Introduction
Computed tomography (CT)-guided transthoracic needle
biopsy (TNB) has become a well-established procedure. It has been
reported that CT-guided TNB is a relatively safe and accurate
method of diagnosing suspected thoracic lesions (1–4). The
general procedure of CT-guided TNB is as follows (3–10):
With CT guidance, a coaxial needle (consisting of an outer cannula
and an introducer stylet used for positioning the outer cannula) is
inserted through the skin until it reaches the thoracic lesion. The
introducer stylet is then removed while keeping the outer cannula
in position. A biopsy gun or needle is then passed through the
outer cannula for the sampling procedure. The precise insertion of
the coaxial needle into the target lesion is a crucial step for
avoiding injuries to important adjacent structures during CT-guided
TNB. The precise insertion requires the operator to be
exceptionally skillful. In 2003, the British Thoracic Society
recommended that operators retrospectively audit their own practice
(11). Tsai et al (5) further suggested a post-procedural
review of biopsy cases in order to check how closely the procedure
followed the plan of the operator. There are many factors involved
in CT-guided TNB auditing, including monitoring the adequacy of
biopsies, complications, pathological results and how closely the
procedure follows the plan of the operator (i.e., the precision of
coaxial needle placement, PCNP). However, to the best of our
knowledge, no index for assessing the PCNP has been reported.
In the present study, a set of self-designed
measurement protocols for PCNP were proposed and applied in a
CT-guided TNB audit of an interventional radiologist to determine
whether the PCNP was commensurate with the experience of the
operator.
Materials and methods
Concepts
Biopsy area
Prior to coaxial needle placement, chest CT images
were carefully reviewed in order to select an appropriate biopsy
area that avoided the necrotic region.
Most appropriate route
The most appropriate route of needle insertion was
determined prior to coaxial needle placement in order to lower the
probability of complications and to ensure that tissue sampling was
successful. An appropriate route leads towards the biopsy area
while avoiding bones, adjacent vital organs, large vessels or
bronchi, bullae and emphysematous areas.
Predefined sampling position
(PSP)
The PSP is the target position of the coaxial needle
tip at the end of the most appropriate route. The PSP was
identified in a transverse CT image (slice thickness, 1–2 mm) prior
to coaxial needle placement. For patients with lesions close to or
adjoining a pleura, the distance between the PSP and the visceral
pleura should not be <0.5 cm. This separation ensures that the
