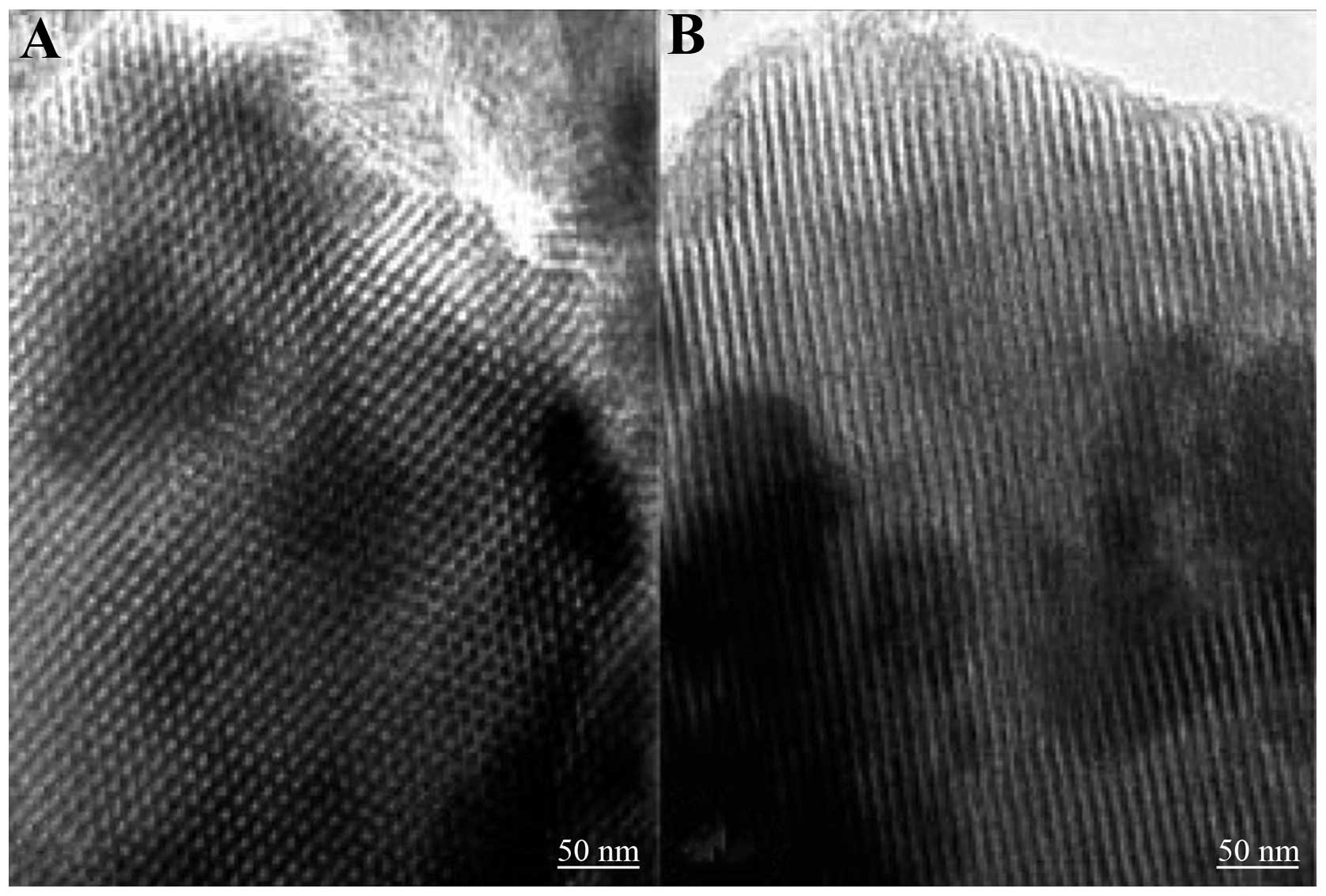
Introduction
Methods for the repair of bone loss include the
filling of bone tissues and the replacement of bone materials. The
participation of protein factors promotes bone formation. In recent
years, the clinical application of artificial bone materials,
including calcium hydroxyapatite, tricalcium phosphate and
bioactive glass, has been studied (1–4). In
addition to mediating ossification, ideal bone replacement
materials may also store and release (delayed release) active
substances required for bone formation. Therefore, an effective
concentration of activity proteins in the filled area may promote
the repair of bone disunion and loss. Several scholars have used
synthetic materials, including calcium hydroxyapatite, tricalcium
phosphate, calcium sulfate and bioactive glass with activity
protein factors, including bone morphogenetic proteins (BMPs) and
transforming growth factor, to repair bone loss (5–7). In
addition, a number of scholars have integrated drugs, such as
Ag+ and antibiotics, into bone filler materials to cure
osteomyelitis and to study the controlled release process (8,9).
