Introduction
Endometriosis (EMs) is a common gynecological
disease that frequently occurs in females within childbearing age,
with the clinical manifestations of abdominal pain, dysmenorrhea,
infertility and irregular menstruation. The disease has shown an
increasing incidence in recent years, resulting in a serious impact
on the health of females. EMs is a type of hormone-dependent
disease. There are a number of methods used in the treatment of
EMs; however, the effects are not satisfactory and the recurrence
rate remains high. The pathogenesis of EMs has not yet been fully
elucidated. The endometrial implantation theory (1), which has been accepted by the
majority of the scientific community, proposes that the endometrium
that is shed during menstruation is transported by retrograde flow
with the blood through the fallopian tubes and into the abdominal
cavity, where the endometrial tissue subsequently grows on the
ovary and adjacent pelvic peritoneum and evolves into an ectopic
endometrium. Based on this theory, a study investigated the manner
of the endometrial implantation in the abdominal cavity, and
revealed that the degradation and reconstruction of the
extracellular matrix (ECM) (2)
were the central features of endometrial implantation. Furthermore,
matrix metalloproteinases (MMPs) were identified to be important in
the degradation and reconstruction of the ECM.
Xiaochaihu Tang (XCHT) is a classic formulation,
which is described in a ‘Treatise on Cold Pathogenic and
Miscellaneous Diseases’ as having the effects of anti-inflammatory,
gastric protection and regulation of the immune system (3). Previous studies have shown that XCHT
exerted a good therapeutic effect on EMs in rats (3–5);
however the mechanisms underlying the effect were not clear. In the
present study, the mechanisms of XCHT in the treatment of EMs were
investigated by observing the effect of XCHT on the expression
levels of MMP-2 and MMP-9 in EMs tissues in a rat model of the
disease.
Materials and methods
Experimental animals
Forty-eight specific-pathogen-free (SPF) female
Sprague-Dawley (SD) rats, weighing 200–220 g, were provided by the
Animal Laboratory Center of Zhengzhou University (Zhengzhou,
China), with license no. SCXK (Henan) 2005-0001. This study was
carried out in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (8th edition, 2011). The animal use protocol
was reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) of Xinxiang Medical University (Xinxiang,
China).
Drugs
XCHT was obtained with reference to Zhang
Zhongjing’s ‘Treatise on Cold Pathogenic and Miscellaneous
Diseases’, and comprised seven crude drugs: 24 g Bupleurum
chinense, 9 g Scutellaria baicalensis, 6 g ginseng root,
9 g Pinellia, 5 g licorice, 9 g ginger and 4 g jujube. These seven
herbs were purchased from Henan Zhang Zhong Jing Pharmacy
(Zhengzhou, China). The mixture of herbs was dipped in water for 1
h. Following this, the herbs were heated to continuously boil for
30 min, prior to the residue being decocted again for a further 30
min. The two decoctions were subsequently mixed and concentrated to
1 g/ml (crude dosage). Gestrinone capsules (2.5 mg/particle, lot
no. 53110602) were purchased from Beijing Zizhu Pharmaceutical Co.,
Ltd. (Beijing, China).
Animal model
Rats with a normal estrous cycle were selected using
a vaginal smear method and were subcutaneously injected with
stilbestrol (0.1 ml/kg) to synchronize the estrous cycle. The
animal model was established according to the method described by
Jones (6). Each rat was injected
with 10% chloral hydrate (300 mg/kg) for anesthesia. Following
routine disinfection, the abdominal cavity was opened and the
uterine vessel was ligated. An incision of ~1 cm was made in the
left horn of the uterus, prior to the endometrium being separated
in Rockwell nutrient solution and cut into fragments of 5×5 mm. The
fragment was adhered to the intimal surface of the abdominal
muscles, attached to the two sides of the abdominal wall and the
incision was then sutured. The remainder of the membrane was sent
for pathological inspection to confirm that the tissues were
endometrial. Following surgery, the rats were injected with 0.1 ml
penicillin daily for five days, in order to prevent infection. The
normal control group underwent sham surgery, consisting solely of
the abdominal cavity being opened. Twenty-one days subsequent to
the model being established, the ectopic endometrium was observed
by opening the abdominal cavity under aseptic conditions. If the
volume of the ectopic endometrium was observed to have increased
and if a cystic capsule with transparent liquid and blood vessels
was apparent, it was considered that the EMs model was successfully
established. In the present experiment, out of the 40 rats that
were used, 33 survived, with a success percentage of 82.5%.
