Introduction
Colorectal cancer (CRC) is the third most common
type of malignant tumor in Western countries, with an estimated
total of 143,000 cases in the United States in 2010 (1). At present, against the declining
trend of incidence of gastric cancer, the prevalence of CRC has
maintained an upward momentum in China, which is partly
attributable to acquiring the Western lifestyle. With the
development of the algorithm of surgery and pan-operation systemic
therapies, particularly chemotherapy and certain targeted
therapies, the overall prognosis of patients has improved. However,
certain patients, either in the curative setting or the metastatic
one, with relatively similar clinical features may have varied
clinical courses and their prognoses are difficult to predict. The
underlying mechanisms of this phenomenon remain largely unknown.
Therefore, prognostic markers are required in order to help
stratify patients according to their risk, enable follow-up
schedules to be individualised and tailor eligibility criteria for
trials of neoadjuvant and adjuvant therapies. Tumor stage,
pathological grade, lymph node involvement and lymphovascular
invasion are commonly used as known prognostic factors in the
clinic (2–4). However, all of these are
postoperative factors. In addition, though certain abnormal
tumor-associated genetic molecules were identified as being able to
predict the prognosis of the patients (5,6),
their measurement is to a certain extent time-consuming and complex
and frequently not integrated into clinical practice. Therefore,
identifying pre-treatment prognostic factors, including a number of
serum biomarkers, would offer the opportunity for more objective
and reproducible measurement and risk stratification prior to
surgery. Thus, due to the characteristics of simplicity,
cost-effectiveness and availablility in daily practice and the
association with cancers, the measurement of serum C-reactive
protein (CRP) levels has gained increasing attention.
CRP, a typical systemic inflammation marker, was
first discovered in the plasma of patients during the acute phase
of pneumococal pneumonia (7). It
is produced almost exclusively in hepatocytes in response to
inflammatory cytokines, such as intereukin (IL)-1, tumor necrosis
factor (TNF)-α and, in particular, IL-6, within a few hours
following insults such as infection, trauma or cardiovascular
diseases (8,9). In recent decades, mounting evidence
has demonstrated that elevated CRP levels were associated with an
increased risk of malignancy (10–13).
Moreover, elevated levels of CRP have been described as a
prognostic factor in various types of human malignancy, including
ovarian, and gastroesophageal (14–17).
However, the correlation between CRP levels and prognosis in
patients with CRC remains to be clarified (18,19).
To the best of our knowledge, few systematic studies
focusing on the clinical significance of CRP in Chinese CRC
patients have been reported. Therefore, in the present study, the
potential clinical and prognostic significance of pre-treatment
high sensitivity-CRP (hs-CRP) levels in Chinese patients with CRC
of all stages and histological subtypes has been comprehensively
analyzed.
Patients and methods
Patients
From 2005 to 2008, a total of 150 CRC patients were
initially enrolled in the present study, including a number of
cases from a previous retrospective study that investigated the
clinical significance of platelet count in CRC (20). Patients underwent either en bloc
colorectomy or palliative resection, and further chemotherapy in
the inoperable patients was studied retrospectively. None of the
patients had received preoperative chemotherapy. The histological
tumor subtype was determined according to the 1997 UICC
classification and staging was based on the 2002 TNM
classification. Patients with active concurrent infection or who
took non-steroidal anti-inflammatory drugs were excluded from the
present study. Patients were also excluded if their pre-treatment
CRP levels were unavailable. Information on patient and tumor
characteristics, such as age, gender, stage, presence of regional
lymph node or distant metastases, histological grade and CRP value,
was obtained from the databases of Taizhou People’s Hopital
(Taizhou, China). Only 123 patients (70 males and 53 females met
the required inclusion criteria). Follow-up information, including
the cause of mortality, was ascertained through a review of
clinical notes and direct or family contact.
The Ethics Committee of Taizhou People’s Hospital
approved the study. Written informed consent was obtained from all
of the patients according to the guidelines approved by the
Institutional Research Board of the hospital.