Therefore, active drugs may be provided in infected areas for a
longer time period and the effectiveness of osteomyelitis treatment
may be enhanced (10–12). However, few researchers have
studied the long-term delayed release of bioactive glass-adsorbed
BMPs (13).
BMPs are special bone growth factors that may induce
the formation of bone and cartilage in vivo and in
vitro. BMP-2 and BMP-7 have high ossification activities and
are used extensively in bone repair (14–16).
In solution, BMP-2 flows and diffuses easily in vivo.
However, BMP-2 is also easily degraded by proteases and is unable
to produce a long-term marked effect in local areas, thereby
affecting the effectiveness of the treatment. The use of BMPs in
bone repair involves loading active BMPs with sufficiently high
purity into a bone fill material with delayed release properties,
and implanting the material in vivo. The BMPs are released
by degradation and replaced with bone material. BMPs enhance bone
formation when maintained at a high concentration in local areas
for a long time period.
Bioactive glass has good biocompatibility and
surface bioactivity. Such material has dual mechanisms of action,
specifically, the induction and mediation of ossification.
Bioactive glass is a suitable material for the repair and
replacement of lost bone. A mesoporous bioactive glass with ordered
nanopores (80S MBG) was manufactured by Yan in 2005 (17). 80S MBG has pores with a diameter of
5–20 nm, a specific pore volume of 0.40 cm3/g and
specific surface area of 300 m2/g (Fig. 1). This material has the ability to
absorb and delay the release of active proteins, and also has good
bioactivity and tissue compatibility. 80S MBG promotes ossification
by modulating IGF-11 gene expression in ossification cells
(18,19). The current study aims to: i) test
the efficacy of delayed release and adsorption of BMP-2 by 80S MBG,
ii) investigate the effective association of MBG and BMPs, and iii)
collect information concerning the delayed release of BMP-2 by 80S
MBG relevant to its use as a filler for bone loss, for stimulating
and inducing ossification, effectively aiding fracture healing
disorders and repairing bone loss.
Materials and methods
Characteristics of 80S MBG
The component molar ratio of 80S MBG (provided by
the Inorganic Chemistry Teaching and Study Department of Fudan
University, Shanghai, China) was 80:15:5
(SiO2:CaO:P2O5). The ordered
mesopores were 5–20 nm. The 80S MBG appeared gray in color. MBG (50
mg) was made into a tablet with diameter of 4 mm, depth of 3.5 mm.
This material was composed of microparticles with a diameter of
38–76 μm.
Preparation and identification of
125I-rhBMP-2
Tagging
To one Iodogen test tube (Sigma, St. Louis, MO, USA)
was added 20 μg/10 μl recombinant human BMP (rhBMP)solution (Dahui
Bioengineering Company, Guangzhou, China; purity >95%) and 1.5
μl Na 125I solution (365 μCi; Radiomedicine Institute,
Shanghai, China). After 10 min of mixing and reaction at room
temperature, the reaction was terminated by the addition of a 0.01
mol/l pH 7.4 phosphate-buffered saline (PBS) solution. Samples were
obtained when the tagging reaction had been performed for ~10 min.
A silica gel instant thin-layer chromatography paper (ITLC/SG)
method was used to analyze the tagging rate. The radioactive
tagging rate obtained was >90%.
Purification
The aforementioned reaction liquid was purified via
Sephadex G25 column chromatography. One chromato bar (Sigma
Chemical Co., St. Louis, MO, USA) filled with Sephadex G25 gel was
used, with 0.01 mol/l PBS and pH 7.4 buffer solution for elution
and purification. After repeating the elution and purification
twice, a high purity 125I-rhBMP-2 protein tagging
solution was obtained. This solution was analyzed, identified and
quantified (radiochemical purity >95%) by ITLC/SG. Following
aseptic filtration of 0.22 μm, a concentrated solution of
125I-rhBMP-2 was obtained.
Identification
i) ITLC/SG method: ITLC/SG was used as the vehicle.
After spreading with alcohol and water (ratio, 85:15 v/v), each
chromatography paper was cut into small strips with an equal width
of 1 cm. The strips were placed in the radioactive measurement test
tube for assay. The Rf value of 125I-rhBMP-2 protein was
0.0. The Rf value of 125I was 0.7–0.8. The radioactivity
percentage binding rate (Rf value = 0.0) was the radiochemical
purity of 125I-rhBMP-2 protein. ii) Trichloroacetic acid
(TCA) precipitation method: a 100-μl solution of
125I-rhBMP-2 protein was placed in a small centrifuge
tube and 1.5 ml 10% aqueous TCA solution was added. After mixing,
the solution was centrifuged for 5 min at 686 × g. The supernatant
was removed and the radioactivity percentage binding rate (TCA
sediment) was tested. The result indicated the radiochemical purity
of the 125I-rhBMP-2 protein.