Experimental groups and
administration
Thirty-two of the model rats were randomly divided
into four groups, with eight rats in each group: the model control
(untreated), high-dose (15 g/kg) XCHT-treated, low-dose (7.5 g/kg)
XCHT-treated and gestrinone-treated (0.5 mg/kg) groups. A further
eight rats subjected to a sham surgery were used as a normal
control group. The rats in the normal control and untreated groups
were administered an equivalent amount of normal saline, while the
treatment groups were treated with the corresponding drugs. The
saline and drugs were administered daily by gavage for 21 days.
Following the final administration, the abdominal cavity of the
rats was opened under aseptic conditions and the eutopic and
ectopic endometria were separated for analysis of the mRNA and
protein levels of MMP-2 and MMP-9.
Hematoxylin and eosin (H&E)
staining
Fresh tissues were fixed in 10% formalin and
paraffin-embedded, prior to being cut into sections of 4 μm. The
sections were successively treated with xylene, anhydrous ethanol,
90% ethanol and 70% ethanol, then dipped in distilled water for 2
min and used for H&E staining for 5–10 min. Excess dyes were
washed away using tap water. The sections were subsequently
gradient dehydrated in 70 and 90% ethanol for 10 min, respectively,
prior to being stained with eosin for 2–3 min. The tissues were
dehydrated, rendered transparent and mounted prior to being
analyzed under a microscope.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the tissues of the
eutopic and ectopic endometria using TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and
reverse-transcribed into cDNA. The transcription procedure was as
follows: 2 μl RNA, 3 μl oligo(dT) and 9 μl distilled water were
mixed and incubated at 70°C for 5 min, prior to being removed and
promptly placed on ice. Following this, 6 μl 5X buffer, 1 μl
Moloney murine leukemia virus (M-MLV; Promega Corp., Madison, WI,
USA), 1.5 μl deoxyribonucleotide triphosphate (dNTP) and distilled
water were added up to a total volume of 30 μl. The mixture was
subsequently incubated at 42°C for 60 min and then heated at 95°C
for 5 min to deactivate the reverse transcriptase.
The PCR reaction system comprised 2 μl cDNA, 2.5
units Taq DNA polymerase, 5 μl 10X buffer, 1.5 mM MgCl2,
0.2 mM dNTP and 10 pmol/l of each primer, with distilled water
added to provide a total volume of 50 μl. The primers were
purchased from Shanghai Sangon Biotechnology Co. Ltd. (Shanghai,
China); the sequences of the primers are shown in Table I. The PCR conditions were as
follows: one cycle of 95°C for 5 min, 35 cycles of 94°C for 30 sec,
Tm°C for 1 min (Tm was dependent on the primer set, see Table I) and 72°C for 40 sec, and one
cycle of 72°C for 10 min. The PCR products were subsequently
electrophoresed in 1.5% agarose gel, prior to being stained with
ethidium bromide. The images were collected under an ultraviolet
(UV) lamp and analyzed using Quantity One® 1-D Analysis
Software v4.6 (Bio-Rad, Hercules, CA, USA).
 | Table IPCR primers and temperature (Tm). |
Table I
PCR primers and temperature (Tm).