Assay of serum CRP
A sample of the peripheral venous blood of patients
was withdrawn one day prior to treatment. The blood samples were
temporarily stored at 4°C. Immediately after the blood was
centrifuged, serum samples or the supernatant were frozen and
stored at −80°C until use. Pre-treatment hs-CRP values were
measured as part of the clinical routine using a BN ProSpec system
(Siemens Healthcare Diagnostics, Germany) according to the
manufacturer’s instructions. Normal serum levels were defined as ≥5
mg/l by the manufacturer’s instructions. When investigating the
correlation between the levels of hs-CRP with clinicopathological
characteristics, the pre-treatment hs-CRP values were classified
into the CRP ≥5 mg/l and >5 mg/l groups, according to the
suggestion of Saito et al(21)and Stein et al(22).
Statistical analysis
Comparisons between the two groups, the high levels
of the hs-CRP and normal levels groups, were performed using the
t-test for quantitative variables, the χ2 test for
categorical clinical variables, and the Fisher’s exact test when
appropriate. Overall survival was measured from the date of
diagnosis until the date the patient succumbed due to disease or of
the final follow-up. Survival curves were obtained according to the
Kaplan-Meier method. Comparison of the survival curves was carried
out using the log-rank test. Variables that were significant at
P<0.05 in the univariate analysis were also included in the
multivariate analysis. Multivariate survival analysis of the group
variables was performed using the Cox proportional hazard model.
Mortalities up to the end of May 2013 were included in the
analysis. To remove a variable from the model, the corresponding
P-value had to be >0.10. Analysis was performed using SPSS
software, version 19.0 (SPSS Inc., Chicago, IL, USA) and two-tailed
values of P<0.05 were accepted as indicating a statistically
significant difference.
Results
Patient characteristics
In total, 123 patients with CRC in this cohort (70
male and 53 female) met the inclusion criteria. The details of the
baseline clinicopathological parameters of the patients studied are
listed in Table I. The mean age of
the group whose hs-CRP levels were >5 mg/l was 62.07±12.51
years, while that of the normal group was 60.85±11.21 years, which
did not manifest a significant difference (P=0.616). A minor
dominance of male cases (n=70) was observed compared with their
female counterparts (n=53) overall, but no significant difference
of gender distribution was identified between the two groups. In
addition, most of the patients were classified as stage III,
followed by stage II, according to the TNM classification, and only
five patients with stage IV and two with stage I were included in
this cohort study. In total, 56 patients, 25 in the hs-CRP >5
mg/l group and 31 in the hs-CRP <5 mg/l group, exhibited
regional lymph node involvement. However, significantly more cases
of lymph node-negative patients were identified in the hs-CRP
levels group compared with the normal group (P<0.001). Partly
attributable to the surgical indication, there were only five
patients with distance metastasis which had palliative resection
performed on them in this cohort of cases, of which four cases had
significantly elevated levels of hs-CRP.
 | Table IAssociation between pretreatment
hs-CRP levels and baseline clinicopathological variables in
patients with CRC. |
Table I
Association between pretreatment
hs-CRP levels and baseline clinicopathological variables in
patients with CRC.
| Groups | |
|---|
|
| |
|---|
| Variables (n) | hs-CRP ≥5 mg/l | hs-CRP >5
mg/l | P-value |
|---|
| Total no. of
cases | 93 | 30 | |
| Age (year; mean ±
SD) | 60.85±11.21 | 62.07±12.51 | 0.616 |
| Gender |
| Male | 54 | 16 | 0.676 |
| Female | 39 | 14 | |
| Lymph node
invasion |
| Positive | 31 | 25 | <0.001 |
| Negative | 62 | 5 | |
| Distant
metastasis |
| Positive | 1 | 4 | 0.012 |
| Negative | 92 | 26 | |
| Vascular
invasion |
| Positive | 8 | 15 | <0.001 |
| Negative | 85 | 15 | |
| Perineural
invasion | | | |
| Positive | 8 | 17 | <0.001 |
| Negative | 85 | 13 | |
| Histological
grade |
| Low | 39 | 17 | 0.022 |
| Moderate | 37 | 13 | |
| High | 17 | 0 | |
| TNM stage |
| I | 2 | 0 | 0.001 |
| II | 24 | 1 | |
| III | 66 | 25 | |
| IV | 1 | 4 | |
Clinicopathological significance of
hs-CRP
The cut-off point for measurements of serum hs-CRP
levels was set at 5.0 mg/l in the hospital, which is concurrent
with previous studies (21,22).