Following purification with Sephadex G25 by column
chromatography, the radiochemical purity of the
125I-rhBMP-2 protein was evaluated and a result of ≥95%
was obtained.
Radioactive measurement
The gauge used was an SH-682 radioimmunity γ-ray
counter (Shanghai Nucleus Institute Rihuan Apparatus No. 1 Factory,
Shanghai, China). A sample applicator was placed at the bottom of a
12×60 mm plastic tube for radioimmunity measurement. Radioactive
counts per min per sample were measured using the SH-682
radioimmunity γ-ray counter.
Loading of quantitation protein on 80S
MBG
A tablet of MBG was placed at the bottom of each
12×60 mm plastic tube for radioimmunity measurement (6 MBG material
tubes). A ~15 μl aliquot of 125I-rhBMP-2 protein
solution (rhBMP-2 protein concentration, 2 mg/ml; load protein
quantity, 30 μg/15 μl/tablet) and was added to the center of each
performing. After dehydration for three days on a 4°C drying dish,
the samples were stored for further use.
Delayed release test
The groups were as follows: test group,
MBG-125I-rhBMP-2, n=6 and control group, MBG blank, n=4.
The dehydrated 80S MBG material preforming (preforming/tube) loaded
with 125I-rhBMP-2 protein was obtained. Simulated body
fluid (SBF) buffer solution (1 ml) was added to each tube. The
tubes were then placed in a 37°C water bath. After a specific
interval (times are provided in Tables
I and II), the liquid was
obtained. A 2 ml SBF buffer solution was used to wash the
preforming solid (twice). Persistent radioactivity [radioactivity
count/min (cpm)] in the preforming solid was tested. The mean dose
released per day was obtained after correction for attenuation
using the following formula: (Measured value at present time-point
- measured value at previous time-point)/release days.
 | Table ICumulative dose of BMP-2 protein
released by MBG at different time-points (n=6). |
Table I
Cumulative dose of BMP-2 protein
released by MBG at different time-points (n=6).
| Time (days) | Mean cumulative
released dose (ng) | SD | Percentage of the
total load |
|---|
| 1 | 6,864 | 314 | 22.88 |
| 2 | 7,688 | 334 | 25.63 |
| 3 | 8,071 | 376 | 26.90 |
| 4 | 8,304 | 387 | 27.68 |
| 6 | 8,404 | 394 | 28.01 |
| 7 | 8,664 | 365 | 28.88 |
| 9 | 8,880 | 388 | 29.60 |
| 13 | 9,068 | 399 | 30.23 |
| 16 | 9,259 | 446 | 30.86 |
| 19 | 9,444 | 463 | 31.48 |
| 23 | 9,600 | 423 | 32.00 |
| 27 | 9,750 | 415 | 32.50 |
| 31 | 9,799 | 434 | 32.66 |
| 37 | 10,130 | 398 | 33.77 |
| 45 | 10,516 | 385 | 35.05 |
| 52 | 10,721 | 380 | 35.74 |
| 59 | 10,968 | 379 | 36.56 |
| 66 | 11,370 | 302 | 37.90 |
| 73 | 11,721 | 306 | 39.07 |
| 80 | 11,734 | 215 | 39.11 |
| 87 | 11,877 | 297 | 39.59 |
| 94 | 11,894 | 246 | 39.65 |
 | Table IIMean dose of BMP-2 protein released
per day by MBG at different time-points (n=6). |
Table II
Mean dose of BMP-2 protein released
per day by MBG at different time-points (n=6).
| Time (days) | Mean dose released
per day (ng) | SD |
|---|
| 1 | 6,864 | 314 |
| 2 | 824 | 80 |
| 3 | 384 | 55 |
| 4 | 232 | 29 |
| 6 | 50 | 5 |
| 7 | 260 | 177 |
| 9 | 108 | 51 |
| 13 | 47 | 10 |
| 16 | 64 | 36 |
| 19 | 61 | 35 |
| 23 | 39 | 17 |
| 27 | 38 | 10 |
| 31 | 12 | 15 |
| 37 | 55 | 7 |
| 45 | 48 | 6 |
| 52 | 29 | 8 |
| 59 | 35 | 6 |
| 66 | 57 | 15 |
| 73 | 50 | 6 |
| 80 | 2 | 31 |
| 87 | 20 | 34 |
| 94 | 2 | 25 |
Construction and analysis of a delayed
release curve
The total amount of rhBMP-2 released and the mean
dose released per day at each time-point in the test and control
groups were used to plot a rhBMP-2 delayed release curve. The
character was analyzed and a general equation for the delayed
release of BMP-2 was determined according to the curve.
Statistical analysis
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze the data, which were expressed as mean ±
standard deviation. One-way ANOVA was used o perform group
comparisons. Partial data were used for further comparison among
means [least significant difference (LSD)]. P<0.05 was
considered to indicate a statistically significant result.
