Introduction
Traditional Chinese Medicine (TCM), such as
Xiao-Qing-Long-Tang (XQLT), is frequently used in Asia for the
clinical treatment of bronchial allergic asthma and allergic
rhinitis (1,2). It has beneficial effects in relieving
allergic inflammation reactions in the airways of allergic animal
models (3–6). XQLT is highly popular in TCM and
complementary medicine. The identification of potential
pharmacological targets of XQLT may provide a basis for evaluating
the effectiveness of XQLT in controlling allergic reactions.
Allergen-induced epithelial-origin nerve growth
factor (NGF) has been demonstrated to be involved in the early
phase of allergic reactions (7–11).
NGF, a member of the neurotrophin family, is a product of activated
Th2 cells (7,8) and is expressed in multiple cells,
including epithelial cells. In allergy, the tissue that is
primarily responsible for allergen presentation is the epithelium.
Epithelial cells present allergens and induce allergy pathways
involving numerous events, including dendritic cell activation and
chemokine secretion (12,13). Moreover, NGF has been observed at
an elevated concentration in patients with allergic diseases.
Toll-like receptor 4 (TLR4), the primary signal that is involved in
the early phase of allergic signal transduction (14), is activated by Dermatophagoides
pteronyssinus (Der p) allergen (15–17),
leading to epithelial thymic stromal lymphopoietin (TSLP)
expression (18). TSLP has been
shown to have a central function in the response to
allergen-stimulated TLR4 signals in lung epithelial cells (19–21).
The TLRs form a large protein family that are apparent in innate
immunity and are responsible for presenting antigens or allergens,
such as lipopolysaccharide (LPS) or Der p (22,23).
The signaling pathways under TLR4 are relatively complicated. The
major factors responsible for TLR4 signal transduction are MyD88
and nuclear factor-κB (NF-κB) (24,25).
It has been demonstrated that a TLR4 signal may lead to the Ser536
phosphorylation of the p65/RelA subunit of NF-κB. Following
phosphorylation, NF-κB translocates into the nucleus to act as a
transcriptional factor.
TLR4 induces NGF and the low-affinity NGF receptor,
p75 neurotrophin receptor (p75NTR), in dendritic cells (DCs)
(26). Dermatophagoides
pteronyssinus group 2 (Der p 2) has been demonstrated to have a
structural homology with MD-2, the LPS-binding component of the
TLR4 complex (27,28). Moreover, a recent study by this
team demonstrated that Der p 2 induced NGF production and reactive
oxygen species in the airway, as well as allergic inflammation,
following direct intratracheal (i.t.) instillation into the lungs
of mice (27). The p75NTR is a
low-affinity receptor for all factors of the neurotrophin family
(29,30). Allergic inflammation and eosinophil
infiltration have been eliminated in p75NTR-knockout mice (29,30).
p75NTR is known for inducing NF-κB activation, which has been
demonstrated to be a major transcriptional factor in the Th2-type
immune response (31,32). NGF may also affect DCs through
p75NTR (33).
This study showed that the oral administration of
XQLT to mice reduced the levels of p75NTR and TLR4 expression in
the lungs of allergic mice. In a HPAEpiC cell line, adding
exogenous recombinant soluble TLR4 (sTLR4) or a rough strain
Salmonella LPS (Re 595) attenuated the XQLT-mediated suppression of
Der p 2-stimulated NGF, p75NTR and TSLP expression. Adding sTLR4
also attenuated the inhibitory effect of XQLT on the Ser536
phosphorylation of NF-κB. These results revealed that the
inhibitory effect of XQLT may be correlated with TLR4. The study
provides evidence useful in further investigations into the
pharmacological targets of XQLT in the treatment of allergic
reactions.
Material and methods
TCM preparation: XQLT
XQLT was obtained and prepared as previously
described (34). Briefly, eight
crude plant ingredients were mixed: Pinellia tuber (6.0 g, root of
Pinellia ternata Breitenbach), Ephedrae herba (3.0 g, stem
of Ephedra sinica Stapf), Schizandrae fructus (3.0 g, a
fruit of Schizandra chinensis Baill), Cinnamonomi cortex
(3.0 g, cortex of Cinnamomum cassia Blume), Paeoniae radix
(3.0 g, root of Paeonia lactiflora Pall.), Asariherba cum
radice (3.0 g, whole plant of Asiasarum heterotropoides F.
Maekawa var. mandshuricum F. Maekawa), Glycyrrhizae radix
(2.0 g, root of Glycyrrhiza uralensis Fisch. Et. DC) and
Zingiberis siccatum rhizoma (1.0 g, steamed root of Zingiber
officinale Roscoe). The mixture was extracted sequentially with
17.5 l and 12.5 l boiling water for 1 h each time, prior to the
extracted liquid being mixed and filtered. Following filtration,
the dregs of the decoction were removed. The filtered liquid was
subsequently lyophilized and crushed into a fine powder. The yield
of dried extract from the crude starting material was 661.8 g
(26.4%, w/w). The batch number was 98041021.
The XQLT mixture was suspended in distilled water at
a fixed concentration for oral administration to the mice, through
a feeding needle and swallowing. The feeding volume was adjusted
within 0.2–0.3 ml to avoid the mice experiencing any pain. For
in vitro use, the XQLT mixture was dissolved in distilled
water and the solution was subsequently centrifuged at 4,500 × g.
Following filtration, the aqueous extract was lyophilized and
weighed. This XQLT extract was then re-dissolved in pyrogen-free
isotonic saline (YF Chemical Corp., Taipei, Taiwan) and filtered
through a 0.2 mm filter (Microgen, Laguna Hills, CA, USA). A sample
of the filtered pyrogen-free solution was lyophilized and weighed,
prior to the final concentration of the filtered pyrogen-free
solution in the sample being estimated. The filtered pyrogen-free
solution was stored at −20°C as a stock solution.
Acute allergic mouse model
Specific pathogen-free, 6–8-week-old BALB/c mice
from the Laboratory Animal Center of the National Science Council
(Taiwan, R.O.C.) were used in this study. The mice were housed in
microisolator cages (Laboratory Products, Maywood, NJ, USA) and
provided with sterile food and water. The care and treatment of the
experimental animals followed the guidelines of the National
Science Council of the Republic of China. The protocol was approved
by the Institutional Animal Care and Use Committee (IACUC) of China
Medical University (Permit No. 101–159-N; Taichung, Taiwan). All
efforts were made to minimize suffering.