When the patients were divided into two groups according to the
individual baseline levels of hs-CRP, 30 patients (24.39%) had
pre-treatment serum hs-CRP levels above the defined cut-off point
of 5 mg/l, ranging from 5.02 to 56.54 mg/l. Conversely, no serum
elevation of hs-CRP levels was recognized in the 93 patients
(75.61%) who were assigned into the normal group (Table I). A close association was observed
between elevated hs-CRP levels (CRP>5.0 mg/l) and clinical lymph
nodal status (P<0.001), distant metastasis (P=0.012), vascular
and perineural invasion (P<0.001 and P<0.001), tumor
differentiation (P=0.022) and clinical stage (P=0.001) (Table I).
The mean duration of follow-up for the survivors
(n=57) was 35.5 months (range, 11–60 months). Overall, more
patients in the high serum hs-CRP levels group had succumbed to
disease (26/30) in comparison to their normal counterpart after
five years of follow-up, of which 60 patients succumbed as a result
of progression of the CRC, two due to surgical complications and
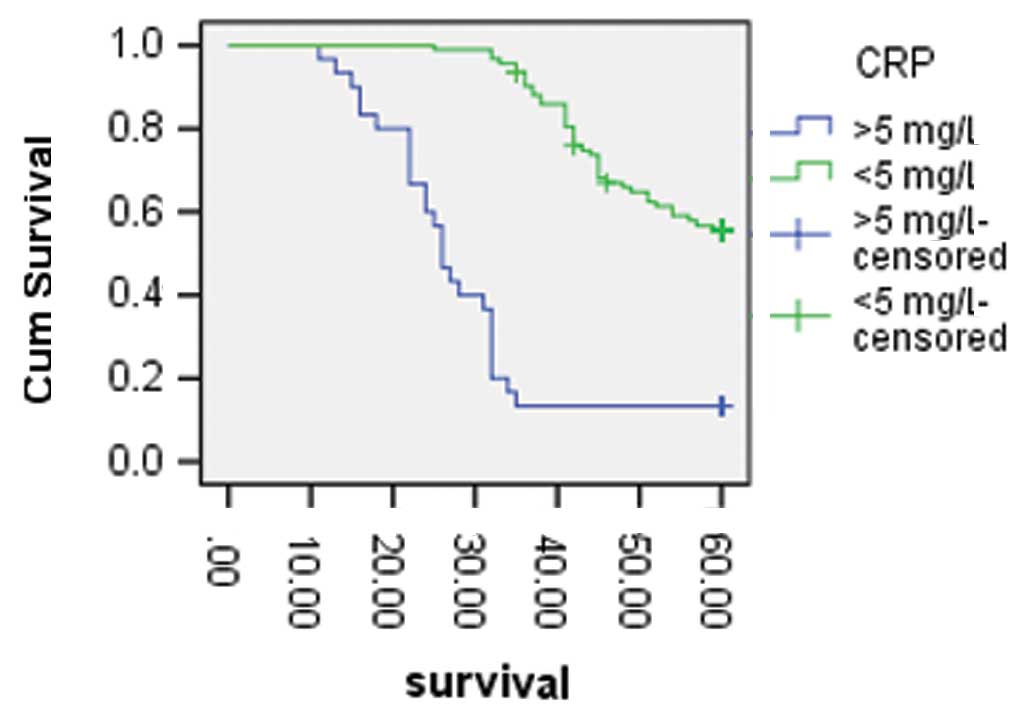
four due to non-cancer causes (data not shown). As shown in
Fig. 1, the five-year survival
rates of the patients with high levels of hs-CRP and normal levels
were 13.30 and 57.0%, respectively (P<0.001). Following the
univariate analysis, along with the conventional prognostic factors
such as lymph node and distant metastasis, vascular and perineural
invasion, clinical stage and pathological grades, serum hs-CRP
levels were also demonstrated to be correlated with the life span
of the patients. Furthermore, considering results of the
multivariate analysis, perineural invasion [hazard ratio (HR)
6.181, 95% confidence interval (CI) 1.264–30.241, P=0.025],
clinical stage (HR 6.86, 95% CI 2.045–23.01, P=0.002) and hs-CRP
>5 mg/l (HR 5.196, 95% CI 2.901–9.309, P<0.001) were also
identified as independent prognosticators in CRC in this study
(Table II).
