Introduction
Tumorigenesis, invasion and metastasis are complex
processes involving several signaling pathways, cell factors and
other tumor-causing factors, and are among the main causes of
mortality. Tumor invasion and metastasis, in addition to the
biological activity of kidney cell transformation and migration,
currently receive considerable attention. Metastatic kidney cancer
is common in patients, and the treatment of renal cell carcinoma is
becoming increasingly difficult. Thus, kidney cancer metastasis and
infiltration are focal points in kidney cancer research.
Biological treatments for kidney cancer have limited
availability. In vitro studies on the side-effects of gene
therapy are limited, and the effects of specific clinical
treatments remain unclear. Epithelial cadherin (E-cadherin), which
is present in epithelial cells, mediates the connections between
calcium-dependent adhesion molecules. E-cadherin, which is
considered to be the most important type of calcium mucin and has
been extensively studied, has the following characteristics: 533
amino acid residues at the N-terminal extracellular domain; 24
amino acid residues composed of highly hydrophobic molecules in the
transmembrane domain; and 150 amino acid residues at the C-terminal
intracellular domain (1).
E-cadherin is vital for maintaining cell morphology (2), cell polarity and the connections
between cells (3). Furthermore,
E-cadherin is an important signal transduction molecule that
participates in the transfer of information between cells (3).
Snail is a zinc-finger transcription factor. The
structure of Snail consists of a variable N-terminal domain and 4–6
zinc fingers with a highly conserved C-terminal region (4). The mechanism of action for Snail
involves the interaction of one of its zinc-finger areas and the
E-cadherin promoter region, E-box sequence (CAGGTG), on the main
chain (5). Thus, Snail becomes a
transcription inhibiting protein and serves as a repressor of
E-cadherin (5).
Previous studies have demonstrated that the
mesenchymal-epithelial transition in tumor infiltration during
migration is closely associated with the development of
epithelia-mesenchymal transition (EMT), and carcinoma cells already
exist in EMT (6,7). Vincent-Salomon and Thiery (8) observed that breast cancer occurs as a
result of infiltration, in which the EMT and migration play
important roles. Similar observations have been made in colon
(9) and liver cancers (10). The indispensable transformation of
epithelial cells into mesenchymal cells results in their
acquisition of interstitial cell characteristics. Hence, the cells
become invasive. It has been confirmed that, in tumorigenesis, EMT
affects numerous transduction factors, including Snail, which
represses E-cadherin expression (11); this is considered to be a key link
in tumorigenesis. Snail expression is observed in tumors showing
features of EMT which causes the downregulation of the
E-cadherin.
Snail expression and E-cadherin repression in clear
cell renal cell carcinoma (CCRCC) are rarely studied. The present
study used the immunohistochemical streptavidin-peroxidase (SP)
method for the combined detection of Snail and E-cadherin in CCRCC,
in the normal tissues adjacent to the tumor and in normal tissues.
The expression characteristics in the CCRCC were analyzed using
clinical and pathological factors. Correlation between these
factors would increase our understanding of the CCRCC infiltration
and migration mechanism and may provide a new theoretical basis for
future targeted therapy for the prevention and treatment of CCRCC
infiltration and migration.
Materials and methods
General data
Patients aged 27–79 years (mean age: 58.41 years)
were recruited from the Fuzhou General Hospital of Nanjing Military
Command of Chinese PLA (Fuzhou, China) from October 2010 to June
2011. Patients were classified according to the American Joint
Committee on Cancer (AJCC; 2002) TNM staging system, and the
conditions were staged and graded using the Fuhrman grading system:
G1, G2, G3, G4, representing high differentiation, differentiation,
low differentiation, undifferentiated, respectively.. Tissue
samples from 69 patients with CCRCC who had undergone radical
nephrectomy were obtained preoperatively prior to the
administration of any related cancer treatment. Postoperative
diagnosis was performed following the pathological examination of
CCRCC. Subsequently, 58 samples from normal tissues adjacent to the
tumor and 10 samples from normal renal tissue were obtained and
compared. This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Fuzhou General Hospital of Nanjing Military Command of Chinese
PLA. Written informed consent was obtained from all
participants.