The establishment of the acute allergic mouse model
was based on a previous study (3)
and comprised two stages, the sensitization and the acute allergy
induction stages. In the sensitization stage, the mice were
administered with a subcutaneous injection at the base of the tail
of 50 ml Der p (1 mg/ml) emulsified in incomplete Freund’s adjuvant
(IFA; Difco Laboratories, Inc, Detroit, MI, USA); one further
boosting injection was administered with the same dose of Der p on
day seven. Fourteen days later, the acute allergy induction stage
was initiated. The mice were lightly anesthetized with an
intraperitoneal (i.p.) injection of 60 mg/kg body weight (BW)
sodium pentobarbital (Nembutal; Abbott Laboratories, Chicago, IL,
USA). Following this, an i.t. instillation of 50 ml Der p (2 mg/ml)
was administered to the mice as an allergen challenge (AC). Acute
Der p allergen-challenged mice, which comprised the DP group, were
fully established following this protocol. To evaluate the effects
of XQLT on this model, mice in the post-allergy curing protocol
group (PAC) were treated with 1 g/kg BW XQLT once, 24 h subsequent
to the AC. The mice in the pre-allergy treatment protocol group
(PAT) were treated with 1 g/kg BW XQLT every two days, from day two
until day 12, a total of six times, prior to the AC on day 14. Mice
in the control group (XQ) were treated with 1 g/kg BW XQLT every
two days, from day two until day 12, a total of six times, without
the AC on day 14. The naive mice group (NA) consisted of animals
without Der p sensitization, challenge or XQLT treatment. All the
mice were sacrificed on day 16. The XQLT dose was based on the
results of our previous investigation (3). Each group comprised six mice. The
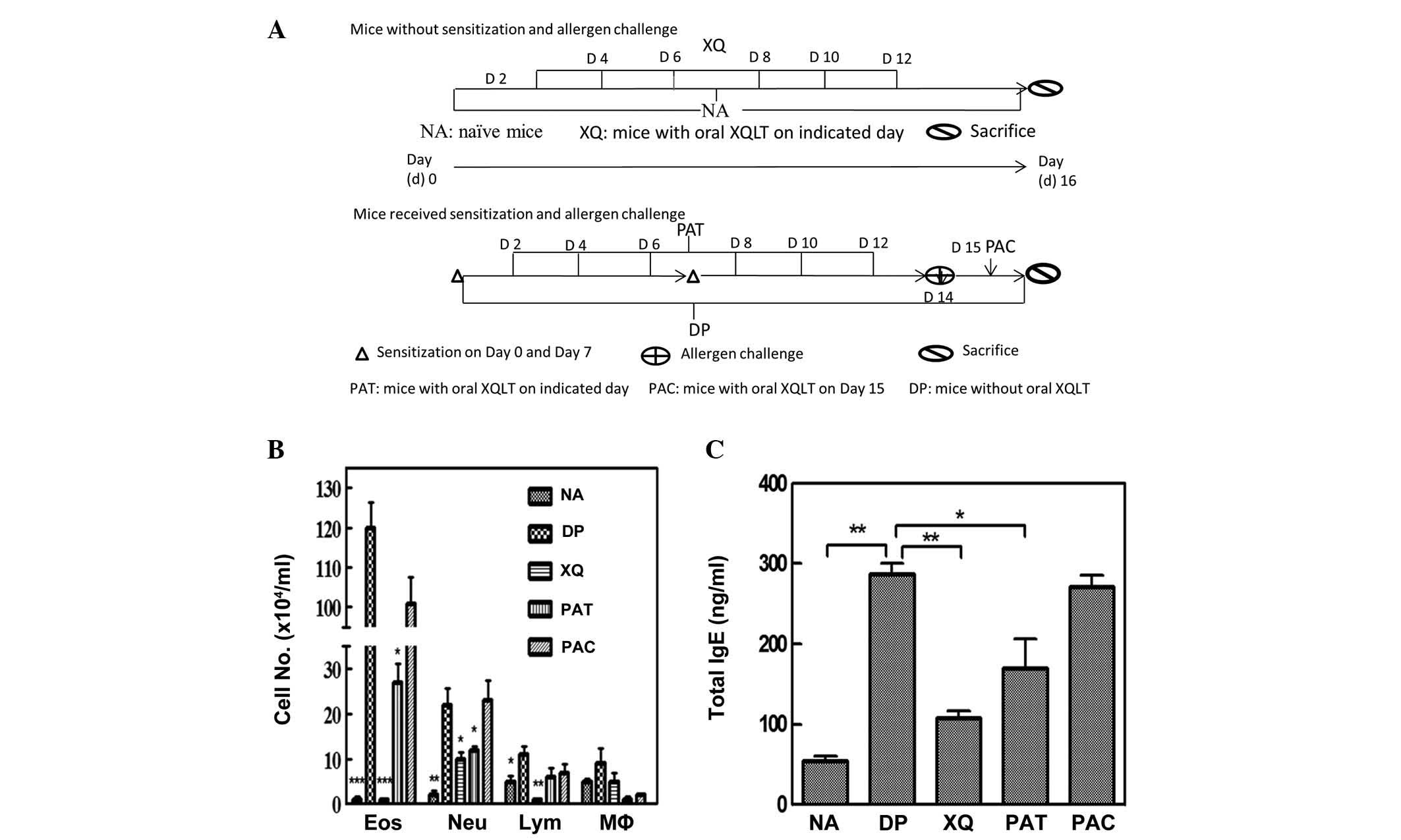
groups are schematically depicted in Fig. 1A.
Collection of BALF and serum
The mice were sacrificed by administering an
overdose of sodium pentobarbital (20 mg/ml) subsequent to the Der p
challenge. Following the sacrifice, BALF was collected by flushing
the lungs with two separate saline washes, through the trachea,
with ~1 ml BALF recovered each time. The BALF samples were
centrifuged at 200 × g for 5 min at 4°C to collect the cells. The
collected cells were subsequently washed with red blood cell lysis
solution and were diluted with RPMI-1640 medium (Gibco-BRL,
Invitrogen Life Technologies, Inc., Gaithersburg, MD, USA). The
total leukocyte content of the BALF was determined by cytometry to
be 1×105 cells/ml. Blood was collected either through
the axillary artery or directly from the heart. The collected blood
was left to stand for 1 h at room temperature to clot.
Centrifugation at 18,000 × g removed the clotted matter to leave
the serum.
Total immunoglobulin (Ig) E concentration
in the serum
The IgE concentration in the serum was measured
using an enzyme-linked immunosorbent assay (ELISA) kit (Mouse IgE
ELISA Quantitation Set, cat. no. E90–115; Bethyl Laboratories,
Inc., Montgomery, TX, USA), in accordance with the manufacturer’s
instructions. Absorbance was measured at a wavelength of 450 nm on
an ELISA plate reader.
Differential cell counting in the
BALF
BALF cells were spun down onto a glass slide at 360
rpm for 8 min by cytospinning. Then, the slides were dried and
stained by eosinophil-specific staining methods (Eosinophil-Mast
Cell Stain kit, CEM-1-IFU; ScyTek Laboratories, Inc., Logan, UT,
USA). A total of >200 cells were counted under a photomicroscope
to determine the numbers of lymphocytes, macrophages, neutrophils
and eosinophils, as percentages of total BALF cells.
Immunohistochemistry of p75NTR/TLR4 in
lung slices
The paraffin-embedded lung slices were mounted on
glass slides, and the paraffin was then depleted away at 60°C. The
slices were treated using a number of reagents in the following
sequence: xylene, ethanol, 3% H2O2 (80%
methanol) (v/v), and 0.01 M sodium citrate buffer (pH 6.0, 95°C).