| Gene | Primer sequence | Tm (°C) | Product size
(bp) |
|---|
| MMP-2 | Sense:
5′-GGCCCTGTCACTCCTGAGAT-3′
Antisense: 5′-GGCATCCAGGTTATCGGGGA-3′ | 56 | 326 |
| MMP-9 | Sense:
5′-GATGCGTGGAGAGTCGAAAT-3′
Antisense: 5′-CACCAAACTGGATGACGATG-3′ | 58 | 273 |
| GAPDH | Sense:
5′-CAGGGCTGCTTTTAACTCTG-3′
Antisense: 5′-GATGATCTTGAGGCTGTTGTC-3′ | 58 | 385 |
Western blotting
The tissues of the eutopic and ectopic endometria
were mixed using radio-immunoprecipitation assay (RIPA) lysis
buffer at a volume ratio of 1:10. The lysates were homogenized
using an ultrasonic homogenizer, and then maintained on ice for 10
min prior to centrifuging at 12,000 × g for 20 min. Following this,
the supernatant was collected and the concentration of protein was
determined using the Coomassie Brilliant Blue method. Protein
samples were separated using sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene
difluoride (PVDF) membrane. The membrane was then dipped in 5%
skimmed milk powder for 2 h to block the nonspecific binding sites
prior to incubation with the primary antibody (for MMP-2, 1:2,000;
for MMP-9, 1:2,000; or for GAPDH, 1:4,000) at 4°C overnight. The
MMP-2 and MMP-9 antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). GAPDH and secondary
antibodies were purchased from Shanghai Kangcheng Biotechnology Co.
Ltd. (Shanghai, China). After rinsing in Tris-Buffered Saline and
Tween 20 (TBST) three times, the membrane was incubated with the
secondary antibody (1:5,000), which was labeled with horseradish
peroxidase (HRP), for 4 h, and then rinsed in TBST a further three
times. Images were obtained using electrochemiluminescence (ECL),
prior to being analyzed using Quantity One® 1-D Analysis
Software v4.6.
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using SPSS 12.0 statistical software
(SPSS, Inc., Chicago, IL, USA). An analysis of variance (ANOVA)
test was used to compare the scores of different groups. Post hoc
(least significant difference, LSD) tests were performed for
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
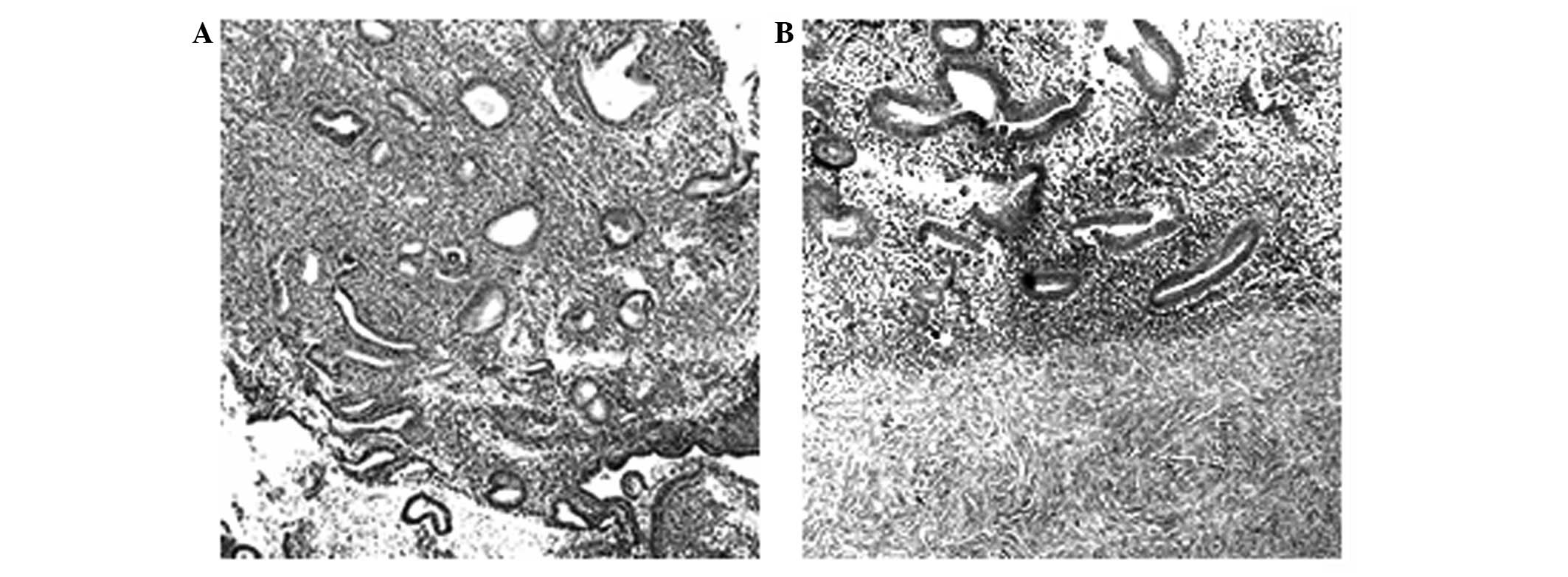
Histological staining of endometrial
tissues
H&E staining showed that the ectopic endometrium
was covered with connective tissues and that the glands and
intercellular substances grew well and intensively. The intima was
thick and the glandular and superficial epithelia formed a high
column. Evident hyperplasia and angiopoiesis were observed, as
shown in Fig. 1.
