Introduction
The resistance of tumor cells to chemotherapeutic
agents is one of the major barriers suppressing the effectiveness
of anticancer drugs in cancer treatment. It has been demonstrated
that cancer stem cells, which are important in carcinogenesis,
tumor progression, invasion and metastasis, are also responsible
for chemoresistance (1). In
vitro studies revealed that stem-like cells from breast cancer
cell lines were less sensitive to paclitaxel and 5-fluorouracil
(2), and a side population of
cells in hepatocellular carcinoma (HCC) exhibited resistance to
doxorubicin (3).
We have previously reported that in the laryngeal
carcinoma cell line HEp-2, ~1.5–3.5% of cells were
CD133+ cancer stem cells, which are responsible for
chemoresistance to cisplatin (3,4).
CD133+ cancer stem cells may be enriched after cisplatin
treatment, leading to chemoresistance (5,6). In
addition to chemoresistance, platinum-based chemotherapy may have
other dose-dependent side-effects that limit the application of
platinum-based drugs in cancer treatment (7). As platinum-based chemotherapy is
currently widely used to treat a number of cancer types,
particularly head and neck cancers (8), it is necessary to develop an optimal
therapy to reduce chemoresistance, suppress tumor recurrence and
enhance effectiveness.
Curcumin (diferuloylmethane) is extracted from the
rhizome of Curcuma Longa, which has been used as a
traditional Chinese medicine for centuries (9). Curcumin is also the major component
of turmeric, a spice widely used in Asian cuisines. Previous
studies have revealed that curcumin has protective and anti-cancer
effects in several types of human cancer (10). Curcumin may function through a
series of signaling pathways implicated in cancer development and,
since it is a promising anticancer drug, it is important to
evaluate the beneficial effect of curcumin as a single treatment or
when administered in combination with other conventional anticancer
drugs (11). As curcumin is
insoluble in water, any form of inorganic solution or saline,
previous studies have focused on liposomal formulations that may
aid the delivery of curcumin (12).
In the present study, we investigated the anticancer
effect of treatment with a combination of curcumin and cisplatin.
The ability of curcumin to enhance the effectiveness of cisplatin
in HEp-2 cells and induce the sensitivity of CD133+
cancer stem cells to cisplatin by suppressing ATP-binding cassette
sub-family G member 2 (ABCG2)-mediated chemoresistance in
vitro was evaluated.
Materials and methods
Chemicals and reagents
Cisplatin,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and
1,2-dimyristoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DMPG) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Curcumin was
obtained from Dingguo Biotech Inc. (Beijing, China). The glycine
buffer used in the MTT assay was prepared with 0.1 M glycine and
0.1 M NaCl, and adjusted to pH 10.5.
Liposomal curcumin preparation
A lipid mixture was prepared by mixing DMPC and DMPG
in a ratio of 9:1. Curcumin was added to the lipid mixture with
sterile water to create a liposomal curcumin solution with a final
lipid:curcumin ratio of 10:1. The liposomal curcumin solution was
then filtered and lyophilized. The lyophilized liposomal curcumin
was suspended with 0.9% NaCl to achieve a stock concentration of
100 mmol/l.
Cell culture and treatment
A human laryngeal squamous cancer cell line, HEp-2,
was provided by the Second Hospital of Jilin University (Changchun,
China). Cells were cultured in Dulbecco’s modified Eagle’s medium
with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2
incubator. 100 U/ml penicillin and 100 μg/ml streptomycin
were used to prevent microbial contamination. Cisplatin was used at
an optimal dose of 5 μg/ml. The liposomal curcumin was
applied to the growth medium at a concentration of 5 μM.
MTT assay
An MTT assay was applied to determine the viability
of HEp-2 cells following various treatments. After 48 h of exposure
to the treatment, the cells were incubated with 100 μl MTT
(5 mg/ml) solution for 3 h. Subsequently, the MTT solution was
replaced with 100 μl dimethyl sulfoxide (DMSO) and 25
μl glycine buffer. The absorbance at 570 nm of untreated
HEp-2 cells was considered as 100% cell viability.