occurrence of pneumothorax is avoided following withdrawal of the
introducer stylet. The PSP is identified using anatomical features,
including spiculation and lobulation of a lesion edge, and
calcification and cavitation within a lesion and in adjacent
bronchi or vessels.
Repeat coaxial needle placement
(RCNP)
When the position of the coaxial needle tip was
incorrect, the needle was withdrawn from the thoracic wall and then
inserted again to the same PSP.
PCNP measurement protocols
The CT images of the coaxial needle placement were
transferred to a Siemens workstation (Leonardo; Siemens Medical
Solutions, Erlangen, Germany). A multiplanar reformation (MPR)
software environment was used. When the left button on the
corresponding position of the PSP in the transverse CT image was
clicked, the origin of the coordinates automatically shifted to the
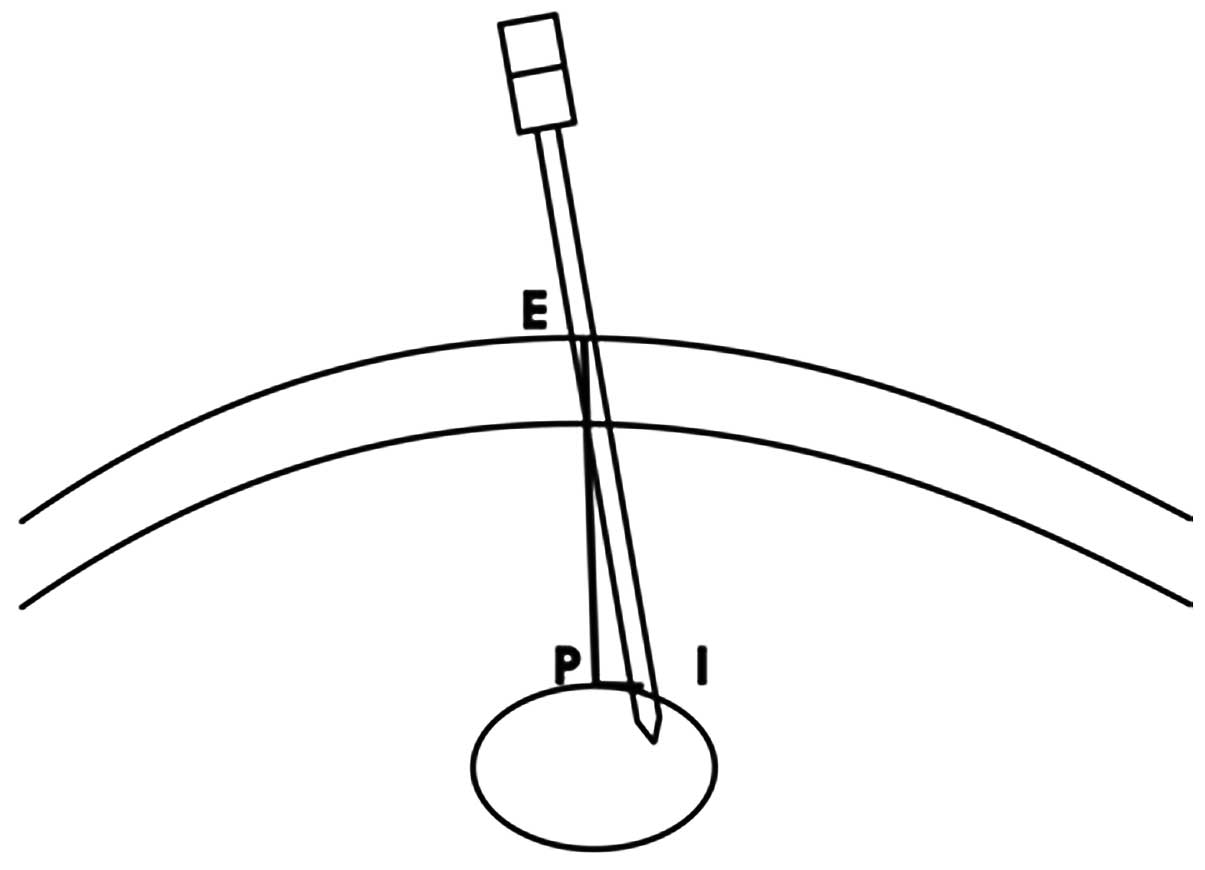
PSP position. The plane (Fig. 1)
containing the tip of the coaxial needle, the skin entry site and
the origin of coordinates (i.e., PSP) were defined by carefully
rotating the three axes while keeping the origin of the coordinates
in position. The PSP depth is the length of the line segment EP,
the coaxial needle deviation from PSP is the length of the line
segment PI (perpendicular to line segment EP) and the PCNP is the
ratio of the coaxial needle deviation from the PSP to the PSP depth
(i.e., PI/EP). The PCNP was classified into four grades as follows:
grade I, PCNP<0.1; grade II, 0.1≤PCNP<0.2; grade III,
0.2≤PCNP<0.3; and grade IV, PCNP≥0.3.
Subjects
Between January 2009 and December 2010, a staff
interventional radiologist (R.H.) was designated to carry out the
CT-guided TNB procedures in Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China).
During this period, a total of 102 patients (98 with lung lesions
and four with mediastinum lesions) agreed to receive a CT-guided
TNB. The indication for the procedure was determined by the
physician who referred the patient and the interventional
radiologist was required to agree with the procedure. The records
of these patients were extracted from the institutional electronic
archives and divided into two groups based on appointment date.
Group A consisted of the first 51 patients (33 male and 18 female;
mean age, 54.0 years; age range, 19–81 years), whereas group B
comprised the latter 51 patients (37 male and 14 female; mean age,
51.5 years; age range, 17–73 years). This study was conducted in
accordance with the Declaration of Helsinki and with approval from
the Ethics Committee of Tongji Medical College, Huazhong University
of Science and Technology. Written informed consent was obtained
from all participants prior to CT-guided TNB.