Following this, 10% non-fat milk was used to block the cooled
slices, and rabbit polyclonal anti-p75NTR antibody (Abcam plc,
Cambridge, UK) or rabbit anti-TLR4 antibody (Abcam plc) was used
for immunostaining (4°C, overnight). Anti-rabbit IgG antibody
[fluorescein isothiocyanate (FITC) or phycoerythrin-conjugated;
Abcam plc] was used as a secondary antibody to develop
fluorescence. The developed slice was observed under a light
microscope and the area density of fluorescence was analyzed using
an inverted fluorescence microscope (Olympus IX71®;
Olympus, Center Valley, PA, USA) and VisionWorks®LS Analysis
Software (UVP, Upland, CA, USA). The results concerning
fluorescence density are presented as a bar graph.
NGF and TSLP production in human
pulmonary alveolar epithelial cells following Der p 2
stimulation
The human lung epithelial cell line, HPAEpiC,
comprising alveolar type I and type II epithelial cells, was
purchased from Sciencell Research Laboratories (Carlsbad, CA, USA).
HPAEpiC cells were cultured in Dulbecco’s modified Eagle medium
(DMEM) F12 medium (Gibco-BRL), containing 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin, in a lysine-coated flask in
an incubator at 37°C with 5% CO2.
Table I (XQLT
dose-response) and Table II (TLR4
competitive assay) show the experimental groups that were
established. The term ‘XQLT pulse 1 h’ referred to the cells being
treated with XQLT for 1 h, prior to the XQLT being washed out. Der
p 2 was then used to stimulate the cells pretreated with XQLT for
the following 24 h. Supernatants was collected for NGF and TSLP
ELISA analysis, respectively. Total protein was extracted from the
treated cells. The LPS source was Salmonella minnesota Re
595 (cat. no. L-9764; Sigma-Aldrich, St. Louis, MO, USA), a known
TLR4 ligand (35). The
commercially available sTLR4 was purchased from USCN®
Life Science Inc. (Houston, TX, USA). Prior to treatment with XQLT,
all cells underwent starvation (serum-free status) for ≥8 h.
Whenever the cells were treated with XQLT or Der p 2, 1% FBS was
added to the cell culture to maintain cell survival.
 | Table I.Xiao-Qing-Long-Tang (XQLT)
dose-response. |
Table I.
Xiao-Qing-Long-Tang (XQLT)
dose-response.
| Treatment | Group
|
|---|
| D | XP0.1 | XP0.5 | XP1 | N |
|---|
| Der p 2 (75
μg/mla) 24
h | + | + | + | + | - |
| XQLT pulse 1 h | - | 0.1 mg/ml | 0.5 mg/ml | 1 mg/ml | - |
 | Table II.Toll-like-receptor-4 (TLR4) competing
assay. |
Table II.
Toll-like-receptor-4 (TLR4) competing
assay.
| Treatment | Group
|
|---|
| N | D | XP | L100 | L200 | T1 | T2 |
|---|
| Der p 2a | - | + | + | + | + | + | + |
| XQLTb | - | - | + | + | + | + | + |
| Treatment combined
with XQLT | - | - | - | LPS
100 ng/ml | LPS
200 ng/ml | +sTLR4
1 μg/ml | +sTLR4
2 μg/ml |
ELISA was used to analyze the concentrations of NGF
and TSLP. The concentration of NGF in the BALF or cell culture
supernatant was measured using an ELISA kit (NGF Emax®
ImmunoAssay Systems; Promega Corporation, Madison, WI, USA), in
accordance with the manufacturer’s instructions. The concentration
of TSLP in the cell culture supernatant was also measured using an
ELISA kit (TSLP: Human TSLP ELISA Ready-SET-Go®;
eBioscience, Inc., San Diego, CA, USA), in accordance with the
manufacturer’s instructions.
Western blot analysis of the levels of
p75NTR and Ser536 phosphorylated p65 (NF-κB)
Cells (1–2 × 106 cells/ml) were lysed
using a Triton X-100-based lysis buffer that contained 1% Triton
X-100, 150 mM NaCl, 10 mM Tris (pH 7.5), 5 mM EDTA, 5 mM
NaN3, 10 mM NaF and 10 mM sodium pyrophosphate. Cell
extracts were separated using sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE), prior to being transferred to a
polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica,
MA, USA). Subsequent to blocking, the blots were developed using a
rabbit polyclonal anti-p75NTR antibody (cat. no. ab8874, Abcam plc)
or rabbit polyclonal anti-phospho-NF-κB p65 (Ser536) antibody (cat.
no. 3031; Cell Signaling Technology, Inc., Danvers, MA, USA). The
blots were subsequently hybridized using horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (Calbiochem, San Diego, CA,
USA) and developed with a chemiluminescence kit (Western Lightning™
Chemiluminescence Reagent Plus; PerkinElmer Life Sciences Inc.,
Boston, MA, USA). The optical density that corresponded with the
p75NTR or Ser536 phosphorylated p65 to total protein ratio was
determined using an image analysis system.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical comparisons were performed using the
Student’s t-test, with P<0.05 or P<0.01 considered to
indicate a statistically significant difference. All statistics are
indicated in the figure legends.
Results
Differential cell counting in BALF and
total IgE production demonstrate that XQLT inhibits allergic
inflammation
The effect of XQLT on allergic inflammation was
studied in an established acute Der p-stimulated allergic mouse
model (3). Fig. 1A shows the experimental animal
groups. Der p-stimulated mice that had been orally treated with
XQLT (PAT and PAC; Fig. 1B) had
fewer infiltrating cells in the BALF compared with the Der
p-challenged mice without oral XQLT administration (DP; Fig. 1B). Further analysis showed that the
oral administration of XQLT to mice, particularly as a pre-allergy
treatment, reduced total IgE production (PAT; Fig. 1C). In addition, XQLT was
demonstrated to reduce allergic inflammation, particularly when
administered orally as a pre-allergy treatment (PAT; Fig. 1B and C). The mice with XQLT
treatment alone (XQ) did not exhibit higher cell infiltration or
total IgE production than the NA group. Therefore, XQLT was shown
to suppress the allergic reaction in the acute Der p-stimulated
mouse model.
XQLT reduces NGF, p75NTR and TLR4
expression in the lungs
In the preceding section, XQLT was demonstrated to
inhibit allergic reaction in a mouse model. NGF promotes the TLR4
signaling-induced maturation of DCs through inducible p75NTR, an
important event in allergy initiation (33). ELISA analysis showed that the oral
administration of XQLT to mice, particularly pre-allergically (PAT;
Fig. 2A) reduced NGF expression in
the BALF. Immunohistochemistry revealed that Der p stimulated
strong p75NTR expression in the lungs of mice (DP; Fig. 2B). The majority of the Der
p-induced NGF receptors were observed around the epithelia. XQLT
treatment reduced the Der p-induced p75NTR expression in the lungs,
particularly in the mice that had received a pre-allergy oral
administration of XQLT (PAT; Fig.