Colony formation assay
Cells of a single-cell suspension (2,000 cells per
well) were inoculated in 6-well plates and incubated for 24 h. The
cells were cultured with various treatments for 2 weeks. The cells
were fixed using ice-cold methanol and stained with crystal violet.
Colonies (>50 cells) were analyzed using a Gel-Pro analyzer
(Media Cybernetics Inc., Rockville, MD, USA).
Apoptosis assay
HEp-2 cells were exposed to different treatments for
48 h. The cells were harvested and stained with the Muse Annexin V
& Dead Cell assay kit (Merck Millipore, Billerica, MA, USA)
according to the manufacturer’s instructions. Cells were analyzed
using a Muse™ Cell Analyzer (Merck Millipore). Late apoptosis was
defined as Annexin V-positive and propidium iodide (PI)-positive
cells.
Flow cytometry assays and
fluorescence-activated cell sorting (FACS) of CD133+
cells
Flow cytometry assays for the CD133+
cells were performed as previously described (3). In brief, following their respective
treatments, the HEp-2 cells were collected and rinsed with PBS. The
number of dissociated cells was counted and 1×107 cells
were subsequently transferred to 100 μl buffer containing
phycoerythrin (PE)-conjugated CD133/2 antibody (Miltenyi Biotec
Inc., Auburn, CA, USA) for 30 min and protected from light. The
cells were then washed and analyzed using a flow cytometer (BD
FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA). The