 | Table IIUnivariate and multivariate analysis
of the clinicopathological parameters in CRC. |
Table II
Univariate and multivariate analysis
of the clinicopathological parameters in CRC.
| Overall
survival |
|---|
|
|
|---|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| | | 95.0% CI | |
|---|
| | |
| |
|---|
| Variables | P-value | Hazard ratio | Lower | Upper | P-value |
|---|
| Age | 0.621 | | | | |
| Gender | 0.544 | | | | |
| hs-CRP >5
mg/l | <0.001 | 5.196 | 2.901 | 9.309 | <0.001 |
| Lymph node
invasion | <0.001 | 1.576 | 0.875 | 2.837 | 0.129 |
| Distant
metastasis | <0.001 | 1.146 | 0.223 | 5.878 | 0.87 |
| Vascular
invasion | <0.001 | 0.585 | 0.126 | 2.724 | 0.494 |
| Perineural
invasion | <0.001 | 6.181 | 1.264 | 30.241 | 0.025 |
| Histological
grades | 0.003 | 0.972 | 0.63 | 1.499 | 0.897 |
| TNM stages | <0.001 | 6.86 | 2.045 | 23.01 | 0.002 |
Discussion
In 1863, Virchow identified leukocyte infiltration
in neoplastic tissues and suggested these sites of chronic
inflammation were the origin of cancer, which was the first
suggestion of the linkage between inflammation and cancer (23). Since then, epidemiological links
between Helicobacter pylori bacterial infection and gastric
cancer and mucosa-associated lymphoid tissue lymphoma, the
so-called MALToma, as well as inflammatory bowel disease and CRC
validated this hypothesis (24–26.). Notably, CRP, a systemic
inflammation marker, was reintroduced as a tool for monitoring
malignancies, similar to its use in cardiovascular diseases in
recent decades (9,27,28).
Furthermore, elevated levels of CRP have also been described as a
prognostic factor in various types of human malignancy, including
digestive system cancers (14–16,29,30).
The majority of the previous studies presumed that elevated serum
CRP levels in patients with malignancy were probably a bodily
response, secondary to tumor necrosis, local tissue damage and
associated inflammation through the cytokines released from
leukocytes infiltrating within the tumor microenvironment, in
particular IL-6 (31).
To the best of our knowledge, several studies have
explored the pathological role of CRP in CRC in the Western
countries. Of the nine published retrospective studies up to 2011,
three studies demonstrated positive associations between
circulating CRP levels and CRC incidence (32–34),
while the remaining reports indicated generally null (35–38)
or even inverse (39,40) associations, which purported the
discrepant conclusions presented. However, two individual
meta-analyses which summarized data from 8 and 11 independent
prospective investigations, respectively, concluded that CRP was a
weak, positive risk factor for CRC (41,42).
However, few studies are currently available with regard to this
issue in Chinese CRC patients. Therefore, in the present study, the
clinical significance of hs-CRP was first explored in CRC patients
in the region. In the cohort of cases, 30 patients with high serum
hs-CRP levels according to our cut-off line set as >5 mg/l were
identified. At the same time, no significant association of the
hs-CRP levels with regard to the age and gender of the patients was
noted. However, it is encouraging that hs-CRP levels were notably
increased in the cases with lymph node or distant metastasis and
vascular or perineural invasion, all of which are traditional
pathological prognostic factors. Elevated hs-CRP concentrations in
serum were more common in the patients with advanced stage CRC.
These results suggest the predictable potency of CRP in the
mortality of CRC patients. Therefore, the results of the present
study to a certain extent confirmed the conclusions of a number of
previous studies which considered the positive pathological role of
CRP in CRC (32–34,43).
Possible reasons for the difference between the previous studies
which indicated a negative association between CRP levels and
clinical and pathological features in CRC patients, and other
previous positive studies including the present one are probably
partly attributable to the variable potential biological features,
different stages, distribution of the patient population and the
disparate territory.
Conventional factors, such as pathological clinical
stages and lymph node involvement, are the most important
predictors of poor clinical outcome in clinical practice (44–46).