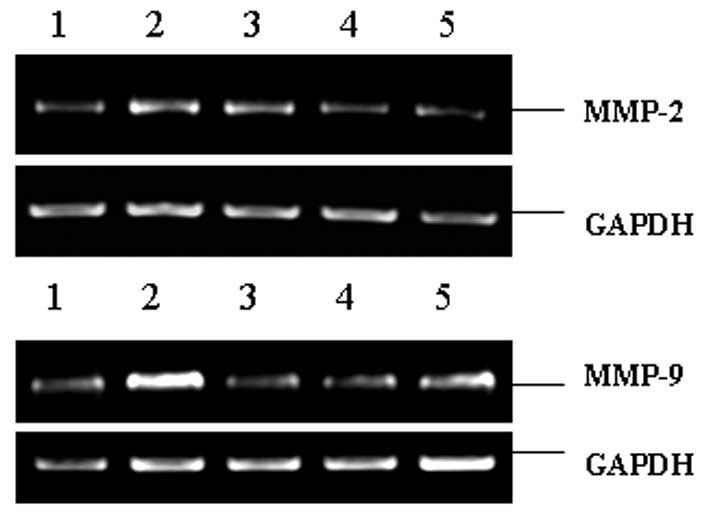
Expression of MMP-2 and MMP-9 mRNA
As shown in Fig. 2
and Table II, the expression
levels of MMP-2 and MMP-9 mRNA were low in the normal endometria,
but increased significantly in the ectopic endometria (P<0.01
and P<0.001, respectively). Following XCHT administration, the
MMP-2 and MMP-9 mRNA levels were significantly decreased compared
with those in the model group (P<0.05 and P<0.001,
respectively, for the 7.5 g/kg group; P<0.01 and P<0.001,
respectively, for the 15 g/kg group). These results indicate that
XCHT reduced the mRNA levels of MMP-2 and MMP-9.
 | Table IIEffect of XCHT on mRNA levels of MMP-2
and MMP-9. |
Table II
Effect of XCHT on mRNA levels of MMP-2
and MMP-9.
