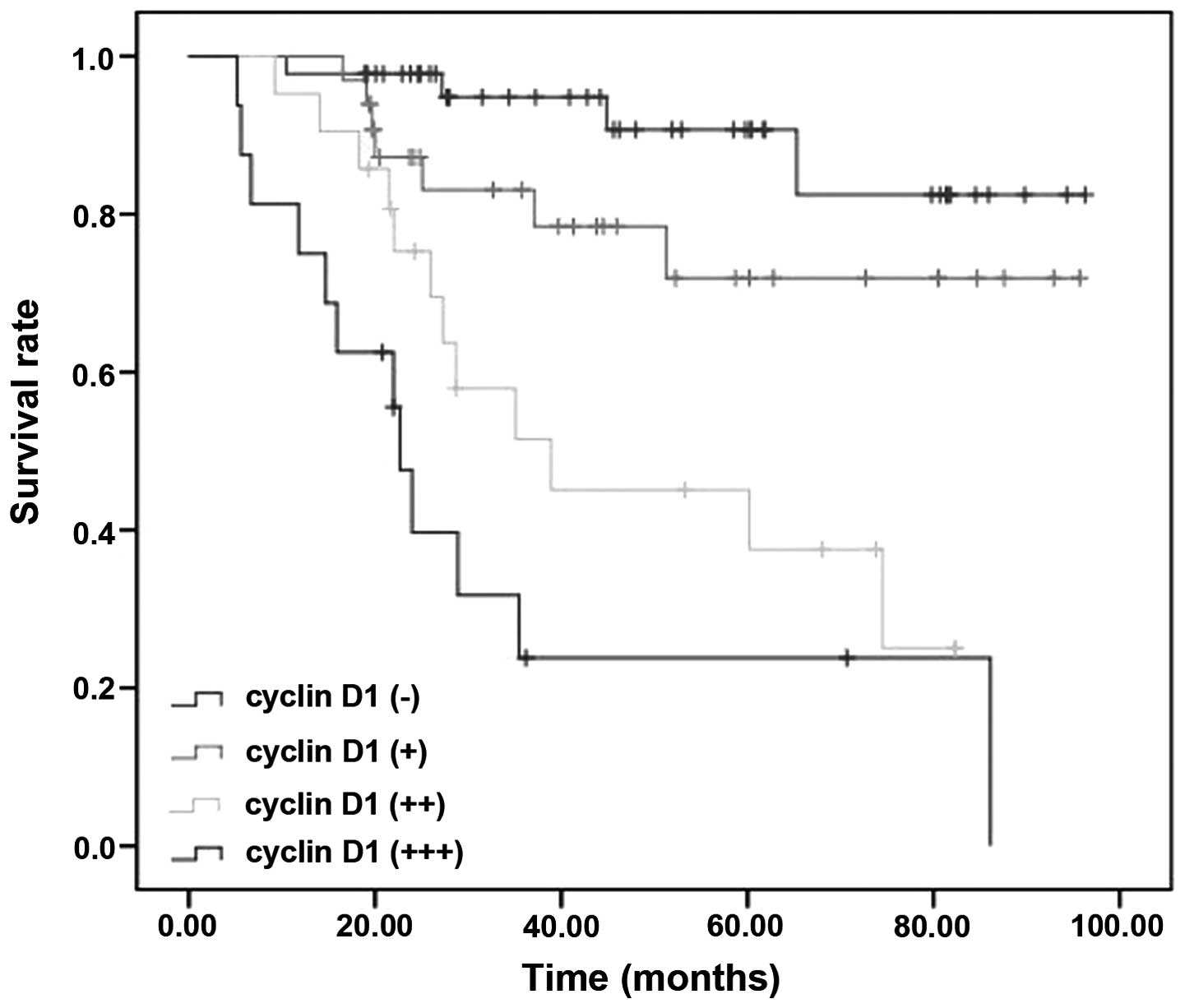
(Figs. 9 and 10). The survival rate of well
differentiated, moderately differentiated and poorly differentiated
groups was 78.3, 66.7 and 0.91%, respectively. The survival rate of
of cyclin D1 −/+/++/+++ groups was 88.9, 76.5, 42.9 and 25.0%,
respectively. The tumor classification and cyclin D1 each were able
to act as independent prognostic factors.