Furthermore, a number of less common serological and molecular
markers have also shown prognostic evidence, such as the estrogen
receptor, progesterone receptor, human epidermal growth factor
receptor-2 in breast cancer and mismatch repair gene in stage II
CRC (6,7). However, these markers are only
available following surgery and their measurement is, to a certain
extent, time-consuming and complex and therefore are often not
integrated into clinical practice. By contrast, serum CRP levels,
measurement of which is relatively inexpensive and easy to quantify
in daily clinical practice, allows classification of patients who
are at a relatively high risk and who are candidates for the latest
intensive treatment. In the last 10 years, an increasing amount of
evidence suggesting the CRP prognostic role in various types of
tumors, including CRC, has been reported (47,48).
Similarly, in the present study, whether CRP had a prognostic role
in this cohort of patients was also investigated. Notably,
following a further univariate analysis, concurrent with lymph node
or distant metastasis, vascular or perineural invasion and clinical
stage, elevated hs-CRP levels were identified as a prognostic
marker in CRC. Patients with high serum levels of hs-CRP had
relatively poorer five-year survival rates in comparison to their
normal hs-CRP counterparts, suggesting that high concentrations of
hs-CRP are a potential prognostic determinant. In addition, when
included in the multivariate analysis, hs-CRP was also indicated as
an independent prognostic factor in the cohort of cases. Together
with perineural invasion, clinical stage was confirmed as another
prognostic factor in the present study. However, although HR was
manifested as >1, the prognostic significance of lymph node and
distant metastasis were not indicated in this study, which was not
in accordance with previous results (19,20).
Relatively fewer patients with distance metastasis recruited in the
present study may have contributed to the contrary result. Overall,
the CRC patients with high levels of pretreatment CRP may
demonstrate high risk potential, thus more attention should be
given this issue in the consideration for more active therapies.
Despite the significant results obtained in this study, it should
be noted that this is a relatively small study in a single center
and further verification in large cohorts in multiple centers in
China is required.
To investigate the potency of CRP in CRC, it is
essential to define CRP, i.e., a participant in the pathogenesis of
CRC or simply a marker of CRC. However, the role of CRP in cancers,
including CRC, remains poorly understood. Several mechanisms of
increased CRP levels in malignant tumors are now known due to
various therories. First, serum CRP levels may reflect the
aggressiveness of the tumor, as they are the result of the immune
response of the host to tumor growth (49,50).
Second, tumor growth causes tissue inflammation in the tumor
microenvironment by increasing the production of inflammatory
proteins, particularly IL-6. Immune and inflammatory cells in the
tumor microenvironment interact with malignant cells in a
complicated manner and the net result of which is stimulation of
tumor growth, invasion and metastasis (51–53).
As a result, the exact underlying role of CRP in various types of
cancer including CRC requires more biological exploratory studies
in the future.
In conclusion, the pre-treatment serum CRP levels
may be a marker of aggressive characteristics of in Chinese CRC
patients. Elevated CRP levels prior to initial treatment were
demonstrated to be a poor prognostic factor for the overall
survival of CRC patients in China. Due to its increased
attractiveness as a routinely available, relatively inexpensive and
objectively measured marker available pre-operatively, CRP may
complement the prognostic value of traditional prognostic factors,
such as stage and performance status, to more accurately stratify
patients with CRC. However, the results of the present study should
await internal or external validation in a number of centers and
prospective exploratory studies prior to being used in clinical
practice in China due to the limitations inherent to a
retrospective study with a small sample size.
References
|
1
|
American Cancer Society. Cancer Facts and
Figures. 2010
|
|
2
|
Corrado F, Mannini D, Ferri C, Corrado G,
Bertoni F, Bacchini P, Lieber MM and Song JM: The prognostic
significance of DNA ploidy pattern in transitional cell cancer of
the renal pelvis and ureter: continuing follow-up. Eur Urol.
21(Suppl 1): 48–50. 1992.PubMed/NCBI
|
|
3
|
Hall MC, Womack S, Sagalowsky AI, Carmody
T, Erickstad MD and Roehrborn CG: Prognostic factors, recurrence,
and survival in transitional cell carcinoma of the upper urinary
tract: a 30-year experience in 252 patients. Urology. 52:594–601.