| Group | Dose (g/kg) | MMP-2/GAPDH | MMP-9/GAPDH |
|---|
| Sham surgery | - | 0.77±0.24 | 0.83±0.36 |
| Model control | - | 1.24±0.35a | 1.87±0.33b |
| XCHT | 7.5 | 1.06±0.37c | 0.89±0.27e |
| XCHT | 15 | 0.72±0.31d | 0.87±0.36e |
| Gestrinone | 0.0005 | 0.64±0.21d | 0.99±0.34e |
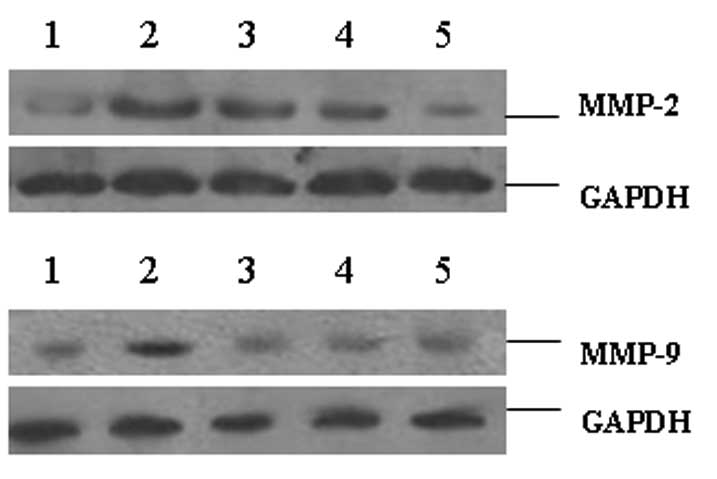
Protein levels of MMP-2 and MMP-9
As shown in Fig. 3
and Table III, the protein
levels of MMP-2 and MMP-9 in the ectopic endometria were
significantly higher than those in the normal endometria
(P<0.001). In the tissues of the XCHT-treated groups, the
protein levels of MMP-2 and MMP-9 were significantly decreased
compared with those in the model control group (P<0.05 and
P<0.001, respectively, for the 7.5 g/kg group; P<0.01 and
P<0.001, respectively, for the 15 g/kg group). These results
indicate that XCHT reduced the protein levels of MMP-2 and
MMP-9.
 | Table IIIEffect of XCHT on protein levels of
MMP-2 and MMP-9. |
Table III
Effect of XCHT on protein levels of
MMP-2 and MMP-9.
| Group | Dose (g/kg) | MMP-2/GAPDH | MMP-9/GAPDH |
|---|
| Sham surgery | - | 0.27±0.06 | 0.32±0.09 |
| Model control | - | 0.87±0.24a | 0.96±0.24a |
| XCHT | 7.5 | 0.69±0.12b | 0.41±0.11d |
| XCHT | 15 | 0.54±0.14c | 0.37±0.09d |
| Gestrinone | 0.0005 | 0.31±0.11d | 0.33±0.12d |
Discussion
MMPs are zinc-dependent proteases that preserve the
function of disassembling the ECM, thereby participating in the
degradation and reconstruction of numerous types of tissues. This
function is also closely associated with the invasion and
metastasis of cancer cells (7–9).
Similar to tumor cells, the ectopic endometrium is able to shed,
diffuse, metastasize and invade surrounding tissues and organs.
Studies have shown that the expression of MMPs is significantly
increased in the ectopic endometrium, indicating that MMPs may
participate in the displacement of the endometrium. Among the MMP
family, MMP-2 and MMP-9 are closely associated with the formation
of EMs (10–12).
Numerous studies have demonstrated that XCHT and its
active components exhibit notable therapeutic effect in EMs
(4,5). Zheng et al(3) observed that XCHT inhibited the growth
and angiogenesis of the ectopic endometrium in a rat model, which
was most likely associated with the regulation of the immune
system. Pan and Zheng (13)
suggested that the effect of XCHT on EMs may have been due to the
decrease in cyclooxygenase (COX)-2 and P450 levels in the ectopic
endometrium and the following reduction of estrogen. Furthermore,
Zhang and Wan (14) demonstrated
that baicalein exhibited a good therapeutic effect in rats with
EMs, with a possible mechanism being through reductions in the
levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
IL-8, and the inhibition of the expression of intercellular
adhesion molecule-1 (ICAM-1) and Bcl-2. Song et al(15) revealed that ginsenoside Rg3
inhibited the expression of inhibitor of DNA binding 1 (ID-1) and
neuropilin-1 (NRP1) in EMs cells. However, it was unclear whether
XCHT was able to reverse the abnormal expression of MMP-2 and MMP-9
in EMs. In the present study, we demonstrated that the mRNA and
protein levels of MMP-2 and MMP-9 were significantly higher in the
ectopic endometrium than those in the normal endometrium, which
further indicated that MMP-2 and MMP-9 were involved in
pathogenesis of EMs. We also showed that XCHT was able to
significantly downregulate the mRNA and protein expression levels
of MMP-2 and MMP-9 in the ectopic endometrium, which suggests that
the therapeutic effect of XCHT on EMs may have been due to the
inhibition of MMP-2 and MMP-9.