CD133+ and CD133− cells were sorted by FACS
as previously described (3).
Western blotting
Protein extracts from CD133+ and
CD133− cells were separated using 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
subsequently transferred to a PVDF membrane. Anti-ABCG2 antibody
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin
antibody (Life Technologies, Grand Island, NY, USA) were used for
detection with an ECL Plus system (GE Healthcare, Pittsburgh, PA,
USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 16 (IBM, Armonk, NY, USA). Data are presented as the mean ±
standard deviation (SD) and evaluated using a t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Curcumin affects the CD133+
population in HEp-2 cells
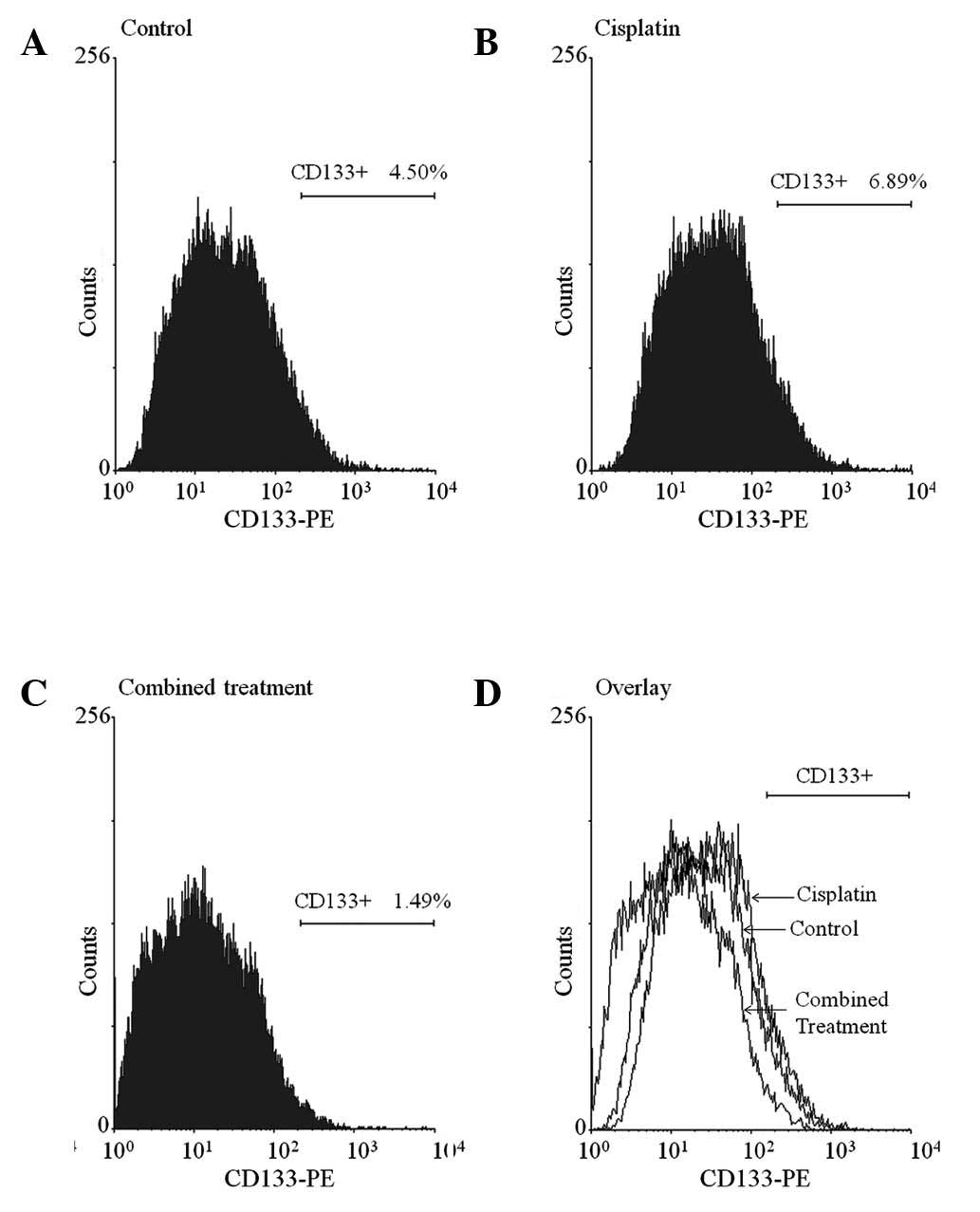
In this study, an average of 4.50% CD133+
cells was detected in untreated HEp-2 cells and the
CD133+ population was increased to 6.89% with cisplatin
treatment. However, when cisplatin was applied with curcumin, the
CD133+ population was markedly reduced to 1.49%
(Fig. 1A–C). As shown in Fig. 1D, the over-laid graph displays a
clear left shift between the CD133 signals of the cisplatin group
and the combined treatment group. This result indicated that
cisplatin led to enrichment of the CD133+ population in
HEp-2 cells and that the enrichment was significantly suppressed by
combined treatment with curcumin.
