Introduction
Fungal keratitis is a serious ocular disease leading
to blindness that frequently occurs among the agricultural
population of developing countries, such as China and India. In
recent years, the incidence of the disease has tended to increase,
and it has become the most common infectious corneal disorder in
certain regions (1–5). Earlier and accurate diagnosis of the
fungal infection in the cornea is crucial for effective treatment
and the prevention of blindness. However, the diagnosis of fungal
keratitis remains dependent upon the laboratory examination. At
present, the diagnosis is dominated by smear cytology and fungal
cultures (6–8). The ‘gold standards’ for the diagnosis
of fungal keratitis are fungal culture and strain identification
(5); however, these are lengthy
processes, usually taking 2–21 days, and making a correct diagnosis
often delays clinical treatment (9–11).
The traditional potassium hydroxide (KOH)-based smear shows a low
positive rate in the diagnosis of fungal keratitis, and thus a high
rate of misdiagnosis. The rapid and accurate diagnosis of fungal
keratitis remains a focus of current investigations (8). To the best of our knowledge, there
have been no previous studies regarding the use of a
methylthioninium chloride (MC) stain for the diagnosis of corneal
fungal keratitis. We suggested the concept based on the ability of
MC to detect gonococcus, thereby enabling the diagnosis and
treatment of gonorrhea (12), and
the fact that an injection of MC in early-stage breast cancer
enables the timely detection of metastasized cancer cells in
sentinel lymph nodes. With regard to its use in cancer, the MC
stain is sprayed directly under the endoscope to detect early
cancer cells, in addition to diagnosing pre-cancerous pathological
changes and early gastric cancer. However, MC has not been used for
the detection of keratitis infected by fungi. The aim of the
current study was to investigate the efficacy of MC staining for
the diagnosis of fungal keratitis. A total of 70 cases of fungal
keratitis were analyzed by MC staining and the positive rates,
sensitivity and specificity were compared with those of a 10%
KOH-based smear.
Materials and methods
Patients
Seventy patients diagnosed clinically with fungal
keratitis were admitted to The First Affiliated Hospital of Henan
University of Science and Technology (Luoyang, China) between
January 2009 and December 2010. The patients included 54 males and
16 females, with an average age of 52.2 years (range, 5–83 years).
The involved eye was OD (right) in 38 cases and OS (left) in 32
cases. The occupation of the patients was ‘peasant’ in 47 cases
(67.1%), ‘worker’ in 14 cases (20%) and ‘other’ in nine cases
(12.9%). The majority of the patients (53 cases, 75.7%) had a
history of ocular trauma; among these 53 cases, 35 (66%) cases were
injured by plants and other such foreign bodies, such as grains,
grass, pieces of wood, bamboo and leaves. In addition, 11 (15.7%)
cases had soil contamination, three cases were injured by welding
sparks and four cases were due to other causes. Seven patients had
a medical history of systemic administration of glucocorticoids and
immunosuppressive agents for systemic disorders. This study was
conducted in accordance with the Declaration of Helsinki and with
approval from the Ethics Committee of the Henan University of
Science and Technology. Written informed consent was obtained from
all participants.
Sample collection
The affected eyes were treated with the topical
anesthetic Benoxil (Invitrogen Life Technologies Carlsbad, CA,
USA), and the skin around the eye was sterilized with 75% alcohol.
The eye was opened with eyelid retractors. The surface, peripheral
parts and basal substance of the corneal ulcer were scraped with a
sterilized lancet and collected as smears for MC staining
(Invitrogen) and KOH-based smears, in order to detect the fungus.
The specimens were also sent for culture of the fungus.
Detection of the fungus by KOH-based
smears
The specimens scraped from the corneal ulcer were
removed and spread on slides to form as thin a layer as possible.
One drop of 10% KOH was added to each slide, and a coverglass was
used to cover the slide for 10 min in order to remove impurities.
Following this, a low-power microscope was initially used to locate
the species position, prior to a high-power microscope being used
to detect the hyphae and spores.
Detection of the fungus by MC
staining
The specimens scraped from the corneal ulcer were
removed and spread on slides. MC (20 μl) was added to each
slide and a coverglass was used to cover the slide for 5 min for
staining. Following this, a low-power microscope was initially used
to locate the species position, prior to a high-power microscope
later being used to detect the hyphae and spores.