Results
Amount of 125I-rhBMP-2
released by 80S MBG per day
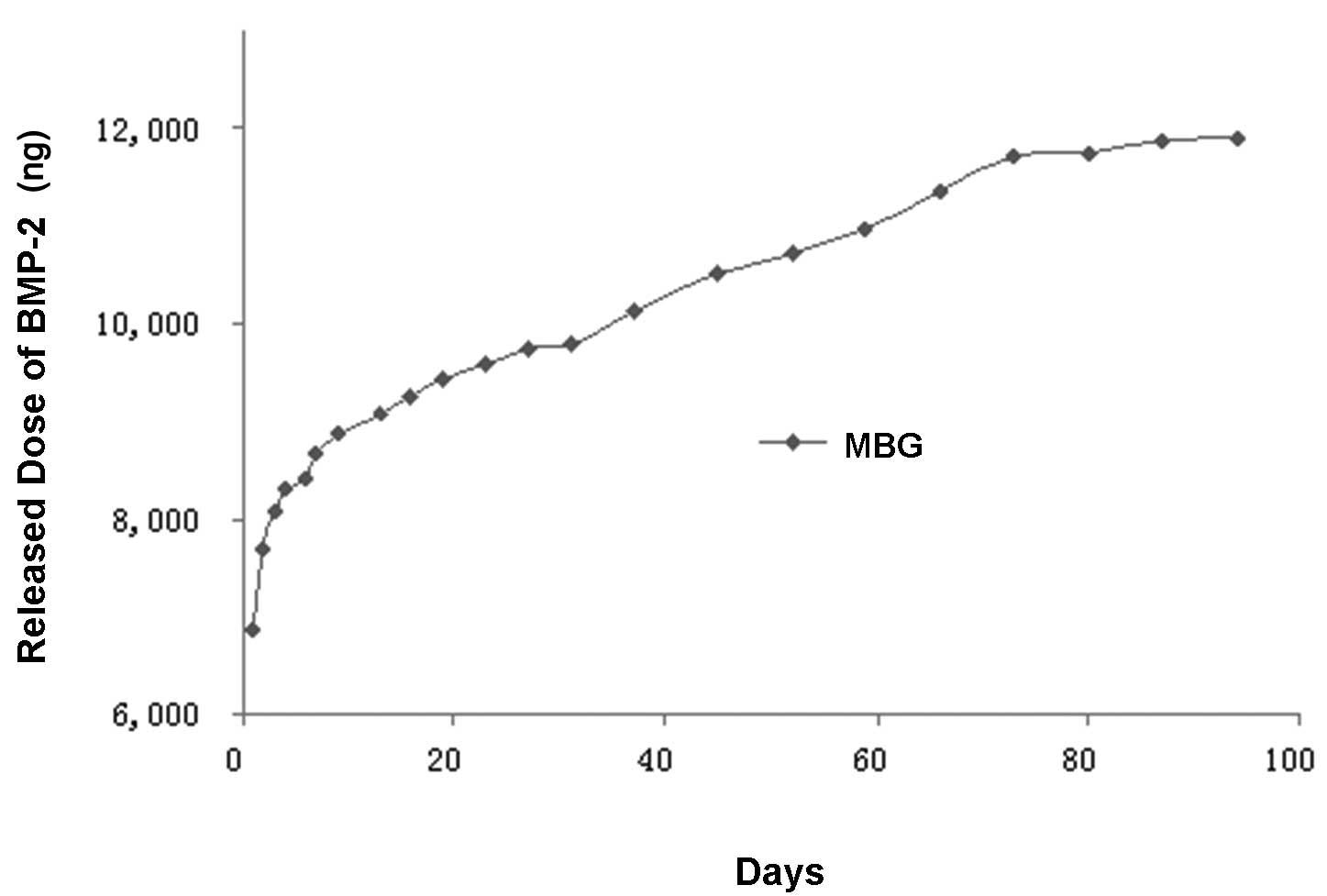
Table I and
Fig. 2 show the cumulative amount
of 125I-rhBMP-2 released at each time-point. The mean
amount of 125I-rhBMP-2 released each day is listed in
Table II. The amount released
during the first day was 6.9 μg, which was 23% of the total load.
The amount released during the second day was 0.8 μg, which was
2.7% of the total load amount. From the third day, a period of
relatively steady delayed release was achieved. By the 94th day,
the total amount released was 11.9 μg, which was 39.7% of the total
load.