Curcumin induces apoptosis of HEp-2
cells
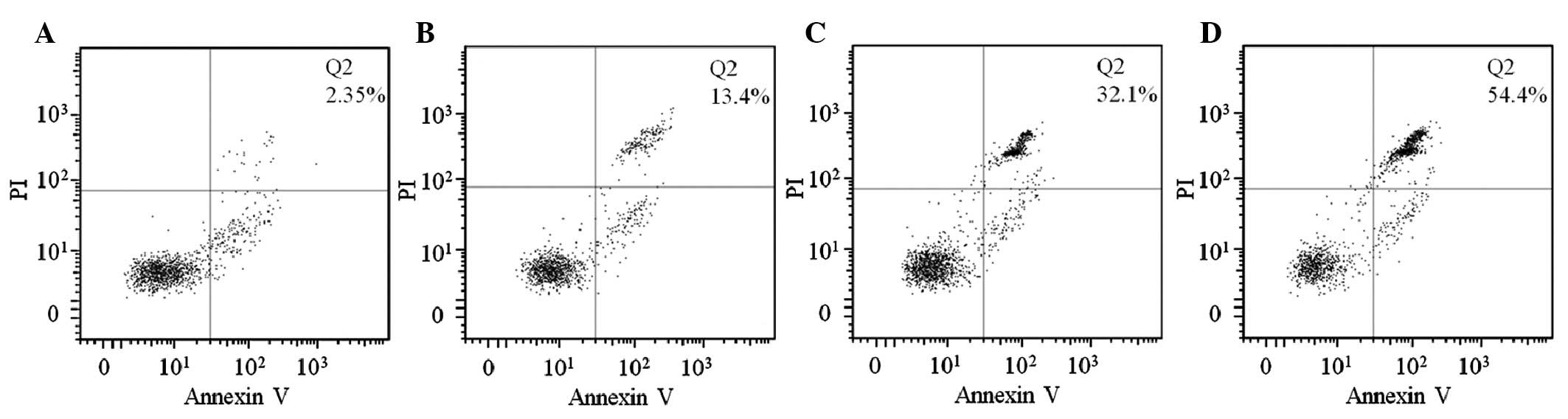
The results of the Annexin V/PI assay demonstrated
that curcumin and cisplatin individually induced the apoptosis of
HEp-2 cells following 48 h exposure to the drugs and the effect was
enhanced when these two drugs were applied simultaneously as a
combined treatment. The percentage of late apoptosis in the
untreated HEp-2 cells was 2.35% (Fig.
2A, Q2), whereas following treatments with curcumin and
cisplatin the percentages of late apoptosis were increased to 13.4
and 32.1%, respectively (Fig. 2B and
C, Q2). Following combined treatment with cisplatin and
curcumin, the percentage of late apoptosis was significantly
increased to 54.4% (Fig. 2D,
Q2).