2B). The XQ mice (oral XQLT alone) did not exhibit a greater
expression of p75NTR than the NA mice. XQLT appeared to
downregulate the Der p-induced NGF and p75NTR expression
simultaneously. It is speculated that XQLT downregulated NGF and
p75NTR expression through an upstream pathway and that TLR4 may be
one of the targets of XQLT.
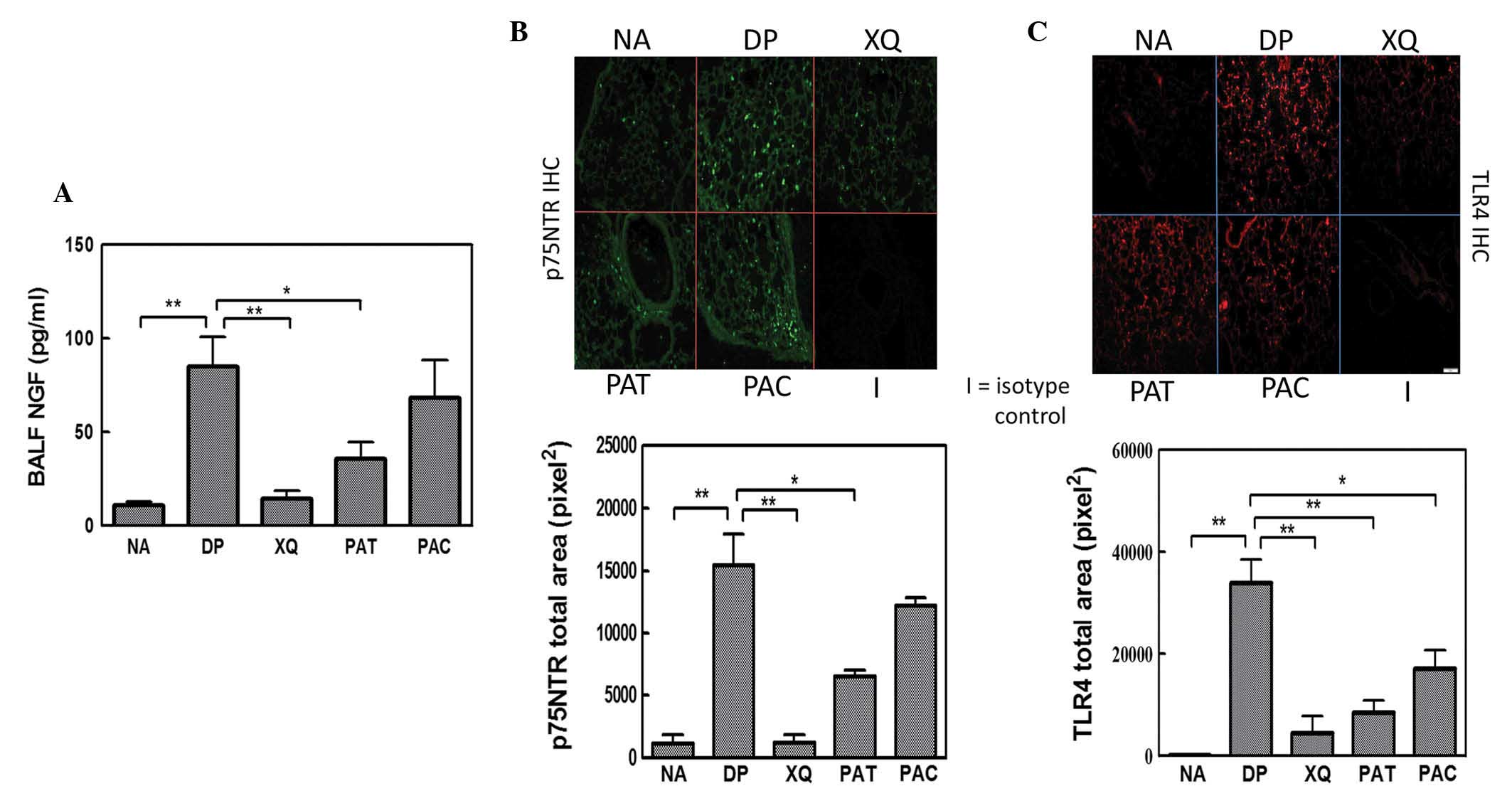 | Figure 2.Enzyme-linked immunosorbent assay
(ELISA) and immunohistochemistry showing the inhibitory effects of
Xiao-Qing-Long-Tang (XQLT) on Dermatophagoides pteronyssinus
(Der p)-induced nerve growth factor (NGF)/p75 neurotrophin receptor
(p75NTR)/Toll receptor-like 4 (TLR4) expression in the lungs. Der p
allergen significantly induced NGF/p75NTR/TLR4 expression in the
lungs in the the allergen-challenged mice without oral XQLT (DP
group). XQLT oral administration, particularly as a preventative
strategy (PAT group), decreased (A) NGF, (B) p75NTR and (C) TLR4
expression. The average area of fluorescence density in
immunohistochemistry was calculated from the fluorescence of 36–50
vision fields in each group. Values represent the mean ± standard
deviation; n=6 mice for each group. P<0.01, statistics of all
groups were versus group DP. NA, naive mice; XQ, mice with XQLT
treatment, but without allergen challenge; PAC, post-allergy curing
protocol group; BALF, broncho-alveolar lavage fluids.
*P<0.05, **P<0.01. |
Immunohistochemistry showed that Der p stimulated
marked TLR4 expression in the lungs of mice (DP; Fig. 2C). The majority of the expressed
TLR4 was focused around the epithelium. The pre- and post-allergy
oral administration of XQLT to mice reduced the expression of TLR4
in the Der p-stimulated lung. The XQLT-inhibited TLR4 expression
was not fully consistent with the XQLT-inhibited NGF receptor
expression, particularly in the PAC mice. The relationship between
the inhibitory effects of XQLT on TLR4 and NGF receptor expression
remains unclear.
XQLT appeared to simultaneously inhibit the
expression of NGF and p75NTR in the lung. To study the effects of
XQLT on NGF and its receptors, Der p 2, a known inducer of NGF and
the TLR4 signal, was used to stimulate an XQLT-treated cell line
that originally morphologically and physiologically resembled lung
cells.