Discussion
Similar to the majority of solid tumors, laryngeal
SCC induction and development is subject to various factors,
including modulation of tumor angiogenesis and the cell cycle. As a
member of the angiopoietins, Ang-2 exerts a regulatory effect on
angiogenesis (7). Cyclin D1 is
classified as an oncogene. Once cyclin D1 mutates or expresses
excessively during the process of cell proliferation, cyclin D1
promotes the induction and development of a tumor (8).
The Ang-2 protein is composed of 496 amino-acids
with a molecular weight of 70 kDa, the gene for which is located at
chromosome 8p23.1. The protein comprises a signal peptide,
amino-end α-spiral hinge and carboxy-end fibrous protein sample
regions (9). Ang-2 mainly exists
in the form of a homodimer and is able to combine with vascular
endothelium Tie-2 without causing phosphorylation of the receptor.
However, Ang-2 is able to competitively block the effect of Ang-1,
relax endothelial cells and perivascular sertoli cells, and
accelerate vascular bed degeneration and extravascular substrate
degradation, which renders the vascular net unstable, thus
providing favorable conditions for endothelial cell division and
new blood vessel reconstruction (7).
The study by Yan et al (10) on 64 cases of colorectal cancer has
observed that, as the differentiation decreases, the expression
rate of Ang-2 increases, which indicates that Ang-2 propelled the
deterioration of the tumor. A study by Chen et al (11) on 51 cases of hepatocellular
carcinoma suggested that Ang-2 is a marker of hepatocellular
carcinoma. Research by Wang et al (12) on 50 cases of cervical cancer has
demonstrated that as the differentiation decreases, the expression
rate of Ang-2 tended to increase. In addition, the microvessel
density rose markedly, which indicated the involvement of Ang-2 in
hepatocellular carcinoma development (13). A study of 335 cases of non-small
cell lung cancer by Anderson et al (14) studied the correlation between the
expression of Ang-2 and prognosis, which showed that Ang-2 was a
signal of tumor prognosis. Hashizume et al (15) suggest that Ang-2 may slow down the
growth of tumors and therefore accelerate tumor cell death.
The results of the present study demonstrated that
Ang-2 expression in laryngeal SCC specimens was markedly higher
than that in atypical hyperplasia and vocal cord polyp tissues
(P<0.05). Ang-2 was mainly located in the cytoplasm of laryngeal
SCC cells. Therefore, we suggest that tumor cells generate Ang-2,
which functions through autocrine and paracrine systems. Ang-2
expression in cancer tissues was markedly higher than that in
normal laryngeal mucosal tissues. As the differentiation decreased,
the expression of Ang-2 increased. We hypothesize that Ang-2
induces the split and shift of endothelial cells, permitting new
vessels to grow by budding to promote the occurrence and
development of a tumor. This study also showed that as the clinical
stage advanced, Ang-2 expression increased. The expression of Ang-2
in the moderately and poorly differentiated groups was higher than
that in the well differentiated group. Expression was higher in the
lymph-metastasis group than in the non-lymph-metastasis group,
which indicates that Ang-2 may promote laryngeal SCC infiltration
and lymph metastasis.
Cyclin D1, the key protein of the G1 stage during
cell proliferation, may interact with multiple proteins and
transition the cell into the S stage. Therefore, it is considered a
significantly positive regulator in the G1/S transition in the cell
cycle (16). When the level of
cyclin D1 increases, the G1/S stage shortens, with cell
proliferation and canceration. There are various ways for the
cyclin D1 gene to mutate within tumors, including gene
amplification, chromosome translocation and inversion, among them
gene amplification is the most common. In a study by Marsit et
al (17), 698 cases of head
and neck SCC underwent immunohistochemical testing. The results
showed that, cyclin D1 was expressed in head and neck SCC tissues.
Cyclin D1 was highly expressed in T3+T4 stages, but was rare in
T1+T2. This indicates that cyclin D1 is relevant to tumor stage and
may be regarded as a potential prognostic signal of head and neck
SCC.