Guiding equipment
All procedures were performed under the guidance of
two multislice computed tomography (MSCT) imagers (Sensation 16 and
Somatom Definition AS+; Siemens Medical Solutions). Selection of
the guiding equipment was made according to routine arrangements.
The scan parameters were 100 or 120 kV; 25–180 mAsec (CARE Dose or
CARE Dose 4D technique); kernel B35 or B50; 512×512 matrix; 16×0.75
mm or 128×0.6 mm collimation; 1.0–2.0 mm slice thickness; 1.2
pitch; and a lung window setting (center = −400, width = 1400) or a
mediastinum window setting (center = 0, width = 400).
Pre-procedural preparation
A coagulation screening, which consists of
prothrombin time, activated partial thromboplastin time and
platelet count, was performed before all procedures. Any
anticoagulants or platelet inhibitors, such as aspirin, were
withheld. All available thoracic images were reviewed by the
operator prior to the biopsy procedure. The patients were
instructed to refrain from moving, coughing or breathing deeply
during the procedure. The importance of the patient holding their
breath following the instructions of the operator at the end of a
normal inhalation was explained to each patient. The technique was
practiced before the procedure until the operator was
satisfied.
Biopsy procedures
The patients fasted for at least 4 h prior to the
procedure. The procedure was performed with the patient in a prone,
supine or lateral decubitus position, depending on the location of
the lesion. The biopsy procedure was standardized. A skin marker (a
radiopaque thin stick or grid) was placed on the body surface
corresponding to the target lesion. Preliminary unenhanced MSCT
images (slice thickness, 1–2 mm) were obtained through the thoracic
lesion when the patient held their breath at the end of a normal
inhalation. The biopsy area and the most appropriate route, which
included the skin entry site and PSP, were identified in the
transverse CT images prior to coaxial needle placement. The patient
was advanced into the gantry again and stopped at the skin entry
site. The selected skin entry site was marked using the skin marker
and the integrated laser beam. The skin entry site was then
prepared and draped in a sterile manner. A local anesthetic was
injected through the skin entry site into the level of the parietal
pleura. The anesthetic needle was kept in an extrapulmonary
position and a CT-scan was then performed to verify if the position
of the skin entry site was correct. The anesthetic needle was then
replaced with a 17-gauge coaxial needle (TruGuide; Bard Biopsy
Systems, Tempe, Arizona, USA). In between breaths held by the
patient at the end of a normal inhalation phase, the coaxial needle
was gradually advanced to the PSP. The needle position was checked
several times during the procedure by using intermittent CT
guidance (slice thickness, 1–2 mm). If the position of the coaxial
needle tip was confirmed to be correct, a sampling of the lesion
was performed at least once using a matching 18-gauge biopsy gun
(Max-Core, Bard Biopsy Systems). If the position of the coaxial
needle tip was confirmed to be incorrect even after manipulation,
an RCNP was then performed. The specimens were fixed in 10%
formalin for pathological evaluation. Sections of the specimens
were placed in a normal saline solution for microbiological
examination to check for inflammatory disease. The procedure was
successfully carried out in all cases.
Post-procedure imaging and care
Approximately 10 min after the coaxial needle was
withdrawn, an unenhanced MSCT scan (slice thickness, 10 mm) was
performed on the whole thorax to check for immediate pneumothorax
and hemorrhage. The patients were placed in a puncture-side-down
position and then monitored in a respiratory ward for at least 24
h. If a small, asymptomatic, immediate pneumothorax developed, the
patient was conservatively treated by the administration of
supplemental oxygen. A follow-up chest radiography was performed to
evaluate the stability of the pneumothorax. Patients whose
pneumothorax was worsening or was accompanied by symptoms, such as
respiratory distress, shortness of breath, pain and decreased
oxygen saturation, received chest tube insertions. Patients with
hemoptysis, lung hemorrhage and hemothorax received hemostasis.