XQLT pretreatment reduced the Der p
2-induced expression of NGF and TSLP in the cell line model
To evaluate the effects of XQLT on the Der p 2
signaling pathway in a cell line model, the expression of NGF,
p75NTR and TSLP was tested. HPAEpiC, a human cell line comprising
type I and type II alveolar cells, was used to evaluate the ability
of Der p 2 to induce the expression of NGF, p75NTR and TSLP in
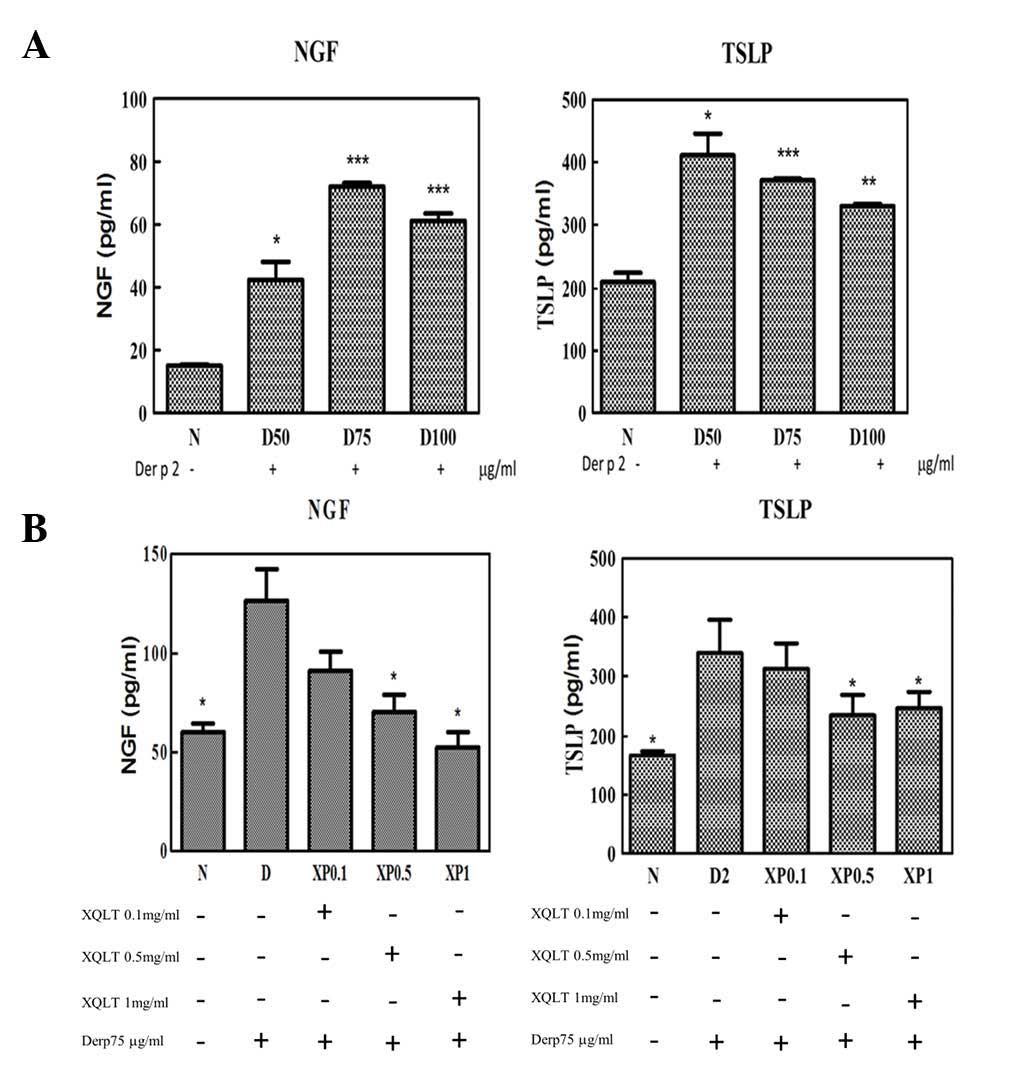
vitro. Der p 2 exhibited a concentration-dependent ability to
induce the expression of NGF and TSLP in the HPAEpiC cell line
(Fig. 3A). Table I shows the experimental design used
to study the effect of varying XQLT concentrations on the Der p
2-induced expression of NGF, TSLP and p75NTR in the cell line
model. Briefly, XQLT was used to pretreat the cells for 1 h, prior
to the XQLT being washed out. Cells were subsequently stimulated
with Der p 2 for 24 h. Pretreatment with XQLT reduced the
expression of NGF and TSLP in the Der p 2-stimulated cell line,
with a concentration-dependent response (Fig. 3B). Furthermore, western blot
analysis showed that pretreatment with XQLT inhibited the
expression of p75NTR in Der p 2-stimulated cell lines (data not
shown). All the results demonstrated that XQLT inhibited Der p 2
signaling, indicating the inhibition of TLR4 and its downstream
signaling.
Adding sTLR4 and rough strain Salmonella
LPS into cell lines attenuates the reduction in NGF and TSLP
expression by XQLT
To study the correlation between XQLT and the TLR4
signaling pathway, a competitive assay was performed to evaluate
whether it was possible to attenuate the inhibitory effect of XQLT
on the expression of NGF and TSLP. Table II shows in detail all the relevant
protocols (TLR4 competitive assay). A TLR4 signaling rough strain
of Salmonella LPS (Re 595) and exogenous recombinant sTLR4
were used in the competitive assay.
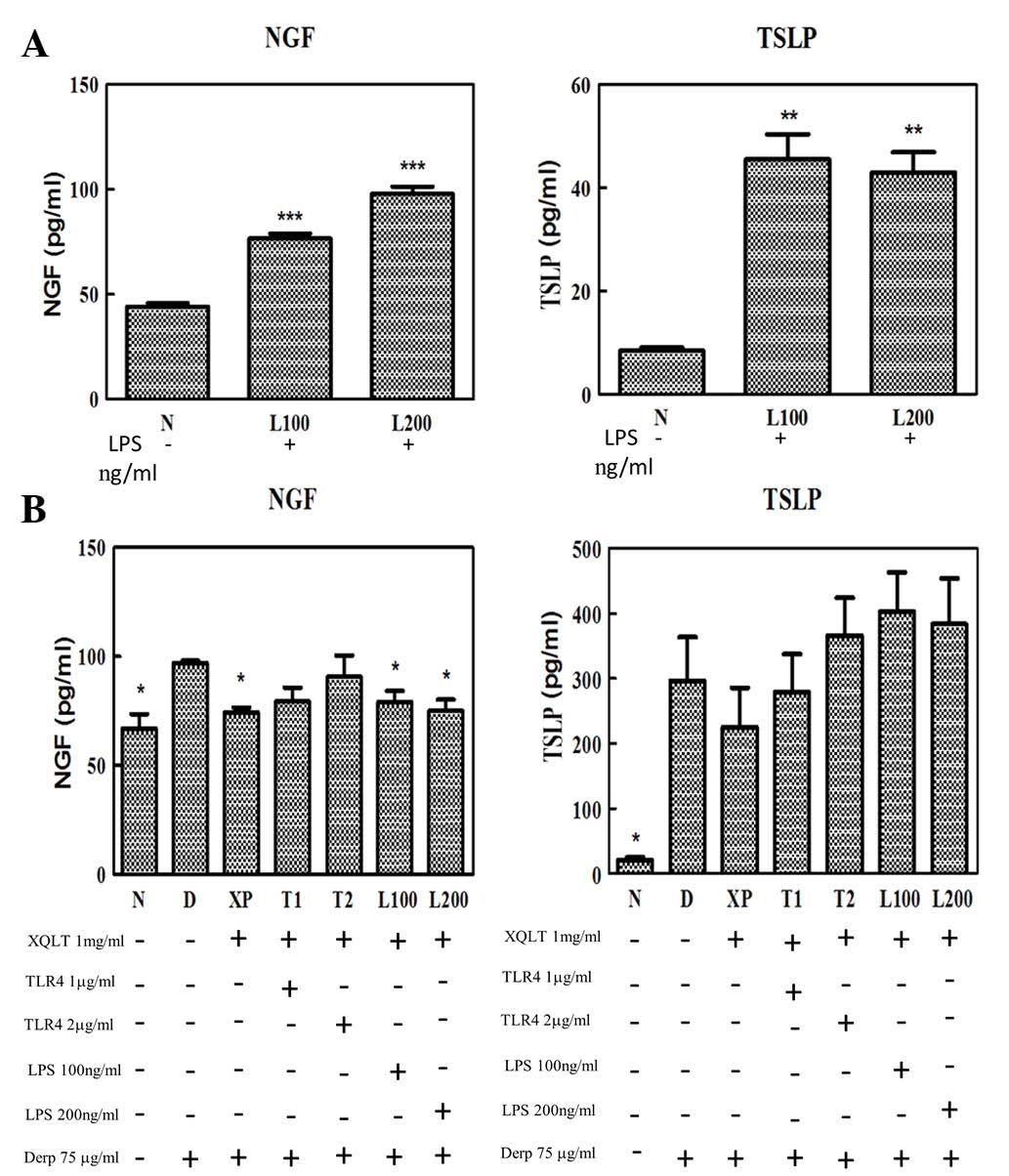
XQLT inhibited the expression of NGF and TSLP in the
Der p 2-stimulated HPAEpiC cells (XP; Figs. 3B and 4B). Although the Salmonella LPS
induced the production of NGF in the 24 h HPAEpiC cell culture
(Fig. 4A), LPS (L100 and L200;
Fig. 4B) did not attenuate the