1998.PubMed/NCBI
|
|
4
|
Kikuchi E, Horiguchi Y, Nakashima J,
Hatakeyama N, Matsumoto M, Nishiyama T and Murai M: Lymphovascular
invasion independently predicts increased disease specific survival
in patients with transitional cell carcinoma of the upper urinary
tract. J Urol. 174:2120–2123. 2005. View Article : Google Scholar
|
|
5
|
Onitilo AA, Engel JM, Greenlee RT and
Mukesh BN: Breast cancer subtypes based on ER/PR and Her2
expression: comparison of clinicopathologic features and survival.
Clin Med Res. 7:4–13. 2009. View Article : Google Scholar
|
|
6
|
Hutchins G, Southward K, Handley K, Magill
L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M,
Kerr D, Gray R and Quirke P: Value of mismatch repair, KRAS, and
BRAF mutations in predicting recurrence and benefits from
chemotherapy in colorectal cancer. J Clin Oncol. 29:1261–1270.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tillett WS and Francis T: Serological
reaction in pneumonia with a non-protein somatic fraction of
pnemoncoccus. J Exp Med. 52:561–571. 1930. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castell JV, Gómez-Lechón MJ, David M,
Fabra R, Trullenque R and Heinrich PC: Acute-phase response of
human hepatocytes; regulation of acute-phase protein synthesis by
interleukin-6. Hepatology. 12:1179–1186. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zimmerman MA, Selzman CH, Cothren C,
Sorensen AC, Raeburn CD and Harken AH: Diagnostic implications of
C.reactive protein. Arch Surg. 138:220–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Uchida A and Sudo A: Clinical significance of
pretreatment serum C-reactive protein level in soft tissue sarcoma.
Cancer. 15:1055–1061. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA,
Kim BG, Kim SG, Kim SH, Jang JS, Kim MC, Kim KH, Han JY and Kim HJ:
Clinical significances of preoperative serum interleukin-6 and
C-reactive protein level in operable gastric cancer. BMC Cancer.
9:1552009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pine SR, Mechanic LE, Enewold L,
Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso
NE and Harris CC: Increased levels of circulating interleukin 6,
interleukin 8, C-reactive protein, and risk of lung cancer. J Natl
Cancer Inst. 103:1112–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee S, Choe JW, Kim HK and Sung J:
High-sensitivity C-reactive protein and cancer. J Epidemiol.
21:161–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hefler LA, Concin N, Hofstetter G, Marth
C, Mustea A, Sehouli J, Zeillinger R, Leipold H, Lass H, Grimm C,
Tempfer CB and Reinthaller A: Serum C-reactive protein as
independent prognostic variable in patients with ovarian cancer.
Clin Cancer Res. 14:710–714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crumley AB, McMillan DC, McKernan M, Going
JJ, Shearer CJ and Stuart RC: An elevated C-reactive protein
concentration, prior to surgery, predicts poor cancer-specific
survival in patients undergoing resection for gastrooesophageal
cancer. Br J Cancer. 94:1568–1571. 2006.
|
|
16
|
Nagaoka S, Yoshida T, Akiyoshi J, Akiba J,
Torimura T, Adachi H, Kurogi J, Tajiri N, Inoue K, Niizeki T, Koga
H, Imaizumi T, Kojiro M and Sata M: Serum C-reactive protein levels
predict survival in hepatocellular carcinoma. Liver Int.
27:1091–1097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polterauer S, Grimm C, Tempfer C, Sliutz
G, Speiser P, Reinthaller A and Hefler LA: C-reactive protein is a
prognostic parameter in patients with cervical cancer. Gynecol
Oncol. 107:114–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishizuka M, Nagata H, Takagi K and Kubota
K: C-reactive protein is associated with distant metastasis of T3
colorectal cancer. Anticancer Res. 32:1409–1415. 2012.PubMed/NCBI
|
|
19
|
Lee WS, Baek JH, You DH and Nam MJ:
Prognostic value of circulating cytokines for stage III colon
cancer. J Surg Res. 182:49–54. 2012.PubMed/NCBI
|
|
20
|
Lin MS, Huang JX, Zhu JY and Shen HZ:
Elevation of platelet count in patients with colorectal cancer
predicts tendency to metastases and poor prognosis.