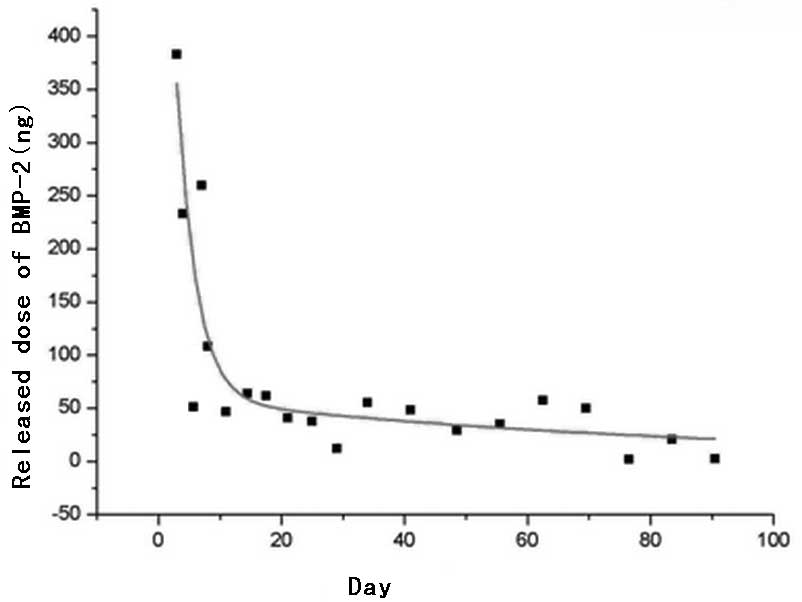
According to the time curve of the amount of
125I-rhBMP-2 released per day (Fig. 3), the amount released per day was
stable at ~2–64 ng/day after 10 days of release. The mean amount
released was 37.42±18.67 ng/day and the curve was steady. ANOVA-LSD
analysis (Tables III and
IV) show that the mean amount
released per day had no significant differences after 10 days
(P>0.05), which indicated that the amount of
125I-rhBMP-2 released per day was steady.
 | Table IIIANOVA of the groups. |
Table III
ANOVA of the groups.
| Comparison | Sum of squares | df | Mean square | F | Significance |
|---|
| Between groups | 264917816.403 | 21 | 12615134.114 | 1856.171 | <0.001 |
| Within groups | 747595.160 | 110 | 6796.320 | | |
| Total | 265665411.563 | 131 | | | |
 | Table IVMultiple comparisons for dependent
variables: released dose/day (least significant difference). |
Table IV
Multiple comparisons for dependent
variables: released dose/day (least significant difference).
| (I) Day | (J) Day | Mean difference
(I–J) (lower bound) | Standard error
(upper bound) | Significance (lower
bound) | 95% Confidence
interval |
|---|
|
|---|
| Lower bound | Upper bound |
|---|
| 13 | 1 | −6817.255a | 47.597 | <0.001 | −6911.580 | −6722.930 |
| 2 | −776.655a | 47.597 | <0.001 | −870.980 | −682.330 |
| 3 | −336.810a | 47.597 | <0.001 | −431.135 | −242.485 |
| 4 | −185.230a | 47.597 | <0.001 | −279.555 | −90.905 |
| 6 | −5.072 | 47.597 | 0.907 | −98.537 | 87.649 |
| 7 | −213.460a | 47.597 | <0.001 | −307.785 | −119.135 |
| 9 | −61.078 | 47.597 | 0.202 | −155.404 | 33.247 |
| 16 | −16.943 | 47.597 | 0.723 | −111.269 | 77.382 |
| 19 | −14.482 | 47.597 | 0.762 | −108.807 | 79.844 |
| 23 | 7.888 | 47.597 | 0.869 | −86.437 | 102.214 |
| 27 | 9.213 | 47.597 | 0.847 | −85.112 | 103.539 |
| 31 | 34.808 | 47.597 | 0.466 | −59.517 | 129.134 |
| 37 | −8.323 | 47.597 | 0.862 | −102.649 | 86.002 |
| 45 | −1.258 | 47.597 | 0.979 | −95.584 | 93.067 |
| 52 | 17.662 | 47.597 | 0.711 | −76.664 | 111.987 |
| 59 | 11.558 | 47.597 | 0.809 | −82.767 | 105.884 |
| 66 | −10.477 | 47.597 | 0.826 | −104.802 | 83.849 |
| 73 | −3.240 | 47.597 | 0.946 | −97.565 | 91.085 |
| 80 | 45.007 | 47.597 | 0.346 | −49.319 | 139.332 |
| 87 | 26.517 | 47.597 | 0.579 | −67.809 | 120.842 |
| 94 | 44.490 | 47.597 | 0.352 | −49.835 | 138.815 |
50% delay in release time of rhBMP-2 by
80S MBG
The square root of the amount of protein released by
80S MBG at days 3–94 was used to create a scatter diagram. A line
was constructed using linear regression analysis (Fig. 4). The equation of the line is as
follows: y = 490.55×1/2 + 7268.82 or × = [(y -
7268.82)/490.55]2. The calculation shows the half-life
of 30 μg BMP-2 loading, where × = [(15,000 -
7,268.82)/490.55]2 = 248.38 days.