Fungal cultivation
An appropriate quantity of ulcer extract was
collected and then inoculated in a tube of Sabouraud dextrose agar
(Gibco Co., Gaithersburg, MD, USA) at 25°C for 3–21 days. The
cultures were observed at certain intervals for fungal growth. The
strains of fungi were identified according to the appearance of the
colony, growth rate and the morphological characteristics of the
hyphae, spores or bacteria.
Data collection and statistical
analysis
The result of the fungal cultivation was used as the
‘gold standard’ for the diagnosis of fungal keratitis. The results
from the MC staining and KOH-based smears were analyzed to
determine the positive rate, sensitivity, specificity, false
positive rate, false negative rate, accuracy index, positive
predictive value and negative predictive value for the fungi.
Analyses were performed using SPSS for Windows
version 10.0 (SPSS, Inc., Chicago, IL, USA), with tests including
the Wilcoxon non-parametric rank sum test and the Spearman rank
related test. P<0.05 was considered to indicate a statistically
significant difference.
Results
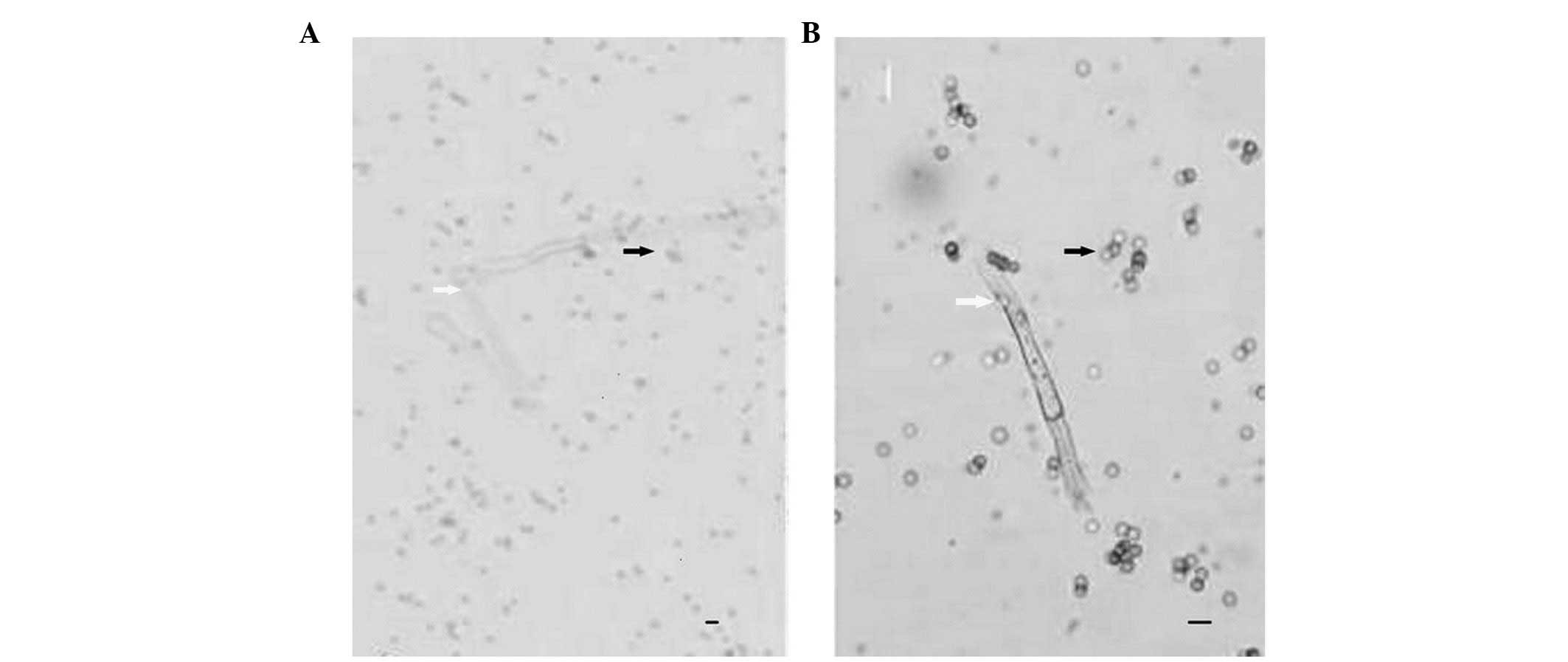
The fungal hyphae in the KOH-based smears were
visible as transparent streak-structures, which were poorly
contrasted against the background (Fig. 1A). The fungal hyphae observed using
MC staining were blue tubular structures, which were strongly
contrasted against the background (Fig. 1B). Among the 70 cases clinically
diagnosed with fungal keratitis, 58 cases were shown by fungal
culture and strain identification to have fungi, and the positive
rate was 82.86%. The positive rate obtained with the MC staining
was 62.86%, with a sensitivity of 70.69% and a specificity of
34.61%. The false positive and negative rates were 25.00 and
29.31%, respectively, the accuracy index was 5.30% and the positive
and negative predictive values were 93.18 and 34.61%, respectively
(Table I). The positive rate
obtained with the KOH-based smears was 44.29%, the sensitivity was
44.83% and the specificity was 17.95% (P<0.05). The false
positive and negative rates were 41.67 and 55.17%, respectively,
the accuracy index was −37.22% and the positive and negative
predictive values were 83.87 and 17.95%, respectively (Table II).