inhibitory effect of XQLT on NGF expression (XP; Fig. 4B) in the Der p 2-stimulated HPAEpiC
cells. All statistical calculations were performed with reference
to the data from the cells stimulated with Der p 2 alone (D;
Fig. 4). By contrast, LPS (L100
and L200) was able, to a certain degree, to attenuate the
inhibitory effect of XQLT on TSLP expression (XP). LPS was
therefore demonstrated to exhibit a differential effect on the
XQLT-mediated inhibition of NGF and TSLP expression.
Recombinant sTLR4 (T1 and T2; Fig. 4B) attenuated the inhibitory effect
of XQLT on Der p 2-stimulated NGF and TSLP expression. Although the
reversal effects of the sTLR4 require further evaluation, it
appeared that the mechanism by which sTLR4 attenuated the
inhibitory effects of XQLT on Der p 2-stimulated HPAEpiC cells
involved a specific ligand competing effect, although not a
nonspecific massive exogenous protein competing effect. To further
analyze the correlation between XQLT and the TLR4 pathway, western
blot analysis was used to study the p75NTR and Ser536
phosphorylated p65 (NF-κB) in a competing assay.
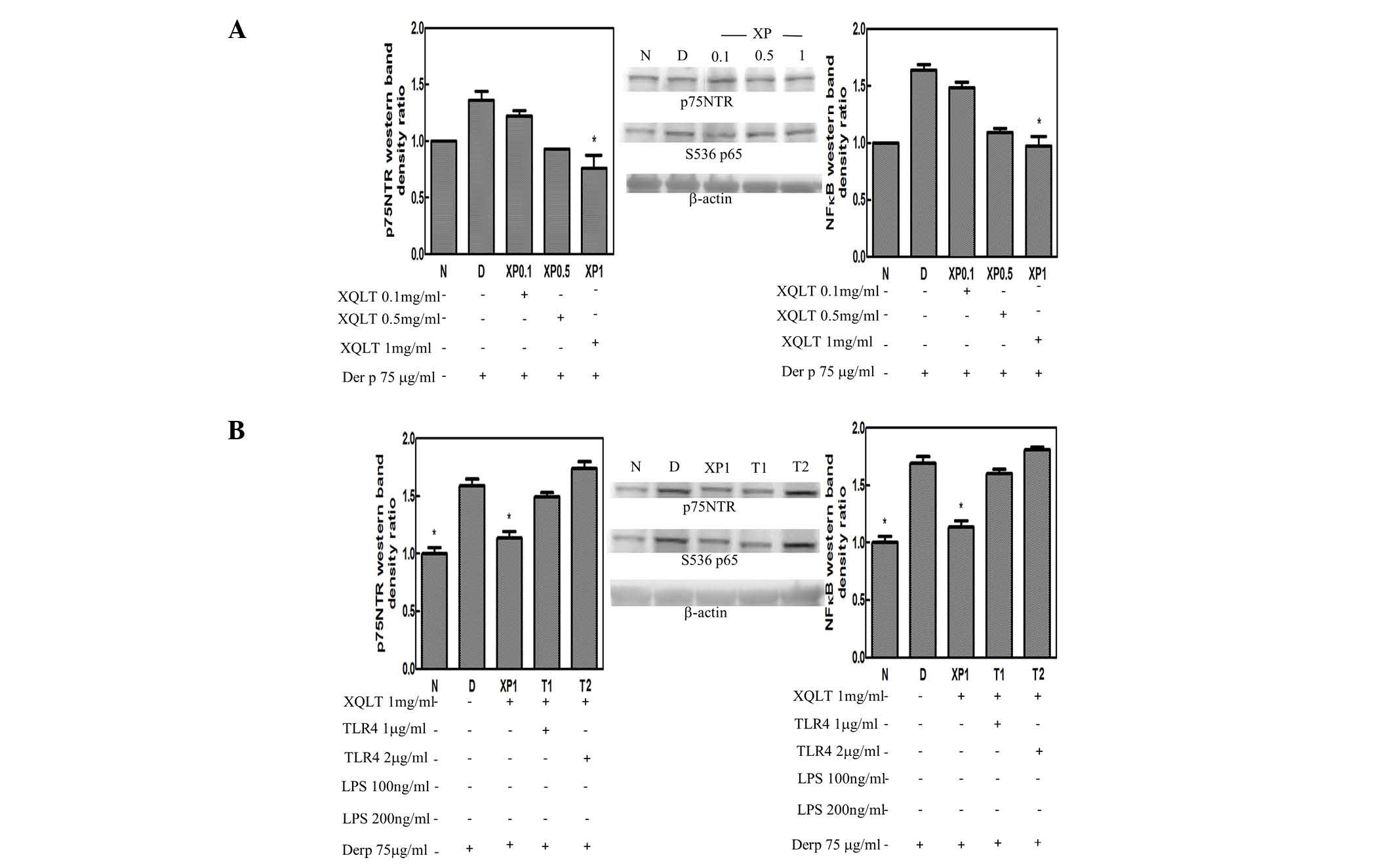
XQLT inhibits p75NTR and Ser536
phosphorylation of p65 (NF-κB) and the inhibition is attenuated by
sTLR4
p75NTR was observed to be induced by a Der p 2
signal, while Ser536 phosphorylation of p65 (NF-κB) is known to be
induced specifically by a TLR4 signal. Using steps described in
Table I, the pretreatment of
HPAEpiC cells with XQLT was observed to inhibit p75NTR and Ser536
phosphorylation of p65 (NF-κB) in concentration-dependent manner
(XP0.1~XP1; Fig. 5A). Results of
the western blotting were analyzed by a densitometer and are
presented as a bar graph with the density value of the negative
group (N in Fig. 5) set as 1. A
concentration of 1 mg/ml XQLT demonstrated the most marked
inhibitory effect on p75NTR and Ser536 phosphorylation of p65
(NF-κB). Furthermore, using the steps described in Table II, adding sTLR4 (T1 and T2;
Fig. 5B) was shown to attenuate
the inhibitory effects of 1 mg/ml XQLT on p75NTR and Ser536
phosphorylation of p65 (NF-κB). The reversal effect of sTLR4 also
demonstrated a concentration-dependent response.