Discussion
80S MBG has ordered nanometer-sized mesopores and a
large pore volume and specific surface area. This material is
expected to have a good adsorption function. Aside from the air
space between particles, the large pore volume and specific surface
area will attach more BMPs and further increase the enduring load
ability for BMPs of MBG. In SBF, recrystallization may be observed
on the surface of MBG through rapid ion exchange. Hydroxyapatite
(HA) crystal coatings are produced on the surface of MBG materials
or at the cavosurface of air spaces (18,20–22),
which makes the aperture of the lumens in the micropore structure
zoom out and the proteins inside cannot be separated quickly. These
types of ‘self-close’ features may contain activity proteins in
vivo and delay their release with the degradation of the
material. The test of long-term controlled release confirmed that
80S MBG possesses the ‘self-close’ attribute, along with good
adsorption capacity and delayed release ability for BMPs.
Initially, BMP-2 was generated by a quick-release
process, in which 6.9 μg was released during the first day and 0.8
μg was released during the second day. A total of 25.67% of the
adsorbed protein was released within two days. This observation may
be attributed to the adsorption of BMP-2 by 80S MBG. Furthermore,
surface adhesion of the protein to the material was weak and thus
the protein was easily separated in the SBF. An amount of HA
crystals sufficient to delay the release of BMP-2, i.e., the
‘self-close’ features that inhibit the absorption of the protein
in vivo, had not yet formed on the surface of the MBG.
Dissolution and precipitation of surface adsorption proteins are
the most significant processes of the rapid release during the
initial stage.
From the third day, the release rate markedly slowed
down. From day 10 to 3 months after the initial observation, the
release of BMP-2 was relatively steady and the mean dose released
per day was maintained between 2 and 64 ng. The mean amount
released per day had no significant differences after 10 days
(P>0.05). This type of release is associated with the particular
attributes of MBG. After the third day, crystal growth
reconstruction on and within the surface of the MBG tablet material
was completed, leading to a typical porous delayed release behavior
of the vehicle. The Higuchi equation (23) expresses the delayed release of a
drug by a porous vehicle as: Mt = AM0
[Cs Deff (2Cd - ɛCs)
t]1/2, where Mt is the dose of drug released
in time t; M0 is the total amount of loaded drug; A is
the surface area of the vehicle material; Deff is the
effective solubility factor of drug in the vehicle micropores;
Cs is the drug solubility; Cd is the drug
concentration and ɛ is the microporosity of the vehicle. The
release behavior of a drug in a porous delayed release vehicle
should be in accordance with the equation. For a known vehicle
material and loaded drug (e.g., 80S MBG and 125I-BMP-2
in this study), M0, Cs, Deff,
Cd and Cs are all constant, whereas A and ɛ
are also fixed at a specific value following the reconstruction of
the MBG material surface. Therefore, this finding is the most
direct and simple manifestation of the equation indicating that
Mt and t1/2 are directly proportional. In the
current study, the square root of time (days) and BMP-2 released
dose exhibited a linear correlation, that is, the Higuchi model
almost exhibits a straight line. This indicates that the release
behavior of 125I-BMP-2 by 80S MBG is in accordance with
the Higuchi equation (24–26).
The adsorption and delayed release behavior of BMP
by MBG has a significant contribution to the repair of fracture
healing disorders and bone loss. MBG is known for demonstrating
efficacy in inducing and mediating ossification. After being used
as a filler to replace the lost bone, the MBG is degraded in
vivo and is gradually replaced by bone tissues. The presence of
BMP-2 further enhances ossification. However, the repair of bone
loss and the curing of non-union is a long-term process. BMP-2 may
produce a marked effect and it must be maintained at a high
concentration for a long time period. Uludag et al(27) observed that the BMP concentration
in a local area is closely associated with the
ossification-inducing potential, thus demonstrating their direct
correlation. In summary, a higher concentration of BMP corresponds
to a stronger ability to induce ossification of the surrounding
osteoblasts (27). The local
delayed release of BMP induces ossification and prevents
neoplasm-like changes caused by exorbitant or ectopic ossification
resulting from BMP moving out of the treated area. The study by
Valentin-Opran et al(28)
shows that a physiological concentration of BMP-2 (2 ng/ml) in a
raw state may be sufficient to complete bone repair. The analysis
of the mean released dose per day in Table II shows a relatively rapid release
in the first 10 days. This corresponds with the hematoma formation
period during fracture repair and reconstruction. The release of a
high concentration of BMP-2 by the vehicle is favorable to
chemotaxis and the accumulation of stem cells in the blood and in
the parenchyma. After two weeks, the protein release rate of the
material may be observed to be steady at ~40 ng and this rate is
maintained for two months. During this stage, bone union enters
into the hematoma organization stage; a metastable BMP-2
concentration is favorable for the further proliferation and cell
differentiation of ossification cells, which are similar to
osteoblasts. After 73 days, the released amount further decreases.