Curcumin suppresses the proliferation of
HEp-2 cells
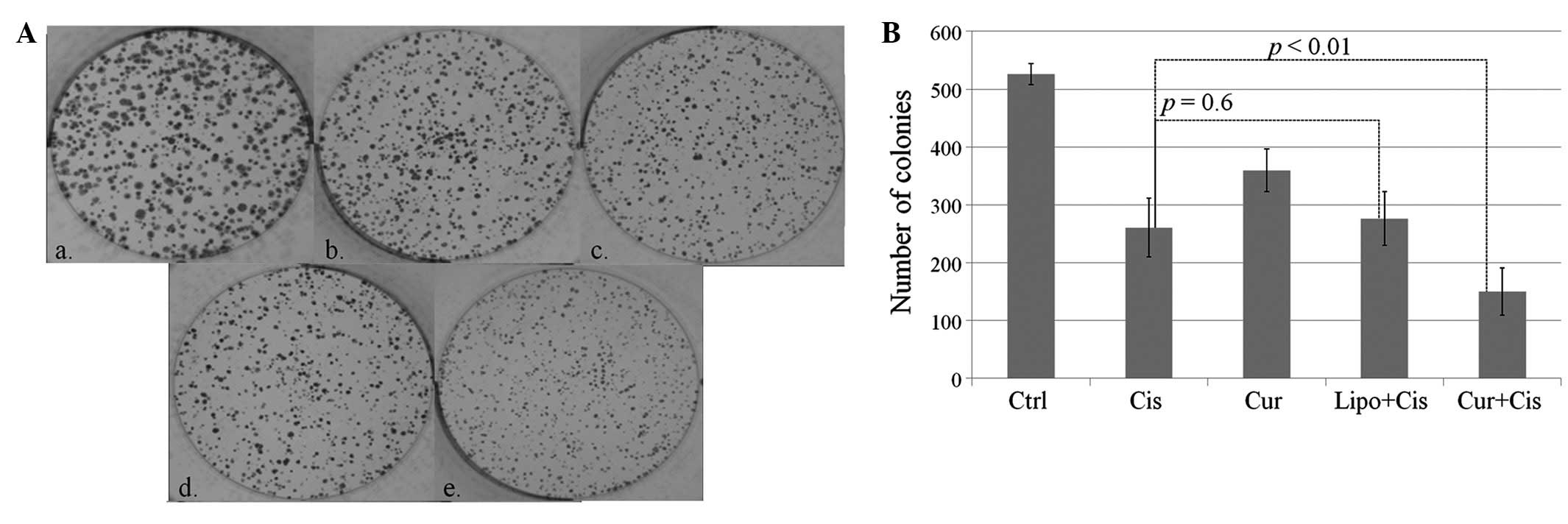
The colony formation assay suggested that, combined
with cisplatin, curcumin synergistically reduced the clonogenicity
and proliferation of HEp-2 cells. Following 2 weeks of incubation,
the average number of colonies (>100 cells) in the untreated
group was 526 and the number of colonies was reduced in the
curcumin and cisplatin groups. When cisplatin was applied with
curcumin, the number of colonies was significantly decreased to 150
(Fig. 3A and B). Furthermore, the
size of the colonies in the combined treatment group was also
reduced (Fig. 3A).
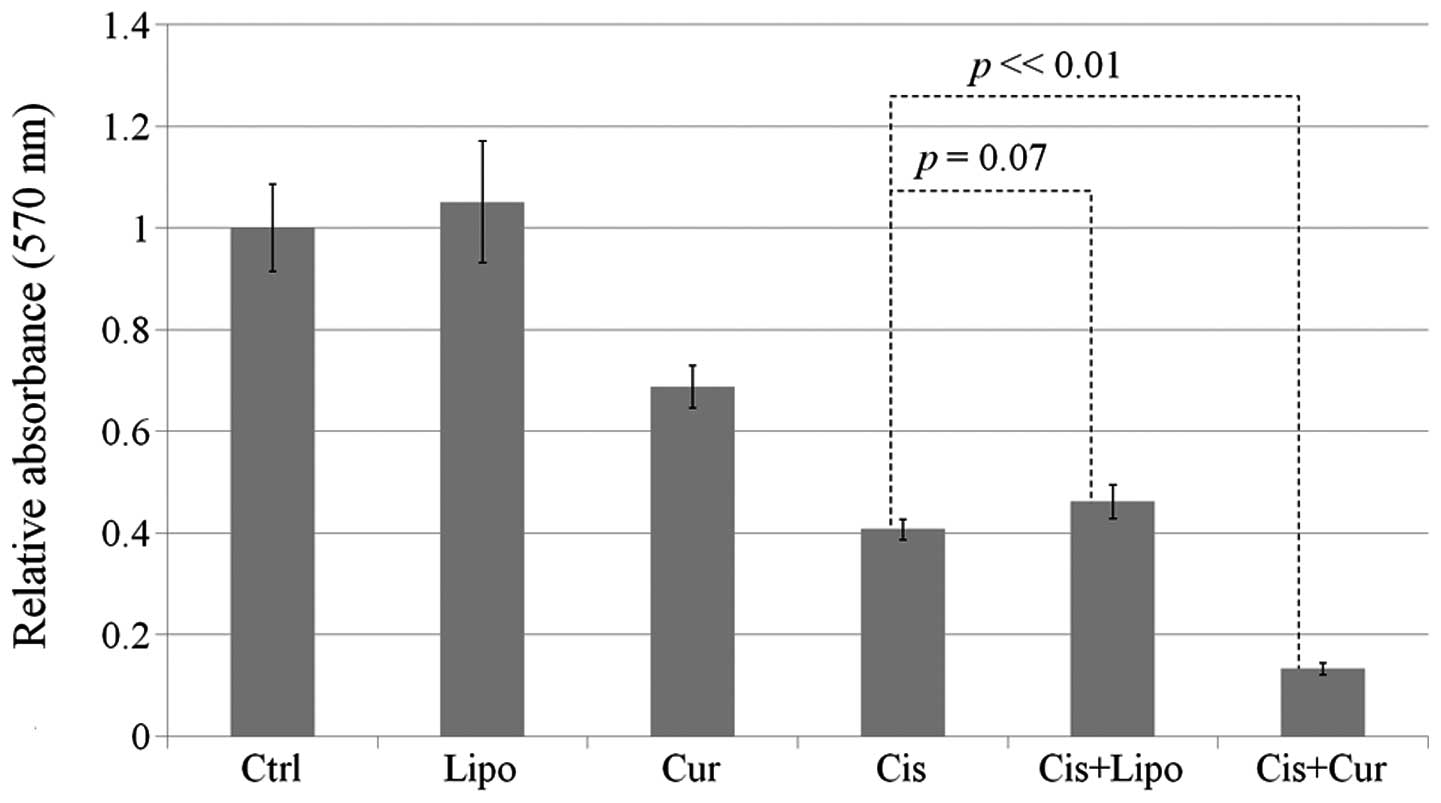
In addition to the colony formation assay, the MTT