Discussion
In this study, the known ability of XQLT to suppress
allergic reactions in allergen-stimulated mice (2–4,34)
was further demonstrated (Fig. 1B and
C). However, XQLT is a herbal product that comprises a complex
mixture of compounds, which presents challenges for studies
investigating its pharmacological action. This study further
identified the potential TLR4-related pharmacological targets of
XQLT action.
TLR4 is a member of the TLR family and is
distributed in the lung and intestinal epithelia (36). It is responsible for detecting
pathogens and allergens. Der p 2 is one of the allergens with a
structure resembling that of the MD-2 in the TLR4 complex. This
study showed that the Der p allergen induced NGF and the
low-affinity receptor, p75NTR, in the allergic mouse model (DP;
Fig. 2A and B), in addition to
inducing the expression of TLR4 (DP; Fig. 2C). XQLT was shown to inhibit the
Der p-induced expression of NGF, p75NTR and TLR4 in the acute
allergic mice model (PAT and PAC; Fig.
2). Der p 2 induced the expression of NGF and TSLP in the
HPAEpiC cell line (Fig. 3A) and
XQLT was demonstrated to suppress the Der p 2-induced expression of
NGF and TSLP in the HPAEpiC cells (XP; Fig. 3B). Salmonella LPS (Re 595)
(L100 and L200; Fig. 4B), a TLR4
agonist, and sTLR4 (T1 and T2; Fig.
4B) were used in a competitive assay to attenuate the
inhibitory effect of XQLT on TSLP expression in the HPAEpiC cells.
sTLR4, but not LPS, attenuated the inhibitory effect of XQLT on NGF
and TSLP expression in HPAEpiC cells (T1 and T2; Fig. 4B). These results suggest that the
active ligand component of XQLT may affect the TLR4 signaling
pathway by binding directly to TLR4, but not to CD14 (the
LPS-binding moiety in the TLR4 receptor complex) (37). We hypothesize that the effects of
XQLT were mediated through TLR4 by different downstream pathways,
leading to the inhibition of TSLP and NGF expression. LPS
attenuated the effects of XQLT on TSLP more markedly than it
attenuated the effects of XQLT on NGF. This may have been due to
different time requirements for LPS to attenuate the effects of
XQLT on NGF and TSLP, respectively. Alternatively, the pathway used
by LPS to attenuate the effects of XQLT on TSLP may have been
different to that used to attenuate the XQLT-mediated inhibition of
NGF. Taking into consideration that LPS reversed the XQLT-mediated
inhibition of TSLP expression, it may be that the signals
associated with CD14 were affected by XQLT, thus leading to reduced
TSLP expression in HPAEpiC cells.
A previous study demonstrated that Der p 2 induced
p75NTR expression through TLR4 (26). The results of the present study,
shown in Fig. 5A, further
indicated that Der p 2 induced p75NTR expression (D). XQLT was
demonstrated to inhibit p75NTR expression in a
concentration-dependent manner. To study whether the TLR4 pathway
was affected, a western blot analysis of Ser536 phosphorylation of
p65 (NF-κB) was performed. The Der p 2-induced Ser536
phosphorylation of p65 (Fig. 5A)
was reduced by XQLT, with a concentration-dependent response. TLR4
has been demonstrated to transduce signals through MyD88 and NF-κB
(24,25). Furthermore, p75NTR signals have
been shown to induce NF-κB (31,32)
and XQLT has been demonstrated to reduce NF-κB translocation into
the nucleus (34). In
consideration of these results, it was suggested that XQLT may
inhibit NGF, p75NTR and TSLP through a pathway involving TLR4.
Using sTLR4 for a competing assay (protocol in Table II), the inhibitory effects of XLQT
on p75NTR and Ser536 phosphorylation of p65 were attenuated
(Fig. 5B). This is the first time,
to the best of our knowledge, that XQLT has been observed to
potentially regulate the early phase of an allergic response.
The inhibitory effects of XQLT on NGF, p75NTR and
TSLP may be associated with the TLR4 pathway, thus providing
pharmacological targets for investigations into how XQLT affects
the early phase of allergic reactions. The present study indicated
that XQLT may have the ability to interfere with the
allergen-presenting process occurring in the epithelium. Further
studies are required to identify how XQLT acts to prevent allergic
reactions and to investigate the effects of XQLT on TLR4. This may
be beneficial in the extension of XQLT usage into other diseases
involving the TLR4 pathway.
In conclusion, XQLT may regulate NGF, p75NTR and
TSLP via the TLR4 pathway in the early phase of an allergic
reaction. Therefore XQLT may provide a preventive benefit in the
control of allergic reaction.
Abbreviations:
|
XQLT
|
Xiao-Qing-Long-Tang;
|
|
sTLR4
|
soluble Toll-like receptor 4
|
Acknowledgements
This study was supported by grants
from the National Science Council, Taiwan (grant no. NSC
101-2320-B-039-056-MY2), China Medical University (grant no.
CMU99-EW-07) and Tainan Sin-Lau Hospital, Taiwan (R.O.C.). The
authors would like to thank Dr Shulhn-Der Wang and Dr Li-Jen Lin
for providing the XQLT pharmaceutics (34).
References
|
1.
|
Kung YY, Chen YC, Hwang SJ, et al: The
prescriptions frequencies and patterns of Chinese herbal medicine
for allergic rhinitis in Taiwan. Allergy. 61:1316–1318. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nagai T, Nakao M, Shimizu Y, et al:
Proteomic analysis of anti-inflammatory effects of a Kampo
(Japanese herbal) medicine ‘Shoseiryuto (Xiao-Qing-Long-Tang)’ on
airway inflammation in a mouse model. Evid Based Complement
Alternat Med. 2011:6041962011.PubMed/NCBI
|
|
3.
|
Kao ST, Wang SD, Wang JY, et al: The
effect of Chinese herbal medicine, xiao-qing-long tang (XQLT), on
allergen-induced bronchial inflammation in mite-sensitized mice.