In conclusion, XCHT is able to decrease the
expression of MMP-2 and MMP-9 in the ectopic endometrium. The
present results may provide a potential theoretical basis for the
therapy of EMs.
References
|
1
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into the venous circulation. Am J Pathol. 3:93–110.
1927.PubMed/NCBI
|
|
2
|
Umezawa M, Saito Y, Tanaka-Hattori N,
Takeda K, Ihara T and Sugamata M: Expression profile of
extracellular matrix and adhesion molecules in the development of
endometriosis in a mouse model. Reprod Sci. 19:1365–1372. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng H, Zuo LD, Li HY, Zhang WJ and Wang
ZN: Effects of Xiaochaihu Tang, a traditional Chinese medicinal
preparation, on ectopic endometrium in rats. Di Yi Jun Yi Da Xue
Xue Bao. 24:1319–1321. 2004.(In Chinese).
|
|
4
|
Zheng H, Zuo LD, Li HY and Jin HY: Effects
of Xiaochaihu Tang on morphology of ectopic endometrium in
experimentally induced endometriosis in rats. Chin J New Drugs Clin
Rem. 24:200–202. 2005.(In Chinese).
|
|
5
|
Zhang WJ, Wang ZN and Zheng PE: Regulation
effects of Xiaochaihu Tang on IL-8 and TNF-α in endometriosis.
Shanxi J Trad Chin Med. 25:468–470. 2004.(In Chinese).
|
|
6
|
Jones RC: The effect of a luteinizing
hormone releasing hormone (LRH) agonist (Wy-40,972),
levonorgestrel, danazol and ovariectomy on experimental
endometriosis in the rat. Acta Endocrinol (Copenh). 106:282–288.
1984.PubMed/NCBI
|
|
7
|
Wang J and Ma X: Effects of estrogen and
progestin on expression of MMP-2 and TIMP-2 in a nude mouse model
of endometriosis. Clin Exp Obstet Gynecol. 39:229–233.
2012.PubMed/NCBI
|
|
8
|
Moroz A, Delella FK, Lacorte LM, Deffune E
and Felisbino SL: Fibronectin induces MMP2 expression in human
prostate cancer cells. Biochem Biophys Res Commun. 430:1319–1321.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Li D, Zhang W, Zhou J, Tang B and Li
L: Matrix metalloproteinase-9 expression correlates with prognosis
and involved in ovarian cancer cell invasion. Arch Gynecol Obstet.
286:1537–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malvezzi H, Aguiar VG, Paz CC,
Tanus-Santos JE, Penna IA and Navarro PA: Increased circulating
MMP-2 levels in infertile patients with moderate and severe pelvic
endometriosis. Reprod Sci. 20:557–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weigel MT, Krämer J, Schem C, et al:
Differential expression of MMP-2, MMP-9 and PCNA in endometriosis
and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol.
160:74–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Q, Qiu N, Pu D, Zhou Y, Li T and Yang
H: Change profiles in matrix metalloproteinase-2 and -9 in induced
endometriosis in mice. J Huazhong Univ Sci Technolog Med Sci.
30:188–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan L and Zheng H: Effect of Xiaochaihu
Soup on the expression of COX-2 and P450arom of endometriosis in
rats. Bull Med Res. 40(8): 126–128. 2011.(In Chinese).
|
|
14
|
Zhang J and Wan SQ: The therapeutic
effects of scutellarin on endometriosis in rats. Lishizhen Med Mat
Med Res. 18:895–897. 2007.(In Chinese).
|
|
15
|
Song ZY, Fu K, Hu LY, Zheng ZR, Ge WJ and
Li ZA: The influence of ginsenoside Rg3 on ID-1 and NRP-1 gene
expression in endometriotic tissues. Chin Rem Clin. 11:768–771.
2011.(In Chinese).
|