Within three weeks after the continuous decrease, the approximate
mean of the amount released/day was 8 ng, which corresponds with
the advanced stage of bone repair. The demand of the organism for
cytokines is reduced; thus, a decreased released dose may prevent
ectopic ossification and neoplasm-like changes, whilst continuously
inducing ossification and promoting repair. After 248 days, or 8
months, the amount of BMP-2 retained by 80S MBG, as derived by the
Hugichi equation, had dropped by 50%, further indicating that the
delayed release function of MBG facilitates the long-term
maintenance of an effective BMP-2 concentration in the filling
area, thereby meeting clinical practice requirements (29).
In summary, 80S MBG exhibits good adsorption and
delayed release effects for BMP-2. The delayed release
characteristics conform to the Higuchi equation. During the most
important first three months of bone healing, an effective
concentration of BMP-2 control released may enhance bone
ossification. This result indicates that 80S MBG is of significant
value in various clinical areas, including tissue engineering, the
controlled release of drugs, dentistry, orthopedics and oral and
maxillofacial surgery. However, in this study, SBF was used as the
release system of the test of 80S MBG. Although its ion
concentration and structure are completely in accordance with those
of body fluids, SBF is far from the true body fluid. The release
action in vivo occurs in a more complex and multivariate
process. The delayed release of one type of protein from the
vehicle may also have an effect on other proteins and cell tissues
in body fluid. Therefore, further tests in vivo are
required.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30571877) and the Natural Science
Foundation of Shanghai (06ZR14127).
References
|
1
|
Pietruska M, Pietruski J, Nagy K, Brecx M,
Arweiler NB and Sculean A: Four-year results following treatment of
intrabony periodontal defects with an enamel matrix derivative
alone or combined with a biphasic calcium phosphate. Clin Oral
Investig. 16:1191–1197. 2012.
|
|
2
|
Nakase T, Fujii M, Myoui A, Tamai N,
Hayaishi Y, Ueda T, Hamada M, Kawai H and Yoshikawa H: Use of
hydroxyapatite ceramics for treatment of nonunited osseous defect
after open fracture of lower limbs. Arch Orthop Trauma Surg.
129:1539–1534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Guo Z, Bai H, Li J, Li X, Chen G
and Lu J: Clinical evaluation of β-TCP in the treatment of lacunar
bone defects: a prospective, randomized controlled study. Mater Sci
Eng C Mater Biol Appl. 33:1894–1899. 2013.
|
|
4
|
Lindfors NC, Hyvönen P, Nyyssönen M,
Kirjavainen M, Kankare J, Gullichsen E and Salo J: Bioactive glass
S53P4 as bone graft substitute in treatment of osteomyelitis. Bone.
47:212–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannink G, Geutjes PJ, Daamen WF and Buma
P: Evaluation of collagen/heparin coated TCP/HA granules for
long-term delivery of BMP-2. J Mater Sci Mater Med. 24:325–332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sibiya SJ, Olivier EI and Duneas N: High
yield isolation of BMP-2 from bone and in vivo activity of a
combination of BMP-2/TGF-β1. J Biomed Mater Res A. 101:641–646.
2013.PubMed/NCBI
|
|
7
|
Waselau M, Patrikoski M, Juntunen M, et
al: Effects of bioactive glass S53P4 or beta-tricalcium phosphate
and bone morphogenetic protein-2 and bone morphogenetic protein-7
on osteogenic differentiation of human adipose stem cells. J Tissue
Eng. 3:20417314124677892012. View Article : Google Scholar
|
|
8
|
Uskoković V and Desai TA: Phase
composition control of calcium phosphate nanoparticles for tunable
drug delivery kinetics and treatment of osteomyelitis. II.
Antibacterial and osteoblastic response. J Biomed Mater Res A.
101:1427–1436
|
|
9
|
Xing J, Hou T, Luobu B, Luo F, Chen Q, Li
Z, Jin H and Xu J: Anti-infection tissue engineering construct
treating osteomyelitis in rabbit tibia. Tissue Eng Part A.
19:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang T, Wen J, Zhou J, Shao Z and Dong J:
Poly (ɛ-caprolactone) coating delays vancomycin delivery from
porous chitosan/β-tricalcium phosphate composites. J Biomed Mater
Res B Appl Biomater. 100:1803–1811. 2012.
|
|
11
|
Jiang P, Qu F, Lin H, Wu X, Xing R and
Zhang J: Macroporous/mesoporous bioglasses doped with
Ag/TiO2 for dual drug action property and bone repair.