assay further confirmed that the combined application of cisplatin
and curcumin was able to suppress the viability of HEp-2 cells. The
application of cisplatin alone reduced the cell viability ~60%.
However, with combined treatment, the reduction was enhanced to
~90% (Fig. 4).
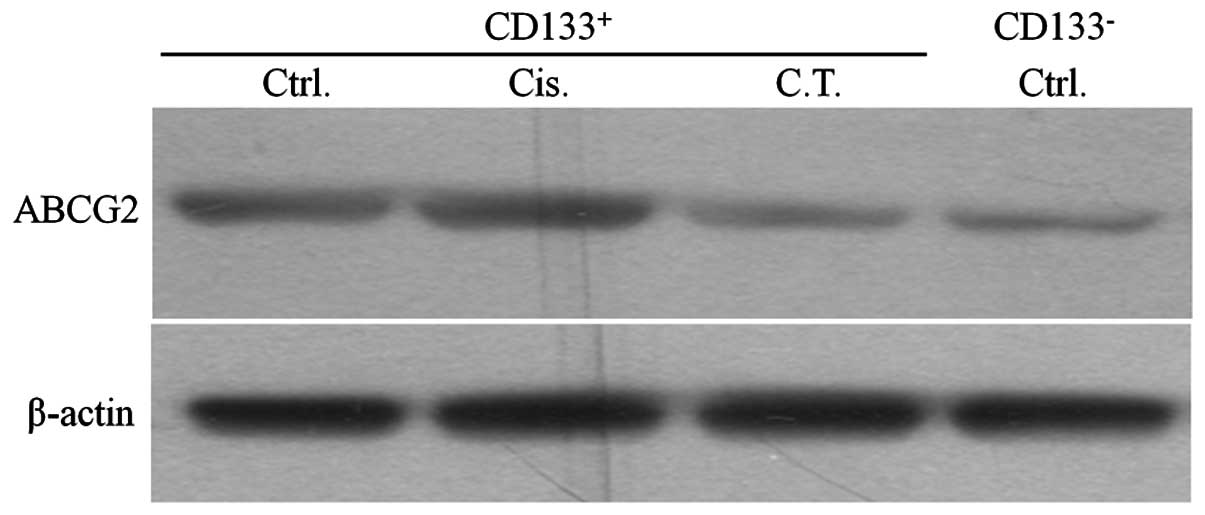
ABCG2 expression was reduced by curcumin
in CD133+ cells
The CD133+ and CD133− cells
were sorted after each treatment and the levels of ABCG2 expression
were investigated. No significant difference in the levels of ABCG2
expression was detected between the untreated and cisplatin-treated
CD133+ cells. However, the expression level was reduced
in the combined treatment group and was as low as that in the
untreated CD133− group (Fig. 5).