Patients without complications or with stable mild complications
were discharged after 24 h of observation. The discharged patients
were instructed to return to the nearest emergency department if
symptoms, including substantial pain and shortness of breath,
developed following dismissal.
Data collection and statistical
analyses
Following biopsy, all CT images that included
information on thoracic lesions, skin entry site, PSP, actual
needle trajectory, RCNP and post-procedural thorax CT scan were
immediately recorded on a compact disc. Thoracic lesion reviews and
PCNP measurements were performed by three staff radiologists
(N.C.J., H.H.L. and Y.H.W. who had seven, four and three years
experience, respectively). Data concerning lesion location (defined
as the location of the lesion center), size (defined as the maximal
axial diameter of the lesion), shape (nodules or tumors, exudation
or consolidation, cavity) and PSP depth (distance from the skin
entry site to PSP) were collected. The PCNP measurement and the
PCNP grading gained after the first needle placement were
determined according to the previously described protocols. Lesion
location and shape were reviewed by the three radiologists and any
differences of opinion were resolved by consensus. The lesion size,
PSP depth and PCNP were independently measured by the three
radiologists without knowledge of the groupings and results.
A thoracic lesion comparison between the two groups
was performed using a Pearson’s Chi-square test. The PCNPs of
groups A and B were compared using a two-tailed Student’s t-test.
The first PCNP grading comparison between the two groups was
performed using a Pearson’s Chi-square test. These tests were
performed using the Statistical Product and Service Solutions
software (version 16.0, SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
The comparison of the thoracic lesions between the
two groups is summarized in Table
I. No significant differences were identified in the lesion
location, size or shape. The number of patients with a PSP depth of
<4 cm was significantly smaller in group B than in group A
(P=0.426).
 | Table I.Comparison of thoracic lesions between
two groups. |
Table I.
Comparison of thoracic lesions between
two groups.
| Variable | Group A | Group B | P-valuea |
|---|
| Lesion location | | | |
| Right upper
lobe | 13 (25.5) | 15 (29.4) | 0.657 |
| Right middle
lobe | 4 (7.8) | 4 (7.8) | 1.000b |
| Right lower
lobe | 8 (15.7) | 11 (21.6) | 0.445 |
| Left upper
lobe | 13 (25.5) | 12 (23.5) | 0.818 |
| Left lower
lobe | 11 (21.6) | 7 (13.7) | 0.299 |
| Mediastinum | 2 (3.9) | 2 (3.9) | 1.000b |
| Lesion size | | | |
| <3 cm | 10 (19.6) | 8 (15.7) | 0.603 |
| 3–6 cm | 22 (43.1) | 20 (39.2) | 0.687 |
| >6 cm | 19 (37.3) | 23 (45.1) | 0.421 |
| Lesion shape | | | |
| Nodules or
tumors | 43 (84.3) | 44 (86.3) | 0.78 |
| Exudation or
consolidation | 5 (9.8) | 2 (3.9) | 0.433b |
| Cavity | 3 (5.9) | 5 (9.8) | 0.713b |
| PSP depth | | | |
| <4 cm | 25 (49.0) | 15 (29.4) | 0.043 |
| 4–6 cm | 21 (41.2) | 25 (49.0) | 0.426 |
| >6 cm | 5 (9.8) | 11 (21.6) | 0.102 |
The first PCNP was 0.19±0.12 in group A and
0.12±0.10 in group B. The difference in the first PCNP between the
two groups was identified to be statistically significant (P=0.003,
two-tailed). The comparison of the first PCNP grading between the
two groups is summarized in Table
II. The number of patients with grade I PCNP in group B was
significantly higher than that in group A (P<0.05). The number
of patients with grade III PCNP in group B was significantly lower
than that in group A (P<0.05).
 | Table II.Comparison of the first PCNP grading
between the two groups. |
Table II.