Immunohistochemical study
All specimens (4-μm-thick sections) were fixed with
10% formalin and embedded in paraffin, followed by preparation of 4
μm sections. 0.01 mol/l citrate buffer was added for antigen
repair. The sections were examined using the SP immunohistochemical
staining method. The procedure with 3,3′-diaminobenzidine staining,
hematoxylin counterstaining and transparent sealing was performed
according to the manufacturer’s instructions. Rabbit anti-human
E-cadherin polyclonal antibody (1:100 dilution) and Rabbit
anti-human Snail antibody (1:150 dilution) were provided by Beijing
Biosynthesis Biotechnology Co., Ltd. (Beijing, China).
Immunohistochemical SP kit was provided by Zymed Laboratories Inc.
(San Francisco, CA, USA). Phosphate-buffered saline instead of
primary antibody was used as negative control.
Judgment standard
Experimental specimens were randomly observed under
a high-power field microscope (magnification, ×400), and the
proportion of positive cells (positivity rate) was calculated based
on the positive expression (demonstrated by yellow staining) of
E-cadherin and Snail in the CCRCC. The samples were determined to
be positive or negative according to positive cell number: positive
cell number ≥25%, positive; no positive staining or positive cell
number <25%, negative (12).
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used to perform the statistical analysis. The rates
and relationships between two groups of variables were compared and
evaluated using the χ2-test and Spearman rank
correlation analysis, respectively. P<0.05 was considered to
indicate a statistically significant result.
Results
Comparison of E-cadherin and Snail
expression
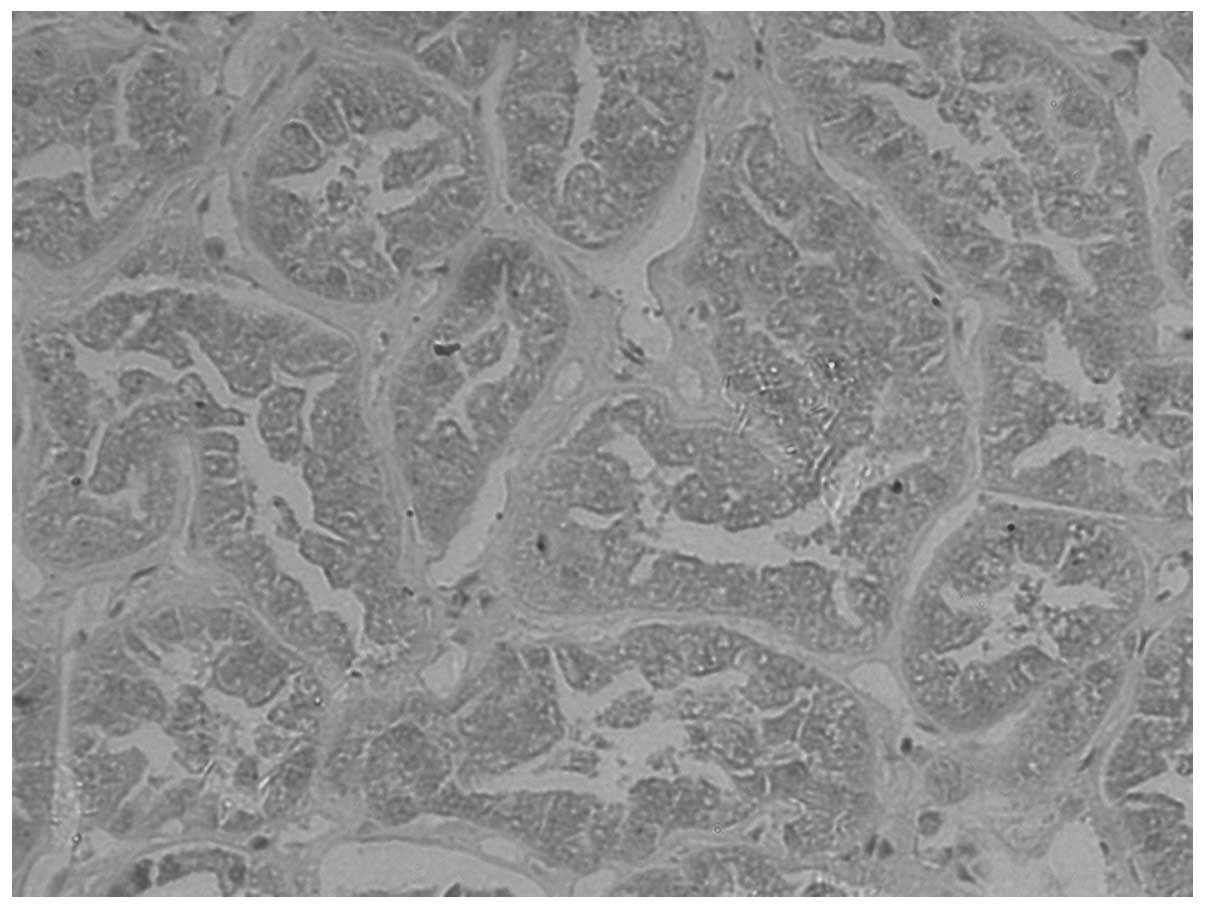
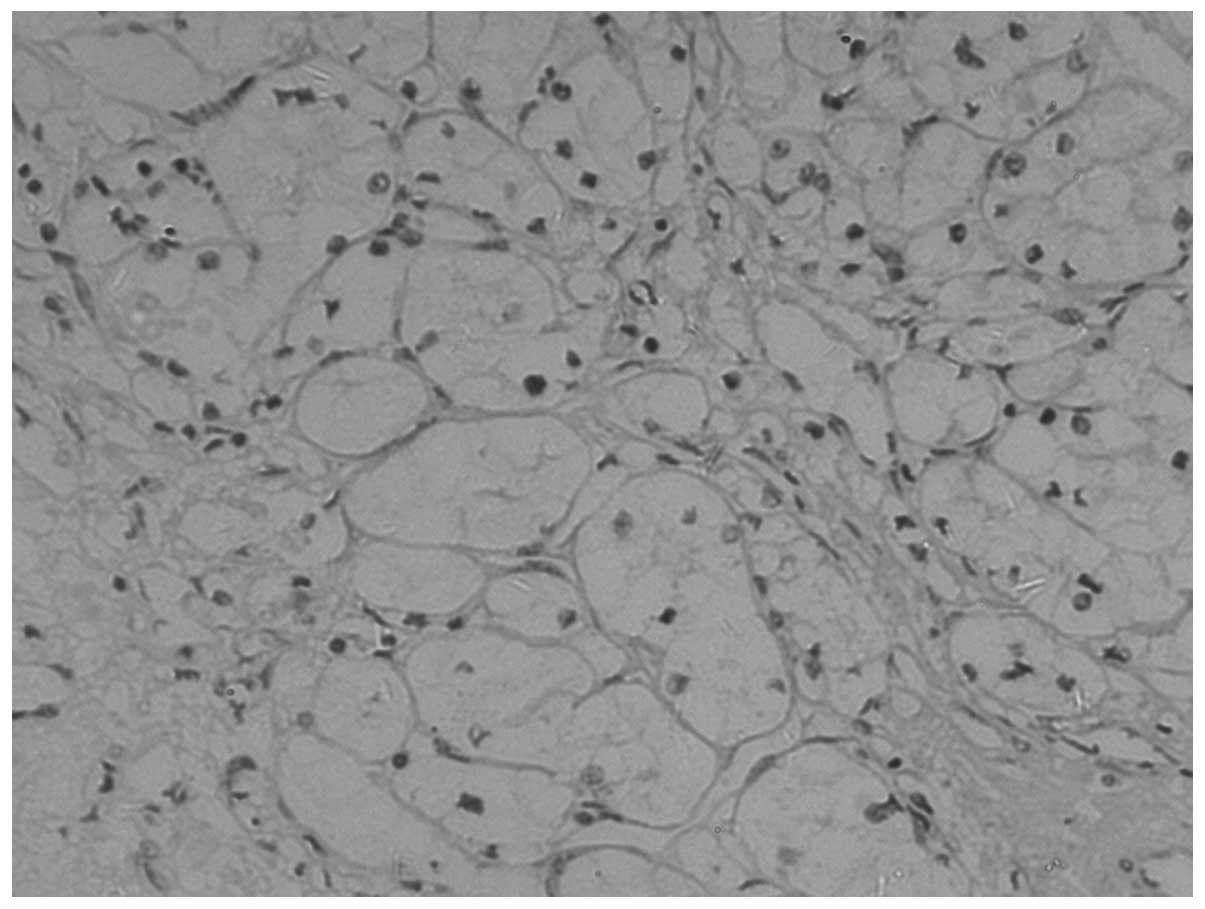
E-cadherin expression was observed as yellow
particles in the cytoplasm or membrane of normal renal cells. A low
level of E-cadherin expression was detected in CCRCC. The