Discussion
Laryngeal carcinoma is a malignant type of head and
neck cancer. Chemotherapy is the main treatment for laryngeal
carcinoma and other head and neck cancers; however, major barriers
to this therapy, including chemoresistance, have prompted
investigation into the underlying mechanisms of anti-cancer
chemotherapy and the development of optimal treatment methods
(13).
The cancer stem cell theory is one of the most
likely explanations for chemoresistance (14). It has been established that CD133
is an important marker for cancer stem cells in the laryngeal
carcinoma cell line HEp-2 (4).
Furthermore, it has previously been reported that in the HEp-2 cell
line, CD133+ cancer stem cells are responsible for drug
resistance to chemotherapeutic agents (3). In the present study, we attempted to
utilize a traditional Chinese medicine and a classical chemotherapy
drug as a combined treatment to induce the sensitivity of
CD133+ stem cells and enhance therapeutic
effectiveness.
MTT and colony formation assays demonstrated the
anti-cancer effect of curcumin. It reduced the clonogenicity and
suppressed the proliferation of HEp-2 cells. The liposomal vehicle
had no effect either when used alone or in combination with
cisplatin (control, Fig. 4), which
indicates that the application of curcumin significantly enhanced
the effectiveness of cisplatin. In this study, curcumin induced the
apoptosis of HEp-2 cells, consistent with a study reporting that
curcumin induced apoptosis in pancreatic carcinoma (12). Moreover, in line with the findings
of the MTT assay, curcumin markedly enhanced apoptosis when applied
with cisplatin.
In order to investigate the curcumin-enhanced
anticancer effect of cisplatin, the chemoresistance of
CD133+ cancer stem cells, one of the major barriers of
cisplatin treatment (15), was
studied. In the HEp-2 laryngeal carcinoma cell line, ~1.5–3.5% of
cells were previously identified to be CD133+ cancer
stem cells (3,4). In the present study, 4.50 and 6.89%
CD133+ cells were detected in untreated and
cisplatin-treated HEp-2 cells, respectively. CD133+ stem
cells were enriched following cisplatin treatment due to the
insensitivity of CD133+ cells to chemotherapeutic
agents. Similar results have also been observed in other cancer
treatments (15), and may reflect
a problem in the current treatment of laryngeal carcinoma; although
chemotherapeutic drugs kill the majority of cancer cells, the
cancer stem cells may also lead to drug resistance and tumor
recurrence. However, when cisplatin was applied with curcumin, the
percentage of CD133+ cells was markedly reduced to
1.49%. The enrichment of CD133+ stem-like cells was
significantly suppressed by combined treatment with curcumin,
indicating that curcumin may increase the sensitivity of
CD133+ cells to cisplatin, leading to the suppression of
chemoresistance of HEp-2 cells.
The ATP-binding cassette transporter, ABCG2,
functions as an ATP-dependent drug efflux pump contributing to drug
resistance (16,17) and is highly expressed in cells with
a side population phenotype (18).
It has been demonstrated that ABCG2 is one of the most important
genes for the chemoresistance of cancer stem cells (19,20).
It has been observed that ABCG2 is highly expressed in
CD133+ HEp-2 cells, leading to chemoresistance (3). In the current study, we demonstrated
that combined treatment with cisplatin and curcumin reduced the
expression of ABCG2 in CD133+ cancer stem cells,
indicating that curcumin may suppress ABCG2-induced chemoresistance
in CD133+ HEp-2 cells.
In conclusion, the present study indicated that the
application of curcumin may induce the sensitivity of
CD133+ cancer stem cells to cisplatin and therefore
enhance the effectiveness of cisplatin on the laryngeal carcinoma
HEp-2 cell line. The reduced expression of ABCG2 in
CD133+ cells may be responsible for the induced
sensitivity of CD133+ cells to cisplatin. The combined
application of curcumin with chemotherapeutic drugs may be a
reliable and effective approach for the treatment of laryngeal
carcinoma. Furthermore, as a major component of the spice turmeric,
dietary curcumin may also be used for the prevention of laryngeal
carcinoma and other types of cancer. Further studies are required
to explore the application of curcumin in the treatment of other
cancer types.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (30973288).