Hepatogastroenterology. 59:1687–1690. 2012.PubMed/NCBI
|
|
21
|
Saito K, Kawakami S, Ohtsuka Y, Fujii Y,
Masuda H, Kumagai J, Kobayashi T, Kageyama Y and Kihara K: The
impact of preoperative serum C-reactive protein on the prognosis of
patients with upper urinary tract urothelial carcinoma treated
surgically. BJU Int. 100:269–273. 2007. View Article : Google Scholar
|
|
22
|
Stein B, Schrader AJ, Wegener G, Seidel C,
Kuczyk MA and Steffens S: Preoperative serum C- reactive protein: a
prognostic marker in patients with upper urinary tract urothelial
carcinoma. BMC Cancer. 13:101–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Helicobacter and Cancer Collaborative
Group. Gastric cancer and Helicobacter pylori: a combined analysis
of 12 case control studies nested within prospective cohorts. Gut.
49:347–353. 2001. View Article : Google Scholar
|
|
25
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: a meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jess T, Gamborg M, Matzen P, Munkholm P
and Sørensen TI: Increased risk of intestinal cancer in Crohn’s
disease: a meta-analysis of populationbased cohort studies. Am J
Gastroenterol. 100:2724–2729. 2005.
|
|
27
|
Kushner I, Rzewnicki D and Damols D: What
does minor elevation of C-reactive protein signify? Am J Med.
119:166.e17–e28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heikkilä K, Ebrahim S and Lawlor DA: A
systematic review of the association between circulating
concentrations of C reactive protein and cancer. J Epidemiol
Community Health. 61:824–833. 2007.PubMed/NCBI
|
|
29
|
Nozoe T, Saeki H and Sugimachi K:
Significance of preoperative elevation of serum C-reactive protein
as an indicator of prognosis in esophageal carcinoma. Am J Surg.
182:197–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanjay P, de Figueiredo RS, Leaver H,
Ogston S, Kulli C, Polignano FM and Tait IS: Preoperative serum
C-reactive proteinlevels and post-operative lymph node ratio are
important predictors of survival after pancreaticoduodenectomy for
pancreatic ductal adenocarcinoma. JOP. 13:199–204. 2012.
|
|
31
|
Slaviero KA, Clarke SJ and Rivory LP:
Inflammatory response: an unrecognised source of variability in the
pharmacokinetics and pharmacodynamics of cancer chemotherapy.
Lancet Oncol. 4:224–232. 2003.PubMed/NCBI
|
|
32
|
Erlinger TP, Platz EA, Rifai N and
Helzlsouer KJ: C-reactive protein and the risk of incident
colorectal cancer. JAMA. 291:585–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gunter MJ, Stolzenberg-Solomon R, Cross
AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR,
Albanes D and Sinha R: A prospective study of serum C-reactive
protein and colorectal cancer risk in men. Cancer Res.
66:2483–2487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Il’yasova D, Colbert LH, Harris TB, Newman
AB, Bauer DC, Satterfield S and Kritchevsky SB: Circulating levels
of inflammatory markers and cancer risk in the health aging and
body composition cohort. Cancer Epidemiol Biomarkers Prev.
14:2413–2418. 2005.PubMed/NCBI
|
|
35
|
Ito Y, Suzuki K, Tamakoshi K, Wakai K,
Kojima M, Ozasa K, Watanabe Y, Kawado M, Hashimoto S, Suzuki S,
Tokudome S, Toyoshima H, Hayakawa N, Kato K, Watanabe M, Ohta Y,
Maruta M and Tamakoshi A; JACC Study Group. Colorectal cancer and
serum C-reactive protein levels: a case control study nested in the
JACC Study. J Epidemiol. 15(Suppl 2): S185–S189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Otani T, Iwasaki M, Sasazuki S, Inoue M
and Tsugane S: Plasma C-reactive protein and risk of colorectal
cancer in a nested case-control study: Japan Public Health
Center-based prospective study. Cancer Epidemiol Biomarkers Prev.
15:690–695. 2006. View Article : Google Scholar
|
|
37
|
Siemes C, Visser LE, Coebergh JW, et al:
C-reactive protein levels, variation in the C-reactive protein
gene, and cancer risk: the Rotterdam Study. J Clin Oncol.