para-cancerous and normal tissue exhibits a higher expression rate,
which is located mainly in the cytoplasm and/or membranes (Figs. 1–3). The positivity rates in the tumor,
normal tissues adjacent to the tumor and normal tissues were 31.88,
91.38, and 100.00%, respectively, with a statistically significant
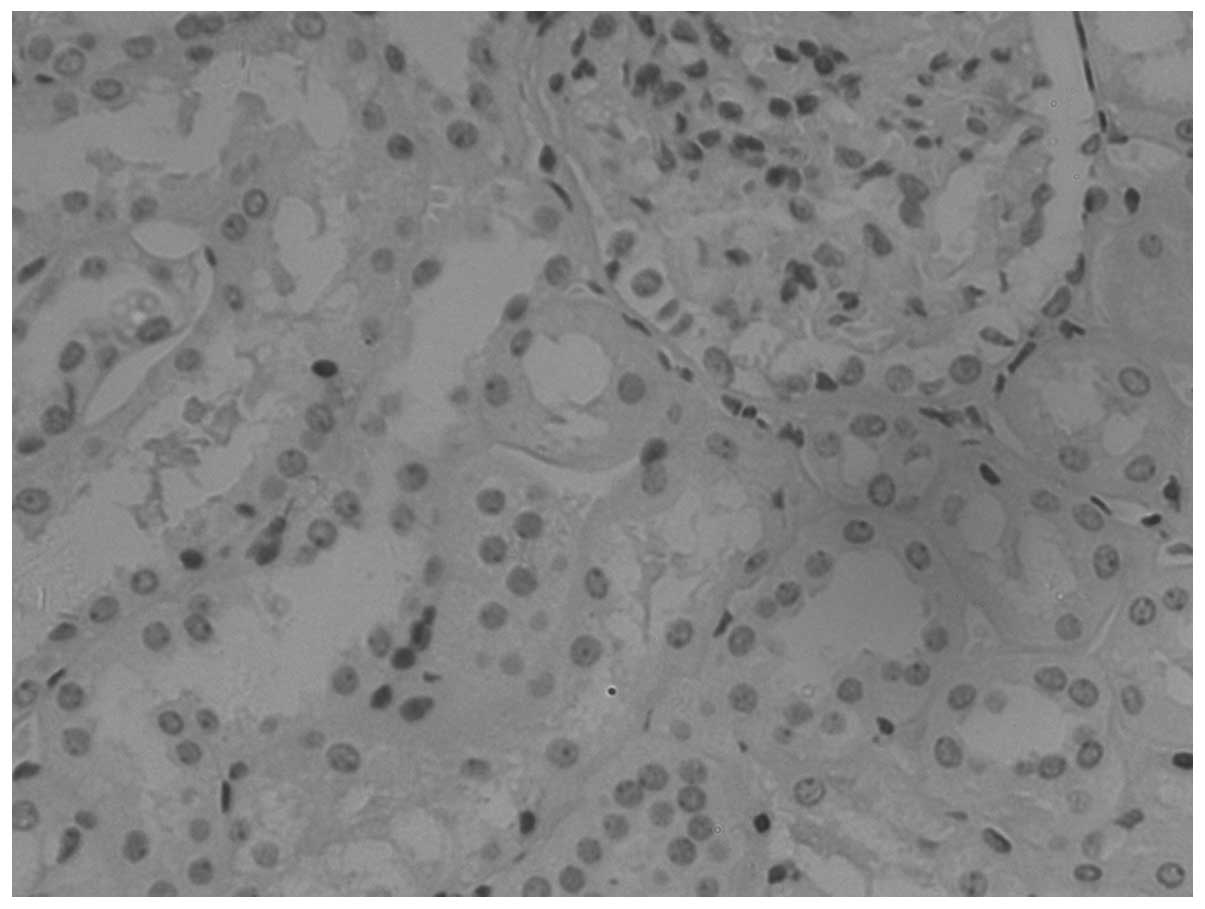
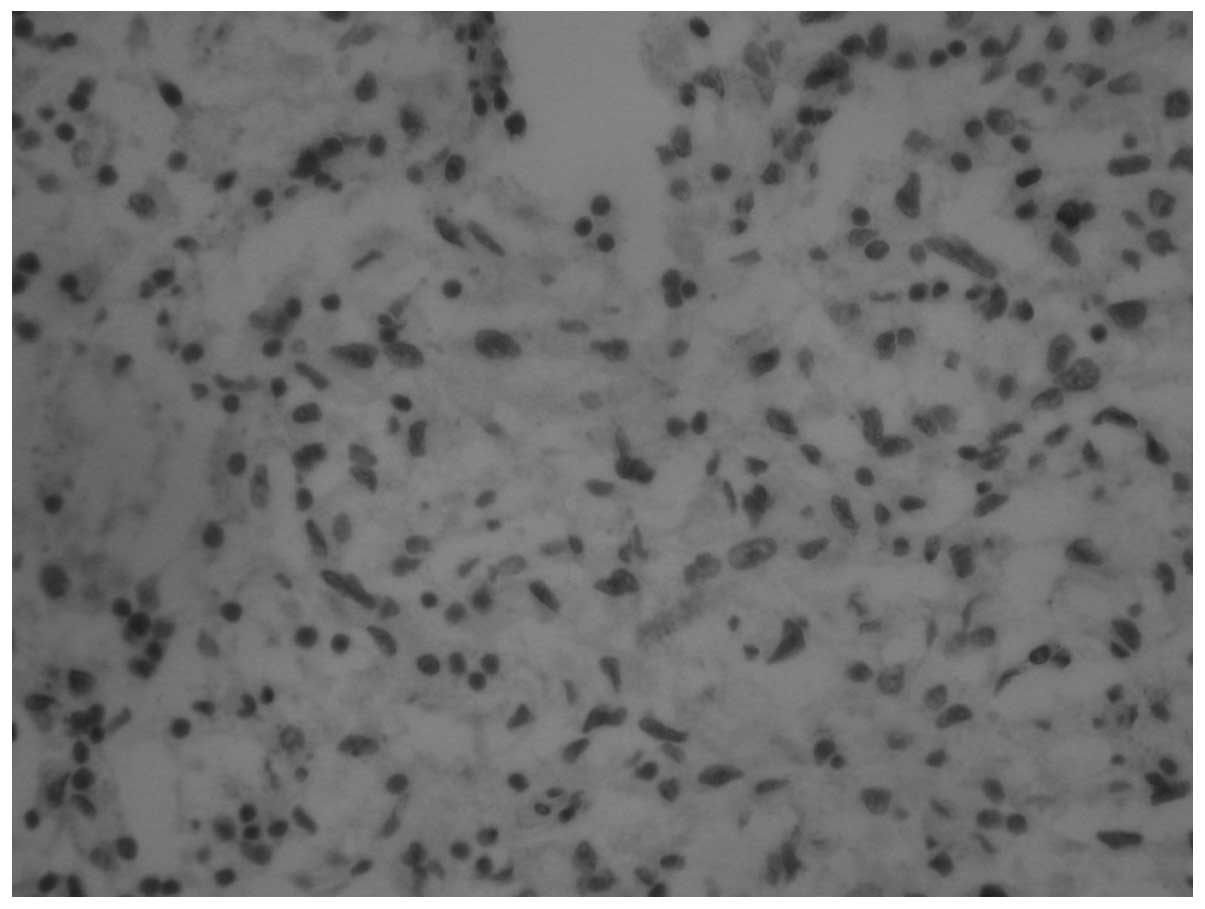
difference (P<0.001). Snail is highly expressed in CCRCC
(Figs. 4–6), with positivity rates of 82.61, 43.10
and 10.00% in the tumor, normal tissues adjacent to the tumor and
normal tissues, respectively. The differences in the expression
rates were statistically significant (P<0.001; Table I).