 | Table I.Comparison between fungal detective
rates of fungal culture and methylthioninium chloride (MC)
staining. |
Table I.
Comparison between fungal detective
rates of fungal culture and methylthioninium chloride (MC)
staining.
| MC staining | Fungal culture
| Total |
|---|
| Positive | Negative |
|---|
| Positive | 41 | 3 | 44 |
| Negative | 17 | 9 | 26 |
| Total | 58 | 12 | 70 |
 | Table II.Comparison between fungal detective
rates of fungal culture and potassium hydroxide (KOH) staining. |
Table II.
Comparison between fungal detective
rates of fungal culture and potassium hydroxide (KOH) staining.
| KOH-based smears | Fungal culture
| Total |
|---|
| Positive | Negative |
|---|
| Positive | 26 | 5 | 31 |
| Negative | 32 | 7 | 39 |
| Total | 58 | 12 | 70 |
Discussion
Fungal keratitis is a serious blindness-causing
keratopathy that occurs as a result of a disease-causing fungus. It
is difficult to diagnose in the clinic and is easily misdiagnosed,
resulting in blindness due to improper treatment. Under normal
circumstances, the fungus does not invade the healthy cornea.
However, under certain conditions, such as when ocular trauma
occurs or surgery is performed, non-disease-causing fungi may
become pathological, giving rise to a corneal secondary fungal
infection. Pathological infection may also occur when antibiotics,
corticosteroids or immunosuppressants are administered over a long
period, when the resistance of the body is reduced or when
keratitis or dry-eye symptoms occur. It may also arise due to the
cornea becoming infected by fungi or fungal crops, such as cereals
and dry grasses, or as a result of wheat stubble injuries. The most
common type of disease-causing organism is Aspergillus,
followed by Fusarium toxin, Candida albicans,
Bacillus and Streptothrix (13,14).
Due to the fact that disease-causing strains are different, the
corneal ulcer morphology varies.
In recent years, the incidence of fungal keratitis
has increased annually and the infection has become an increasingly
serious problem. Therefore, early diagnosis and treatment have a
decisive effect on the prognosis of the disease, particularly with
regard to eyesight. A preliminary diagnosis may be made according
to a history of infection following corneal injury by plants, and
in combination with the characteristics of corneal focus. However,
the accurate diagnosis of fungal keratitis continues to depend upon
the laboratory examination (8).
Gram and Giemsa staining of corneal scraping specimens, and
hematoxylin and eosin staining are commonly used methods at the
early stage of diagnosis, but these stainings lack sufficient
efficacy. The fungal culture may involve the administration of a
blood agar base or a chocolate medium. If corneal smears and fungal
cultures are all negative, despite fungal keratitis being highly
suspected in the clinic, a corneal tissue biopsy may be taken into
account; however, these methods are all lengthy procedures, which
delay the diagnosis. In addition to immunofluorescence staining,
confocal laser microscopy and polymerase chain reaction (PCR)
technology represent a better prospect for application in the field
of fungal keratitis diagnosis, although these methods are
expensive, which reduces their popularity and use (15–17).
When concerned with medical disputes and legal disputes, it is
usually possible to reach an accurate conclusion through
pathological diagnosis, and, therefore, pathological diagnosis
represents the final verdict of diagnosis (17). The early, rapid and effective
diagnosis of fungal keratitis is a focus of current studies
(1,8).