Comparison of the first PCNP grading
between the two groups.
| PCNP grading | Group A | Group B | P-valuea |
|---|
| I | 11 (21.6) | 26 (51.0) | 0.002 |
| II | 19 (37.3) | 15 (29.4) | 0.401 |
| III | 15 (29.4) | 6 (11.8) | 0.028 |
| IV | 6 (11.8) | 4 (7.8) | 0.505 |
In group A, five patients underwent one RCNP. An
improvement in the PNCP was observed in all five patients; however,
only four patients had an increase in the PCNP grade (two patients
from grade II to I, one patient from grade III to I and one patient
from grade IV to III) and one patient remained in the same PCNP
grade. In group B, three patients underwent one RCNP. An
improvement in the PNCP was observed in all three patients, but
only two patients had an increase in the PCNP grade (one patient
from grade III to I and one patient from grade II to I) and one
patient remained in the same PCNP grade. A patient in group B
underwent four RCNPs (the patient had a target pulmonary nodule
that had a diameter of 0.7 cm in the right lower lobe); although
the first PCNP grade was I, the nodule was not reached until the
fourth RCNP was performed.
Discussion
The precise placement of the coaxial needle in the
target lesion area is a crucial step in CT-guided TNB for a number
of reasons. Firstly, given that CT screening is widely used for
lung cancer (12–15), it is important to analyze
unidentified small pulmonary nodules found at an early stage. This
analysis is usually done by sampling via precise placement of the
coaxial needle. Secondly, large pulmonary neoplasms often have
necrotic areas. The cellular components of specimens obtained in
these areas have little diagnostic significance; therefore, the use
of these specimens is not suitable for cytological and pathological
evaluations (16). In the present
study, the operator carefully studied the CT images of the target
lesions and then predetermined the biopsy area suitable for
sampling prior to each CT-guided TNB. Thirdly, the biopsy procedure
may injure pleura, adjacent vital organs, large vessels and
bronchi, and may cause complications, including pneumothorax,
hemothorax, hemoptysis, lung hemorrhage and air embolism (1,2,17–20).
On rare occasions, the procedure may even result in
life-threatening complications (18–20).
The precise placement of the coaxial needle helps to minimize
damage and complications.
PCNP measurement is difficult to evaluate due to
multiple affecting factors, including the lesion size and depth,
the experience of the operator, guiding techniques, the choice of
puncture technique, the type of needle used and the level of
cooperation of the patient. Among these factors, the guiding
techniques, choice of puncture technique and type of needle used
are stable factors in a number of institutes. The maximum
cooperation of a patient may also be achieved via a preprocedural
drill. Therefore, the lesion size and location and the experience
of the operator are considered as the main factors that affect the
PCNP measurement. According to numerous operators, the coaxial
needle placement is more difficult when the thoracic lesion is
deeper or smaller. The PCNP measurement protocols in this study
minimize the effects of thoracic lesion size and depth during
coaxial needle placement. By defining the PCNP as the ratio of the
coaxial needle deviation to the PSP depth, the impact of lesion
depth was reduced to a minimum. By defining the PSP at the end of
the most appropriate route as the target point of the coaxial
needle placement, the impact of lesion size was also significantly
decreased. The three radiologists involved in this study all stated
that the protocols were easy to follow.
The results demonstrated that a better PCNP was
obtained in group B than in group A. This indicates that the PCNP
increases as the experience of the operator increases. The results
were consistent with our expectations.
This set of protocols has mainly been used for
assessing the PCNP of the operator. However, these PCNP measurement
protocols are intended to have further potential uses. New
technologies and methods of CT-guided TNBs are emerging. Thus, the
PCNP measurement protocols may be used to assess objectively if
these new technologies and methods are able to help to improve the
PCNP of the operator.
Although a Siemens image workstation and MPR
software were used in this study, any image workstation or MPR
software would fulfill the demand of the protocols as long as the
origin of the coordinates remains in position while the three axes
are rotated.
The PCNP measurement protocols do have limitations.
For example, the PSP is identified using anatomical features and
inaccuracies are inevitable. The optimal method of avoiding
inaccuracies is for the operator to study the CT images carefully
in advance.
References
|
1.
|
Tomiyama N, Yasuhara Y, Nakajima Y, et al:
CT-guided needle biopsy of lung lesions: a survey of severe
complication based on 9783 biopsies in Japan. Eur J Radiol.