 | Table IE-cadherin and Snail expression in
various tissues. |
Table I
E-cadherin and Snail expression in
various tissues.
| | E-cadherin | Snail |
|---|
| |
|
|
|---|
| Tissue type | n | + | Rate (%) | + | Rate (%) |
|---|
| Carcinoma | 69 | 22 | 31.88 | 57 | 82.61 |
| Tumor-adjacent | 58 | 53 | 91.38 | 25 | 43.10 |
| Normal | 10 | 10 | 100.00 | 1 | 10.00 |
E-cadherin and Snail expression at
various clinical stages
In CCRCC, the expression of E-cadherin and Snail was
correlated with clinical stage and histological grade, in addition
to distant and lymph node metastases (contingency coefficient
r-values are shown in Table II;
P<0.05). The age and gender of the patients demonstrated no
significant correlation (contingency coefficient r-values are
listed in Table II;
P>0.05).
 | Table IIE-cadherin and Snail CCRCC in
different stages and classifications and the relationship between
clinical parameters |
Table II
E-cadherin and Snail CCRCC in
different stages and classifications and the relationship between
clinical parameters
| | E-cadherin | Snail |
|---|
| |
|
|
|---|
| Clinical
parameters | n | + | Rate (%) | χ2 | P-value | r | + | Rate (%) | χ2 | P-value | r |
|---|
| Classification | | | | 7.921 | 0.048 | 0.321 | | | 13.020 | 0.005 | 0.398 |
| G1 | 11 | 7 | 63.63 | | | | 5 | 45.45 | | | |
| G2 | 33 | 10 | 30.30 | | | | 29 | 87.88 | | | |
| G3 | 20 | 5 | 25.00 | | | | 18 | 90.00 | | | |
| G4 | 5 | 0 | 0.00 | | | | 5 | 100.00 | | | |
| TNM | | | | 8.810 | 0.032 | 0.336 | | | 9.217 | 0.027 | 0.343 |
| T1 | 36 | 17 | 47.22 | | | | 25 | 69.44 | | | |
| T2 | 20 | 4 | 20.00 | | | | 19 | 95.00 | | | |
| T3 | 10 | 1 | 10.00 | | | | 10 | 100.00 | | | |
| T4 | 3 | 0 | 0.00 | | | | 3 | 100.00 | | | |
| Age (years) | | | | 0.051 | 0.821 | 0.027 | | | 0.608 | 0.435 | 0.093 |
| <58 | 39 | 12 | 30.77 | | | | 31 | 79.49 | | | |
| ≥58 | 30 | 10 | 33.33 | | | | 26 | 86.67 | | | |
| Gender | | | | 0.725 | 0.394 | 0.102 | | | 0.039 | 0.843 | 0.024 |
| Male | 42 | 15 | 35.71 | | | | 35 | 83.33 | | | |
| Female | 27 | 7 | 25.92 | | | | 22 | 81.48 | | | |
| Lymphaden | | | | 7.773 | 0.005 | 0.318 | | | 5.127 | 0.024 | 0.263 |
| (−) | 51 | 21 | 41.18 | | | | 39 | 76.47 | | | |
| (+) | 18 | 1 | 5.56 | | | | 18 | 100.00 | | | |
| Metastases | | | | 8.972 | 0.003 | 0.328 | | | 4.035 | 0.045 | 0.235 |
| M0 | 54 | 22 | 40.74 | | | | 42 | 77.78 | | | |
| M1 | 15 | 0 | 0.00 | | | | 15 | 100.00 | | | |
Correlation of E-cadherin and Snail
expression in CCRCC
Spearman rank correlation analysis revealed that the
positive expression of E-cadherin is repressed as the positive
expression of Snail increases; the expression of E-cadherin and
Snail in the CCRCC is negatively correlated (r=0.342, P=0.004;
Table III).