MC is widely used in clinics as a chemical
indicator, a dye, a biological staining agent and a biological
antidote. MC is used to stain various organs and has such
advantages as few side-effects, a low cost, a large range of doses
and a high accuracy rate. Therefore, it is suitable for wider
popularization. MC has numerous differences from other dyes,
including its ability to stain finer lymphatic vessels, its clear
blue staining of sentinel lymph nodes (SLN), its relatively low
molecular weight and its rapid excretion rate. Therefore, it is
used more skillfully, and rapidly (18). It has been demonstrated that an
overdose of MC has certain harmful effects on tissues and brings
about tissue necrosis. However, in the present study, MC was
promptly removed following its use for staining and, therefore,
there were no side-effects. Thus, MC was used successfully as a dye
in this group of cases. MC is a blue liquid, which colors hyphae
and spores purple, thereby enhancing their contrast against the
background and enabling them to be detected easily. This improves
the sensitivity, positive rate and the positive predictive value of
the diagnostic experiment. Hyphae stained with MC may be easily
distinguished from corneal fibers and impurities.
In the present study, we demonstrated that the
sensitivity, specificity, positive rate, accuracy index and
positive and negative predictive values of the fungi detected by MC
staining were higher than those obtained using KOH-based smears.
The false positive and negative rates for the fungi obtained using
MC staining were lower than those obtained using KOH-based smears.
The authenticity of the diagnostic experiment includes its
sensitivity and specificity. The sensitivity, i.e., true positive
rate, means that the diagnostic experiment accurately identifies
the true non-keratitis cases when the disease is not present. The
higher the sensitivity and specificity, the higher the accuracy
index and the authenticity of the diagnostic experiment. It was
demonstrated in this study that the sensitivity, specificity and
accuracy index of the detection of fungi by MC staining were higher
than those by KOH-based smears, suggesting that the MC staining had
a higher authenticity in the detection of fungi. The positive
predictive value refers to the proportion of true positive results
among the positive cases in the diagnostic experiment, while the
negative predictive value refers to the proportion of true negative
results among the negative cases in the experiment. Investigations
into the level of disease rate in the population have a great
impact on the positive predictive value of a diagnostic experiment.
The population in the present study included patients clinically
diagnosed with fungal keratitis, i.e., belonging to a high-risk
population, and, therefore, the positive predictive value of the
diagnostic experiment was relatively high. The MC staining was
demonstrated to have a higher positive predictive value than that
of the KOH-based smears, reaching 93.18%. This indicated that the
MC staining had a greater chance of detecting the individuals
positive for fungi among the cases with fungal keratitis, enabling
the guidance of early clinical treatment. The false positive rate
is the rate at which the diagnostic experiment wrongly judges the
true non-keratitis cases to be cases with the disease, i.e., the
misdiagnosis rate. The false negative rate is the rate at which the
diagnostic experiment wrongly judges the true keratitis cases to be
cases without the disease, i.e., the rate of missed diagnosis. It
was demonstrated in this study that the false positive and negative
rates for fungi were lower with MC staining than with KOH-based
smears, indicating that the rate of misdiagnosis and the rate of
missed diagnosis by MC staining were lower, respectively.
Several factors affecting the detectable rate of
fungal smears were considered, one of which was species collection.
When fewer fungi exist in the smears, the detectable rate by MC
staining decreases (5). Fungal
growth is most often located in the peripheral ulcer sites and the
bottom parts of the ulcer, and the sticky white substance in the
center of the ulcer is often the hyphal plexus. There are
relatively few corneal species. Therefore, the sample containing
the white sticky substance in center of ulcer was collected, in
order to improve the detection rate of fungal smears. The other
factor was over-thickness of the smears. When the smears are
over-thick, overlapping of the species causes microscopic
evaluation to become more challenging; therefore, the smear species
should be dispersed as much as possible in the staining liquid, and
if necessary, pressure should be applied to the glass coverslip, in
order to disperse the mass-like substances.
The prognosis of fungal keratitis, with regard to
eyesight, is poor. Only in cases where there is a small corneal
focus that is diagnosed in a timely manner and effectively treated
is the restoration of sight certain. An increase in the severity of
the condition may endanger the eyeball and eventually lead to
blindness (8). The results of the
present study showed that the outcome of cultivation was the ‘gold
standard’ for confirming the diagnosis of fungal keratitis. Among
the 70 cases with fungal keratitis, 58 cases were observed to
possess fungi, and the positive rate was 82.86%. The positive rate
obtained with MC staining was 62.86%, which indicated that the MC
staining had a lower positive rate than cultivation. However, MC
staining, with its low cost, rapid detection of fungi (within a few
minutes), high sensitivity and higher relative positive predictable
value, remains a simple, rapid and effective technique for the
early diagnosis of fungal keratitis. The present study has
conducted preliminarily investigations of the technique using
morphological characterization; however, further investigations
involving molecular biology are required.