Allergy. 55:1127–1133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nagai T, Arai Y, Emori M, et al:
Anti-allergic activity of a Kampo (Japanese herbal) medicine
‘Sho-seiryu-to (Xiao-Qing-Long-Tang)’ on airway inflammation in a
mouse model. Int Immunopharmacol. 4:1353–1365. 2004.
|
|
5.
|
Ko E, Rho S, Cho C, et al:
So-Cheong-Ryong-Tang, tradititional Korean medicine, suppresses Th2
lineage development. Biol Pharm Bull. 27:739–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ko E, Rho S, Lee EJ, et al: Traditional
Korean medicine (SCRT) modulate Th1/Th2 specific cytokine
production in mice CD4+ T cell. J Ethnopharmacol.
92:121–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ehrhard PB, Erb P, Graumann U and Otten U:
Expression of nerve growth factor and nerve growth factor receptor
tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc
Natl Acad Sci USA. 90:10984–10988. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ehrhard PB, Erb P, Graumann U, Schmutz B
and Otten U: Expression of functional trk tyrosine kinase receptors
after T cell activation. J Immunol. 152:2705–2709. 1994.PubMed/NCBI
|
|
9.
|
Glaab T, Hoymann HG, Hecht M, et al:
Effect of anti-nerve growth factor on early and late airway
responses in allergic rats. Allergy. 58:900–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Abram M, Wegmann M, Fokuhl V, et al: Nerve
growth factor and neurotrophin-3 mediate survival of pulmonary
plasma cells during the allergic airway inflammation. J Immunol.
182:4705–4712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shi Y, Jin Y, Guo W, et al: Blockage of
nerve growth factor modulates T cell responses and inhibits
allergic inflammation in a mouse model of asthma. Inflamm Res.
61:1369–1378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hahn C, Islamian AP, Renz H and Nockher
WA: Airway epithelial cells produce neurotrophins and promote the
survival of eosinophils during allergic airway inflammation. J
Allergy Clin Immunol. 117:787–794. 2006. View Article : Google Scholar
|
|
13.
|
Noga O, Peiser M, Altenähr M, et al:
Differential activation of dendritic cells by nerve growth factor
and brain-derived neurotrophic factor. Clin Exp Allergy.
37:1701–1708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Phipps S, Lam CE, G, Kaiko GE, et al:
Toll/IL-1 signaling is critical for house dust mite-specific helper
T cell type 2 and type 17 responses. Am J Respir Crit Care Med.
179:883–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hammad H, Chieppa M, Perros F, et al:
House dust mite allergen induces asthma via Toll-like receptor 4
triggering of airway structural cells. Nat Med. 15:410–416. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ricci A, Greco S, Mariotta S, et al:
Neurotrophins and neurotrophin receptors in human lung cancer. Am J
Respir Cell Mol Biol. 25:439–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Molloy NH, Read DE and Gorman AM: Nerve
growth factor in cancer cell death and survival. Cancers.
3:510–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu YJ, Soumelis V, Watanabe N, et al:
TSLP: an epithelial cell cytokine that regulates T cell
differentiation by conditioning dendritic cell maturation. Annu Rev
Immunol. 25:193–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Al-Shami A, Spolski R, Kelly J, et al: A
role for TSLP in the development of inflammation in an asthma
model. J Exp Med. 202:829–839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liu YJ: Thymic stromal lymphopoietin:
master switch for allergic inflammation. J Exp Med. 203:269–273.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Roan F, Bell BD, Stoklasek TA, et al: The
multiple facets of thymic stromal lymphopoietin (TSLP) during
allergic inflammation and beyond. J Leukoc Biol. 91:877–886. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar
|
|
23.
|
Kenny EF and O’Neill LA: Signalling
adaptors used by Toll-like receptors: an update. Cytokine.
43:342–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kobayashi T, Takaesu G and Yoshimura A:
Mal-function of TLRs by SOCS. Nat Immunol. 7:123–124. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wang J, Cai Y, Shao LJ, et al: Activation
of NF-κB by TMPRSS2/ERG fusion isoforms through Toll-like
receptor-4. Cancer Res. 71:1325–1333. 2011.
|
|
26.
|
Jiang Y, Chen G, Zheng Y, et al: TLR4
signaling induces functional nerve growth factor receptor p75NTR on
mouse dendritic cells via p38MAPK and NF-κB pathways. Mol Immunol.
45:1557–1566. 2008.PubMed/NCBI
|
|
27.
|
Ye YL, Wu HT, Lin CF, et al:
Dermatophagoides pteronyssinus 2 regulates nerve growth
factor release to induce airway inflammation via a reactive oxygen
species-dependent pathway. Am J Physiol Lung Cell Mol Physiol.
300:L216–L224. 2011. View Article : Google Scholar
|
|
28.
|
Trompette A, Divanovic S, Visintin A, et
al: Allergenicity resulting from functional mimicry of a Toll-like
receptor complex protein. Nature. 457:585–588. 2009.PubMed/NCBI
|
|
29.
|
Tokuoka S, Takahashi Y, Masuda T, Tanaka
H, Furukawa S and Nagai H: Disruption of antigen-induced airway
inflammation and airway hyper-responsiveness in low affinity
neurotrophin receptor p75 gene deficient mice. Br J Pharmacol.
134:1580–1586. 2001. View Article : Google Scholar
|
|
30.
|
Kerzel S, Päth G, Nockher WA, Quarcoo D,
Raap U, Groneberg DA, Dinh QT, Fischer A, Braun A and Renz H:
Pan-neurotrophin receptor p75 contributes to neuronal
hyper-reactivity and airway inflammation in a murine model of
experimental asthma. Am J Respir Cell Mol Biol. 28:170–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Bothwell M: p75NTR: a receptor after all.
Science. 272:506–507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Carter BD, Kaltschmidt C, Kaltschmidt B,
et al: Selective activation of NF-κB by nerve growth factor through
the neurotrophin receptor p75. Science. 272:542–545. 1996.
|
|
33.
|
Jiang Y, Chen G, Zhang Y, et al: Nerve
growth factor promotes TLR4 signaling-induced maturation of human
dendritic cells in vitro through inducible p75NTR. J Immunol.
179:6297–6304. 2007. View Article : Google Scholar
|
|
34.
|
Wang SD, Lin LJ, Chen CL, Lee SC, Lin CC,
Wang JJ and Kao ST: Xiao-Qing-Long-Tang attenuates allergic airway
inflammation and remodeling in repetitive Dermatogoides
pteronyssinus challenged chronic asthmatic mice model. J
Ethnopharmacol. 142:531–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Muroi M and Tanamoto KI: The
polysaccharide portion plays an indispensable role in
Salmonella lipopolysaccharide-induced activation of NF-κB
through human toll-like receptor 4. Infect Immun. 70:6043–6047.
2002.PubMed/NCBI
|
|
36.
|
Nishimura M and Naito S: Tissue-specific
mRNA expression profiles of human toll-like receptors and related
genes. Biol Pharm Bull. 28:886–892. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Zanoni I, Ostuni R, Marek LR, et al: CD14
controls the LPS-induced endocytosis of Toll-like receptor 4. Cell.
147:868–880. 2011. View Article : Google Scholar : PubMed/NCBI
|