 | Table IIIRelationship between E-cadherin and
Snail in CCRCC. |
Table III
Relationship between E-cadherin and
Snail in CCRCC.
| E-cadherin
expression | |
|---|
|
| |
|---|
| Snail
expression | Positive | Negative | Total |
|---|
| Positive | 14 | 43 | 57 |
| Negative | 8 | 4 | 12 |
Discussion
Numerous studies have shown that EMT plays an
important role in the development of malignant epithelial tumors
(6). A previous study has shown
that during tumorigenesis and migration in the early stage of
cancer, EMT results in the formation of cancer cells with specific
connective tissue damage and diffused tumor cell invasion into the
surrounding tissue and blood vessels (13). The EMT is also involved in
embryonic neural embryo and gastrula formation and is one of the
most important cell mechanisms during organ development. A number
of studies have demonstrated that EMT also plays an important role
in tumor development in the bladder (14), stomach (15), and colon (9), in addition to the development of
malignant epithelial tumors. The mechanisms of EMT are as follows:
i) Cell polarity is lost such that the expression of molecules
related to cell adhesion is repressed, resulting in the destruction
of the connection between specific cells. ii) The cell morphology
is altered such that similar interstitial cell characteristics are
obtained, accompanied by a series of changes to the molecular
composition, making the cell vulnerable to strong attacks (7).
In the current study, we used a high-power
microscope in order to view the cancer cells and observed that the
arrangement of the cancer cells was looser compared with that of
the normal renal tissue cells such that the connections between the
cancer cells were not closed; the normal, organized kidney
structure was lost. These observations supported the possible
presence of a mechanism that leads to the formation of tumor cell
connections between transformations, resulting in easy diffusion
and migration. This mechanism is likely to be the EMT. Neve et
al(16) and Charafe-Jauffret
et al (17) applied the Affymetrix
gene chip and proteomic principles to analyze a variety of breast
cancer cell lines and observed high expression of interstitial
molecular markers in the highly aggressive cell line, contrary to
the low-invasive and well-differentiated cells in which the
interstitial molecular markers have low expression levels or may be
missing. EMT may close the connection between cells during tumor
development.
Snail is a type of zinc-finger transcription factor,
and its key targets in the occurrence of EMT have been identified.
Snail acts through its zinc-finger area and the E-cadherin promoter
E-box region (sequence CAGGTG), on the main chain, thereby
repressing E-cadherin expression. The downregulation of E-cadherin
directly results in alterations to the cell morphology, loss of
polarity, structural instability of the organisation and the
destruction of the connections between epithelial cells, resulting
in the tumor cells breaking away from the original site of tumor
invasion and metastasis (4).
Blanco et al(18) found
that Snail expression reduced or completely inhibited E-cadherin
expression in breast ductal carcinoma and various other tumors.
E-cadherin, widely distributed in epithelial cells, is stably
expressed in normal epithelial cells, but its expression is
repressed or completely inhibited in gastric, liver and prostate
cancers in addition to various other epithelial malignant tumors
(19). This is consistent with the
findings in the present study in which E-cadherin expression in
CCRCC was observed to be downregulated. Furthermore, we studied
Snail expression in CCRCC, para-cancerous and normal tissues. A
high level of Snail expression was observed in the cancerous tissue
and in the majority of the histological grades, all cases of lymph
node metastasis and stages.
The detection of Snail and E-cadherin expression in
normal tissue, normal tissues adjacent to tumor tissue and in CCRCC
using the immunohistochemical method, in addition to the internal
connection of these two factors, is rarely reported. The results
from the present study demonstrate a high level of Snail expression
in CCRCC with a positive expression rate of 82.61% compared with
those in the normal tissues near the tumor and normal tissues. The
tumor stage, classification, infiltration and metastasis are
correlated with the expression of Snail. Hence, Snail, to a certain
extent, may reflect the ability of the tumor cells to invade. We
also observed that the positive expression of Snail in CCRCC
significantly repressed E-cadherin expression (P<0.05). In
CCRCC, the acceleration of tumor invasion and metastasis by Snail
may be achieved through the inhibition of E-cadherin
expression.