References
|
1.
|
Jurkunas U, Behlau I and Colby K: Fungal
keratitis: changing pathogens and risk factors. Cornea. 28:638–643.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sun GH, Li SX, Gao H, Zhang WB, Zhang MA
and Shi WY: Clinical observation of removal of the necrotic corneal
tissue combined with conjunctival flap covering surgery under the
guidance of the AS-OCT in treatment of fungal keratitis. Int J
Ophthalmol. 5:88–91. 2012.
|
|
3.
|
Pucket JD, Allbaugh RA and Rankin AJ:
Treatment of dematiaceous fungal keratitis in a dog. J Am Vet Med
Assoc. 240:1104–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kymionis GD, Kankariya VP and Kontadakis
GA: Combined treatment with flap amputation, phototherapeutic
keratectomy, and collagen crosslinking in severe intractable
post-LASIK atypical mycobacterial infection with corneal melt. J
Cataract Refract Surg. 38:713–715. 2012. View Article : Google Scholar
|
|
5.
|
Liu X, Zhao Y, Yang Y, et al:
Mycobacterium massiliense keratitis. Optom Vis Sci.
89:E944–E947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Das M, Murthy SI, Dikshit S and Rathi V:
Natamycin and voriconazole in fungal keratitis. Arch Ophthalmol.
129:8142011.PubMed/NCBI
|
|
7.
|
Das S, Sharma S, Kar S, Sahu SK, Samal B
and Mallick A: Is inclusion of Sabouraud dextrose agar essential
for the laboratory diagnosis of fungal keratitis? Indian J
Ophthalmol. 58:281–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Nayak N: Fungal infections of the eye -
laboratory diagnosis and treatment. Nepal Med Coll J. 10:48–63.
2008.PubMed/NCBI
|
|
9.
|
Bashir G, Shah A, Thokar MA, Rashid S and
Shakeel S: Bacterial and fungal profile of corneal ulcers - a
prospective study. Indian J Pathol Microbiol. 48:273–277.
2005.PubMed/NCBI
|
|
10.
|
Galperin G, Berra M, Tau J, Boscaro G,
Zarate J and Berra A: Treatment of fungal keratitis from
Fusarium infection by corneal cross-linking. Cornea.
31:176–180. 2012. View Article : Google Scholar
|
|
11.
|
Kurbanyan K, Hoesl LM, Schrems WA and
Hamrah P: Corneal nerve alterations in acute Acanthamoeba
and fungal keratitis: an in vivo confocal microscopy study. Eye
(Lond). 26:126–132. 2012.
|
|
12.
|
Mathelin C, Salvador S, Croce S,
Andriamisandratsoa N, Huss D and Guyonnet JL: Optimization of
sentinel lymph node biopsy in breast cancer using an operative
gamma camera. World J Surg Oncol. 5:1322007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pradhan L, Sharma S, Nalamada S, Sahu SK,
Das S and Garg P: Natamycin in the treatment of keratomycosis:
correlation of treatment outcome and in vitro susceptibility of
fungal isolates. Indian J Ophthalmol. 59:512–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Barequet IS, Bourla N, Pessach YN, et al:
Staphylolysin is an effective therapeutic agent for
Staphylococcus aureus experimental keratitis. Graefes Arch
Clin Exp Ophthalmol. 250:223–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Menassa N, Bosshard PP, Kaufmann C, Grimm
C, Auffarth GU and Thiel MA: Rapid detection of fungal keratitis
with DNA-stabilizing FTA filter paper. Invest Ophthalmol Vis Sci.
51:1905–1910. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Avunduk AM, Beuerman RW, Varnell ED and
Kaufman HE: Confocal microscopy of Aspergillus fumigatus
keratitis. Br J Ophthalmol. 87:409–410. 2003. View Article : Google Scholar
|
|
17.
|
Xie L, Li S, Shi W and Han D: Clinical
diagnosis of fungal keratitis by confocal microscopy. Zhonghua Yan
Ke Za Zhi. 35:7–9. 1999.(In Chinese).
|
|
18.
|
Zhang B, Bai Y and Chen G: Clincal
significance of sentinel lymph node biopsy in breast Cancer.
Zhonghua Zhong Liu Za Zhi. 22:395–397. 2000.(In Chinese).
|