References
|
1.
|
Spillane JB and Henderson MA: Cancer stem
cells: a review. ANZ J Surg. 77:464–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Fillmore CM and Kuperwasser C: Human
breast cancer cell lines contain stem-like cells that self-renew,
give rise to pheno-typically diverse progeny and survive
chemotherapy. Breast Cancer Res. 10:R252008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yang JP, Liu Y, Zhong W, Yu D, Wen LJ and
Jin CS: Chemoresistance of CD133+ cancer stem cells in
laryngeal carcinoma. Chin Med J (Engl). 124:1055–1060.
2011.PubMed/NCBI
|
|
4.
|
Zhou L, Wei X, Cheng L, Tian J and Jiang
JJ: CD133, one of the markers of cancer stem cells in Hep-2 cell
line. Laryngoscope. 117:455–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Sharma BK, Manglik V, O’Connell M, et al:
Clonal dominance of CD133+ subset population as risk
factor in tumor progression and disease recurrence of human
cutaneous melanoma. Int J Oncol. 41:1570–1576. 2012.
|
|
6.
|
Yin T, Wei H, Gou S, et al: Cancer
stem-like cells enriched in panc-1 spheres possess increased
migration ability and resistance to gemcitabine. Int J Mol Sci.
12:1595–1604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar
|
|
8.
|
Price KA and Cohen EE: Current treatment
options for metastatic head and neck cancer. Curr Treat Options
Oncol. 13:35–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ammon HP and Wahl MA: Pharmacology of
Curcuma longa. Planta Med. 57:1–7. 1991.
|
|
10.
|
Reuter S, Eifes S, Dicato M, Aggarwal BB
and Diederich M: Modulation of anti-apoptotic and survival pathways
by curcumin as a strategy to induce apoptosis in cancer cells.
Biochem Pharmacol. 76:1340–1351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Teiten MH, Eifes S, Dicato M and Diederich
M: Curcumin-the paradigm of a multi-target natural compound with
applications in cancer prevention and treatment. Toxins (Basel).
2:128–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Li L, Braiteh FS and Kurzrock R:
Liposome-encapsulated curcumin: in vitro and in vivo effects on
proliferation, apoptosis, signaling, and angiogenesis. Cancer.
104:1322–1331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yamano Y, Uzawa K, Saito K, et al:
Identification of cisplatin-resistance related genes in head and
neck squamous cell carcinoma. Int J Cancer. 126:437–449. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Visvader JE and Lindeman GJ: Cancer stem
cells: current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bertolini G, Roz L, Perego P, et al:
Highly tumorigenic lung cancer CD133+ cells display
stem-like features and are spared by cisplatin treatment. Proc Natl
Acad Sci USA. 106:16281–16286. 2009.PubMed/NCBI
|
|
16.
|
de Jonge-Peeters SD, Kuipers F, de Vries
EG and Vellenga E: ABC transporter expression in hematopoietic stem
cells and the role in AML drug resistance. Crit Rev Oncol Hematol.
62:214–226. 2007.PubMed/NCBI
|
|
17.
|
Sarkadi B, Ozvegy-Laczka C, Német K and
Váradi A: ABCG2 - a transporter for all seasons. FEBS Lett.
567:116–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
An Y and Ongkeko WM: ABCG2: the key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Hirschmann-Jax C, Foster AE, Wulf GG,
Goodell MA and Brenner MK: A distinct ‘side population’ of cells in
human tumor cells: implications for tumor biology and therapy. Cell
Cycle. 4:203–205. 2005.
|