Our results have demonstrated that the expression of
cyclin D1 in laryngeal SCC tissues is markedly higher than that in
atypical hyperplasia and vocal cord polyp tissues (P<0.05). As
the differentiation decreased, the expression of cyclin D1
increased, which indicated that the upregulated expression of
cyclin D1 was associated with the degree of malignancy of laryngeal
SCC. Solid tumor growth, proliferation and metastasis require new
vessel support. At present, there are few studies concerning the
correlation of cyclin D1 and Ang-2 expression with tumors. The
present study showed that positive correlation exists between
cyclin D1 and Ang-2 expression in laryngeal SCC, which indicates
that both exert a synergic effect on laryngeal SCC occurrence,
development and metastasis. We conducted analysis of 116 laryngeal
SCC patients through log-rank one-way method and observed that
laryngeal SCC prognosis is associated with smoking, TNM stage,
lymph metastasis, tumor classification, tumor location, cyclin D1
and Ang-2, and is not associated with age, gender or drinking habit
(Table 6). Using multi-factor Cox
proportional hazards regression models to analyze TNM stage, lymph
metastasis, tumor classification, tumor location, cyclin D1 and
Ang-2, it was demonstrated that the tumor classification and cyclin
D1 are associated with the survival rate (Figs. 9 and 10). The tumor classification and cyclin
D1 were able to act as independent prognostic factors. Log-rank
one-way method and multi-factor Cox proportional hazards regression
models for tumor classification identified cyclin D1 as the
independent factor affecting patient survival time (Figs. 9 and 10). Furthermore, this study did not
observe any association between cyclin D1 expression and patient
gender, age or drinking habit.
References
|
1.
|
Lv ZH and Luan XY: Dialectic thoughts in
treatment of laryngeal carcinoma. Med Phil. 26:34–35. 2005.(In
Chinese).
|
|
2.
|
O’Reilly MS, Holmgren L, Shing Y, et al:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994.PubMed/NCBI
|
|
3.
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Liu X-Q, Wan H-R, Chen H-S, et al:
Significance of expression of angiopoietin mRNA in the tissue of
in-situ implanted hepatoma. Chin J Gen Surg. 13:196–198. 2004.(In
Chinese).
|
|
5.
|
Thompson L: World Health Organization
classification of tumours: pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
6.
|
Xu LZ and Yang WT: Judging standard of
immunohistochemical results. Chin Oncol. 6:229–231. 1996.(In
Chinese).
|
|
7.
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Flørenes VA, Faye RS, Maelandsmo GM,
Nesland JM and Holm R: Levels of cyclin D1 and D3 in malignant
melanoma: deregulated cyclin D3 expression is associated with poor
clinical outcome in superficial melanoma. Clin Cancer Res.
6:3614–3620. 2000.PubMed/NCBI
|
|
9.
|
Vajkoczy P, Farhadi M, Gaumann A, et al:
Microtumor growth initiates angiogenic sprouting with simultaneous
expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin
Invest. 109:777–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Zuo WZ, Wen CY, Zhang JH, et al:
Expression and value of Ang-1, Ang-2 and receptor Tie-2 in
colorectal cancer tissue. Jilin Med J. 32:2501–2505. 2011.(In
Chinese).
|
|
11.
|
Chen N, Su P and Xiong H: Detection result
and significance of Ang-2 in the serum of patients with
hepatocellular carcinoma. Chin J Integ Trad West Med Liver Dis.
21:102–103. 1292011.(In Chinese).
|
|
12.
|
Wang Z, Hou JQ and Jiang L: Expression and
significance of Ang-2 in cervical cancer and cervical
intraepithelial neoplasia. Chin J Gerontol. 15:2128–2130. 2010.(In
Chinese).
|
|
13.
|
Zadeh G, Koushan K, Baoping Q, Shannon P
and Guha A: Role of angiopoietin-2 in regulating growth and
vascularity of astrocytomas. J Oncol. 2010:6592312010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Andersen S, Donnem T, Al-Shibli K, et al:
Prognostic impacts of angiopoietins in NSCLC tumor cells and
stroma: VEGF-A impact is strongly assiociated with Ang-2. PloS One.
6:e197732011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hashizume H, Falcón BL, Kuroda T, et al:
Complementary actions of inhibitors of angiopoietin-2 and VEGF on
tumor angiogenesis and growth. Cancer. 70:2213–2223.
2010.PubMed/NCBI
|
|
16.
|
Won KA, Xiong Y, Beach D and Gilman MZ:
Growth-regulated expression of D-type cyclin genes in human diploid
fibroblasts. Proc Natl Acad Sci USA. 89:9910–9914. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Marsit CJ, Black CC, Posner MR and Kelsey
KT: A genotype-phenotype examination of cyclin D1 on risk and
outcome of squamous cell carcinoma of the head and neck. Clin
Cancer. 14:2371–2377. 2008. View Article : Google Scholar : PubMed/NCBI
|