24:5216–5222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Trichopoulos D, Psaltopoulou T, Orfanos P,
Trichopoulou A and Boffetta P: Plasma C-reactive protein and risk
of cancer: a prospective study from Greece. Cancer Epidemiol
Biomarkers Prev. 15:381–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang SM, Buring JE, Lee IM, Cook NR and
Ridker PM: C-reactive protein levels are not associated with
increased risk for colorectal cancer in women. Ann Intern Med.
142:425–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ognjanovic S, Yamamoto J, Saltzman B,
Franke A, Ognjanovic M, Yokochi L, Vogt T, Decker R and Le Marchand
L: Serum CRP and IL-6, genetic variants and risk of colorectal
adenoma in a multiethnic population. Cancer Causes Control.
21:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsilidis KK, Branchini C, Guallar E,
Helzlsouer KJ, Erlinger TP and Platz EA: C-reactive protein and
colorectal cancer risk: a systematic review of prospective studies.
Int J Cancer. 123:1133–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo YZ, Pan L, Du CJ, Ren DQ and Xie XM:
Association between C-reactive protein and risk of cancer: a
meta-analysis of prospective cohort studies. Asian Pacific J Cancer
Prev. 14:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gunter MJ, Stolzenberg-Solomon R, Cross
AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR,
Albanes D and Sinha R: A prospective study of serum C-reactive
protein and colorectal cancer risk in men. Cancer Res.
66:2483–2487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bollschweiler E, Metzger R, Drebber U,
Baldus S, Vallböhmer D, Kocher M and Hölscher AH: Histological type
of esophageal cancer might affect response to neo-adjuvant
radiochemotherapy and subsequent prognosis. Ann Oncol. 20:231–238.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
D’Angelo E, Espinosa I, Ali R, Gilks CB,
Rijn M, Lee CH and Prat J: Uterine leiomyosarcomas: tumor size,
mitotic index, and biomarkers Ki67, and Bcl-2 identify two groups
with different prognosis. Gynecol Oncol. 121:328–333.
2011.PubMed/NCBI
|
|
46
|
Ma J, Liu L, Tang L, Zong J, Lin A, Lu T,
Cui N, Cui C and Li L: Retropharyngeal lymph node metastasis in
nasopharyngeal carcinoma: prognostic value and staging categories.
Clin Cancer Res. 13:1445–1452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zacharakis M, Xynos ID, Lazaris A, Smaro
T, Kosmas C, Dokou A, Felekouras E, Efstathios A, Polyzos A,
Sarantonis J, Syrios J, Zografos G, Papalambros A and Tsavaris N:
Predictors of survival in stage IV metastatic colorectal cancer.
Anticancer Res. 30:653–660. 2010.PubMed/NCBI
|
|
48
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar
|
|
49
|
Siemes C, Visser LE, Coebergh JW, Splinter
TAW, Witteman JCM, Uitterlinden AG, Hofman A, Pols HAP and Stricker
BH: C-reactive protein levels, variation in the C-reactive protein
gene, and cancer risk: the Rotterdam Study. J Clin Oncol.
24:5216–5222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramsey S, Lamb GWA, Aitchison M and
McMillan DC: The longitudinal relationship between circulating
concentrations of C-reactive protein, interleukin-6 and
interleukin-10 in patients undergoing resection for renal cancer.
Br J Cancer. 95:1076–1080. 2006. View Article : Google Scholar
|
|
51
|
Nozoe T, Korenaga D, Futatsugi M, Saeki H,
Maehara Y and Sugimachi K: Immunohistochemical expression of
C-reactive protein in squamous cell carcinoma of the
esophagus-significance as a tumor marker. Cancer Lett. 192:89–95.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine serum levels in soft tissue sarcoma
patients: correlations with clinico-pathological features and
prognosis. Int J Cancer. 100:463–471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wigmore SJ, Fearon KC, Sangster K, Maingay
JP, Garden OJ and Ross JA: Cytokine regulation of constitutive
production of interleukin-8 and -6 by human pancreatic cancer cell
lines and serum cytokine concentrations in patients with pancreatic
cancer. Int J Oncol. 21:881–886. 2002.
|