59:60–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Richardson CM, Pointon KS, Manhire AR and
Macfarlane JT: Percutaneous lung biopsies: a survey of UK practice
based on 5444 biopsies. Br J Radiol. 75:731–735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tsukada H, Satou T, Iwashima A and Souma
T: Diagnostic accuracy of CT-guided automated needle biopsy of lung
nodules. AJR Am J Roentgenol. 175:239–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim TJ, Lee JH, Lee CT, et al: Diagnostic
accuracy of CT-guided core biopsy of ground-glass opacity pulmonary
lesions. AJR Am J Roentgenol. 190:234–239. 2008. View Article : Google Scholar
|
|
5.
|
Tsai IC, Tsai WL, Chen MC, et al:
CT-guided core biopsy of lung lesions: a primer. AJR Am J
Roentgenol. 193:1228–1235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
de Bazelaire C, Farges C, Mathieu O, et
al: Blunt-tip coaxial introducer: a revisited tool for difficult
CT-guided biopsy in the chest and abdomen. AJR Am J Roentgenol.
193:W144–W148. 2009.PubMed/NCBI
|
|
7.
|
Wallace MJ, Krishnamurthy S, Broemeling
LD, et al: CT-guided percutaneous fine-needle aspiration biopsy of
small (≤1-cm) pulmonary lesions. Radiology. 225:823–828. 2002.
|
|
8.
|
Loubeyre P, Copercini M and Dietrich PY:
Percutaneous CT-guided multisampling core needle biopsy of thoracic
lesions. AJR Am J Roentgenol. 185:1294–1298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Billich C, Muche R, Brenner G, et al:
CT-guided lung biopsy: incidence of pneumothorax after instillation
of NaCl into the biopsy track. Eur Radiol. 18:1146–1152. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Singh AK, Leeman J, Shankar S and Ferrucci
JT: Core biopsy with curved needle technique. AJR Am J Roentgenol.
191:1745–1750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Manhire A, Charig M, Clelland C, et al:
Guidelines for radiologically guided lung biopsy. Thorax.
58:920–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Henschke CI, Yankelevitz DF, Naidich DP,
et al: CT screening for lung cancer: suspiciousness of nodules
according to size on baseline scans. Radiology. 231:164–168. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lindell RM, Hartman TE, Swensen SJ, et al:
Lung cancer screening experience: a retrospective review of PET in
22 non-small cell lung carcinomas detected on screening chest CT in
a high-risk population. AJR Am J Roentgenol. 185:126–131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wagnetz U, Menezes RJ, Boerner S, et al:
CT Screening for lung cancer: implication of lung biopsy
recommendations. AJR Am J Roentgenol. 198:351–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
New York Early Lung Cancer Action Project
Investigators: CT screening for lung cancer: diagnoses resulting
from the New York Early Lung Cancer Action Project. Radiology.
243:239–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pinstein ML, Scott RL and Salazar J:
Avoidance of negative percutaneous lung biopsy using
contrast-enhanced CT. AJR Am J Roentgenol. 140:265–267. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hiraki T, Fujiwara H, Sakurai J, et al:
Nonfatal systemic air embolism complicating percutaneous CT-guided
transthoracic needle biopsy: four cases from a single institution.
Chest. 132:684–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mokhlesi B, Ansaarie I, Bader M, Tareen M
and Boatman J: Coronary artery air embolism complicating a
CT-guided transthoracic needle biopsy of the lung. Chest.
121:993–996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Glassberg RM and Sussman SK:
Life-threatening hemorrhage due to percutaneous transthoracic
intervention: importance of the internal mammary artery. AJR Am J
Roentgenol. 154:47–49. 1990. View Article : Google Scholar
|
|
20.
|
Yamaura H, Inaba Y, Aral Y, Matsueda K and
Hatooka S: Massive intrathoracic haemorrhage after CT-guided lung
biopsy. Br J Radio. 73:1105–1107. 2000. View Article : Google Scholar : PubMed/NCBI
|