In conclusion, this study evaluated the EMT and the
correlation between Snail and E-cadherin protein expression in
CCRCC using an immunohistochemical method. The presence of Snail
and E-cadherin expression in CCRCC correlates with the CCRCC
staging and grading as well as with the lymph node and distant
metastasis. Therefore, the joint detection of Snail and E-cadherin
expression in CCRCC may be used as a biological marker to monitor
CCRCC infiltration and migration. The effect of EMT may be the
inhibition of tumor infiltration or metastasis. However, the role
of transformation in tumor infiltration requires further study as
the linking mechanism remains unclear. Further studies are required
to confirm the results of this study for the comprehensive
treatment of kidney tumors and provide a theoretical basis for
tumor pathogenesis in the kidney.
References
|
1
|
Takeichi M: Cadherin cell adhesion
receptors as a morphogenetic regulator. Science. 251:1451–1455.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McNeill H, Ozawa M, Kemler R and Nelson
WJ: Novel function of the cell adhesion molecule uvomorulin as an
inducer of cell surface polarity. Cell. 62:309–316. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andersen H, Mejlvang J, Mahmood S, et al:
Immediate and delayed effects of E-cadherin inhibition on gene
regulation and cell motility in human epidermoid carcinoma cells.
Mol Cell Biol. 25:9138–9150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosivatz E, Becker I, Specht K, et al:
Differential expression of the epithelial-mesenchymal transition
regulators Snail, SIP1, and twist in gastric cancer. Am J Surg
Pathol. 161:1881–1891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieto MA: Epithelial-mesenchymal
transitions in development and disease: old views and new
perspectives. Int J Dev Biol. 53:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vincent-Salomon A and Thiery JP: Host
microenvironment in breast cancer development:
epithelial-mesenchymal transition in breast cancer development.
Breast Cancer Res. 5:101–106. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bates RC, DeLeo MJ 3rd and Mercurio AM:
The epithelialmesenchymal transition of colon carcinoma involves
expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res.
299:315–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Zijl F, Zulehner G, Petz M, et al:
Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009.
|
|
11
|
Wang L, Li Z, Wang C, et al: E-cadherin
decreased human breast cancer cells sensitivity to staurosporine by
up-regulating Bcl-2 expression. Arch Biochem Biophys. 481:116–122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gabbert HE, Mueller W, Schneiders A, Meier
S, Moll R, Birchmeier W and Hommel G: Prognostic value of
E-cadherin expression in 413 gastric carcinomas. Int J Cancer.
69:184–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Wever O, Pauwels P, De Craene B, et al:
Molecular and pathological signatures of epithelial-mesenchymal
transitions at the cancer invasion front. Histochem Cell Biol.
130:481–494. 2008.PubMed/NCBI
|
|
14
|
Hou JQ, Wei JX, Chang JK and Li TQ: E -
calcium sticky grain and actin crosslinked protein in bladder
transitional cell carcinoma of the expression and its significance.
Surg. 28:847–848. 2011.(In Chinese).
|
|
15
|
Castro Alves C, Rosivatz E, Schott C, et
al: Slug is overexpressed in gastric carcinomas and may act
synergistically with SIP1 and Snail in the down-regulation of
E-cadherin. J Pathol. 211:507–515. 2007.PubMed/NCBI
|
|
16
|
Neve RM, Chin K, Fridlyand J, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charafe-Jauffret E, Ginestier C, Monville
F, et al: Gene expression profiling of breast cell lines identifies
potential new basal markers. Oncogene. 25:2273–2284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanco MJ, Moreno-Bueno G, Sarrio D, et
al: Correlation of Snail expression with histological grade and
lymph node status in breast carcinomas. Oncogene. 21:3241–3246.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peña C, Garcia JM, Silva J, et al:
E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in
colon cancer: clinicopathological correlations. Hum Mol Genet.
14:3361–3370. 2005.PubMed/NCBI
|