IET Nanobiotechnol. 6:93–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Xie Z, Zhang C, et al: Bioactive
borate glass scaffolds: in vitro and in vivo evaluation for use as
a drug delivery system in the treatment of bone infection. J Mater
Sci Mater Med. 21:575–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergeron E, Marquis ME, Chrétien I and
Faucheux N: Differentiation of preosteoblasts using a delivery
system with BMPs and bioactive glassmicrospheres. J Mater Sci Mater
Med. 18:255–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bosemark P, Isaksson H, McDonald MM and
Little DG: Augmentation of autologous bone graft by a combination
of bone morphogenic protein and bisphosphonate increased both
callus volume and strength. Acta Orthop. 84:106–111.
2013.PubMed/NCBI
|
|
15
|
Draenert ME, Kunzelmann KH, Forriol F,
Hickel R and Draenert K: Primary cancellous bone formation with BMP
and micro-chambered beads: experimental study on sheep. Bone.
52:465–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung MR, Shim IK, Chung HJ, et al: Local
BMP-7 release from a PLGA scaffolding-matrix for the repair of
osteochondral defects in rabbits. J Control Release. 162:485–491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan XX, Deng HX, Yu CZ, et al: Mesoporous
bioactive glasses, synthesis and structural characterization.
Non-Cryst Solids. 351:3209–3217. 2005. View Article : Google Scholar
|
|
18
|
Yan X, Huang X, Yu C, et al: The in-vitro
bioactivity of mesoporous bioactive glasses. Biomaterials.
27:3396–3403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quan Z, Han X, Ye Z, Chenzhong Y and
Wenjun C: Influence of novel nano-mesoporous bioactive glass on the
regulation of IGF-II gene expression in osteoblasts. Cell Biochem
Biophys. 62:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balamurugan A, Benhayoune H, Kannan S, et
al: Cryo-X-ray analysis - A novel tool to better understand the
physicochemical reactions at the bioglass/biological fluid
interface. Microsc Res Tech. 71:684–688. 2008. View Article : Google Scholar
|
|
21
|
Ginsac N, Chenal JM, Meille S, et al:
Crystallization processes at the surface of polylactic
acid-bioactive glass composites during immersion in simulated body
fluid. J Biomed Mater Res B Appl Biomater. 99:412–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nganga S, Zhang D, Moritz N, Vallittu PK
and Hupa L: Multi-layer porous fiber-reinforced composites for
implants: in vitro calcium phosphate formation in the presence of
bioactive glass. Dent Mater. 28:1134–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otsuka M, Fujita H, Nakamura T and Kokubo
T: Effects of ceramic component on cephalexin release from
bioactive bone cement consisting of Bis-GMA/TEGDMA resin and
bioactive glass ceramics. Biomed Mater Eng. 11:11–22. 2001.
|
|
24
|
Chakraborty S, Mitra MK, Chaudhuri MG, Sa
B, Das S and Dey R: Study of the release mechanism of Terminalia
chebula extract from nanoporous silica gel. Appl Biochem
Biotechnol. 168:2043–2056. 2012.PubMed/NCBI
|
|
25
|
Petropoulos JH, Papadokostaki KG and
Sanopoulou M: Higuchi's equation and beyond: overview of the
formulation and application of a generalized model of drug release
from polymeric matrices. Int J Pharm. 437:178–191. 2012.
|
|
26
|
Singh V, Bushetti SS, Raju SA, Ahmad R,
Singh M and Ajmal M: Polymeric ocular hydrogels and ophthalmic
inserts for controlled release of timolol maleate. J Pharm
Bioallied Sci. 3:280–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uludag H, Gao T, Porter TJ, Friess W and
Wozney JM: Delivery systems for BMPs: factors contributing to
protein retention at an application site. J Bone Joint Surg Am.
83-A(Suppl 1 Pt 2): S128–S135. 2001.PubMed/NCBI
|
|
28
|
Valentin-Opran A, Wozney J, Csimma C,
Lilly L and Riedel GE: Clinical evaluation of recombinant human
bone morphogenetic protein-2. Clin Orthop Relat Res. 395:110–120.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giganti MG, Liuni F, Celi M, et al:
Changes in serum levels of TNF-alpha, IL-6, OPG, RANKL and their
correlation with radiographic and clinical assessment in fragility
fractures and high energy fractures. J Biol Regul Homeost Agents.
26:671–680. 2012.PubMed/NCBI
|
|
30
|
Uskoković V and Desai TA: Phase
composition control of calcium phosphate nanoparticles for tunable
drug delivery kinetics and treatment of osteomyelitis. II.
Antibacterial and osteoblastic response. J Biomed Mater Res A.
101:1427–1436
|
|
31
|
Xing J, Hou T, Luobu B, Luo F, Chen Q, Li
Z, Jin H and Xu J: Anti-infection tissue engineering construct
treating osteomyelitis in rabbit tibia. Tissue Eng Part A.
19:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|