Introduction
Recurrent spontaneous abortion (RSA) is defined as
two or more consecutive pregnancy losses prior to 20 gestational
weeks (1). Studies have identified
numerous causes for RSA, including genetic (2), endocrine (3) and autoimmune (4) causes, which account for ~50% of
patients with RSA. The mechanisms for the remaining cases are
unexplained and these cases are known as unexplained recurrent
spontaneous abortion (URSA). In recent years, a number of studies
have shown that a high level of apoptosis in the chorionic villi
and decidua is associated with RSA, revealing that apoptosis may be
one of the causes of RSA (5,6).
p53, a negative cell cycle regulator, is important in numerous
biological processes, such as the cell cycle, DNA repair,
differentiation and apoptosis (7).
p53 has been observed to be expressed abnormally in the chorionic
villi and decidua of females with hydropic, spontaneous or missed
abortions; however, the expression of p53 in the chorionic villi
from patients with URSA has, to the best of our knowledge, yet to
be investigated (8–10). In the present study, p53 expression
in URSA and the corresponding correlation between p53 expression
and URSA were analyzed.
Subjects and methods
Subjects
A total of 53 patients with URSA and 32 control
volunteers were recruited from the First Affiliated Hospital of
Zhengzhou University from June 2010 to June 2012. All patients
included in the study exhibited the following clinical
characteristics: i) A regular menstrual cycle and menstrual blood
volume, with normal color; ii) no chromosomal abnormality or family
history of abortion; iii) no reproductive system diseases; iv)
negative for cardiolipin, sperm and endometrial antibodies; v) no
endocrine diseases; vi) no cardiovascular or venereal diseases;
vii) no long-term medication history, no history of radiation
therapy and no trauma or drug allergy; viii) no mother-child
incompatibility of blood types; ix) no unhealthy habits, such as
smoking; and x) no psychiatric history. The age of the 53 patients
with URSA ranged between 21 and 40 years (mean, 28.8±7.8 years);
the pregnancy duration ranged between 40 and 75 days (mean,
55.3±9.8 days) and the diameter of the gestational sacs ranged
between 1.20 and 4.37 cm (mean, 2.65±1.08 cm). The controls were
volunteers who came to the hospital for an induced abortion. The
age-range of the controls was 20–38 years (mean, 29.1±8.6 years);
the pregnancy duration ranged between 39 and 65 days (mean,
53.8±9.4 days) and the diameter of the gestational sacs ranged
between 1.17 and 4.55 cm (mean, 2.45±1.11 cm). No significant
differences in maternal age, pregnancy duration or gestational sac
size were identified between the controls and the patients
(P<0.05). This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Written informed consent was obtained from all
participants.
Quantitative polymerase chain reaction
(qPCR)
Chorionic villus tissues were homogenized in 1 ml
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and 200 μl
chloroform was added and mixed. The mixture was subsequently
naturally stratified on ice and centrifuged at 15,000 × g for 10
min, prior to the supernatant being transferred and mixed with an
equal volume of isopropanol. Following this, the RNA was collected
by centrifugation at 15,000 × g for 15 min and washed twice with
75% cooling ethanol, prior to being centrifuged for a final time at
10,000 × g for 10 min. The precipitate was subsequently redissolved
in diethylpyrocarbonate (DEPC)-treated sterilized water. The RNA
was converted into cDNA using reverse transcription reagents
(Takara, Dalian, China) and then used for qPCR.
To examine the endogenous mRNA expression of p53,
the qPCR was performed using the following primers: p53-forward:
5′-CCCCTCCTGGCCCCTGTCATCTTC-3′; and p53-reverse:
5′-GCAGCGCCTCACAACCTCCGTCAT-3′. The reaction mixture was prepared
with SYBR-Green master mix (Roche Diagnostics, Basel, Switzerland),
500 nmol/l of each primer and 80–100 μg of cDNA, to provide a final
volume of 20 μl. qPCR was performed using an ABI Prism 7500
instrument (Applied Biosystems, Foster City, CA, USA) with the
following cycle parameters: 30 sec at 95°C, followed by 40 cycles
of 3 sec at 95°C and 30 sec at 60°C. The specificity of the product
was determined using melting curve analysis according to the
manufacturer’s instructions. The data acquired were analyzed using
the 2−ΔΔCT method.
Immunohistochemical analysis
Each tissue sample was paraffin-embedded and cut
into 5-μm sections, prior to being mounted on a glass slide and
dried for 5 min at 70°C. The slides were deparaffinized in xylene,
rehydrated using graded ethanol and washed in phosphate-buffered
saline (PBS; 0.2% Tween-20) three times for 5 min each time. PBST
was used for all subsequent washes. The tissues were quenched in 3%
H2O2-methanol, washed three times and blocked
with PBST containing 10% goat serum for 30 min at 37°C. The slides
were subsequently incubated with mouse-anti-human p53 antibody
(1:200 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA)
at 4°C overnight. Following three further washes to remove excess
antibodies, the slides were incubated with diluted goat-anti-mouse
peroxidase-conjugated antibody (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) for 30 min at 37°C. The slides were then
washed three times, prior to staining with 3,3′-diaminobenzidine
(DAB) as the chromogen. Following this, the slides were
counterstained with hematoxylin, dehydrated using a graded ethanol
series and mounted using mountant. A semi-quantitative method was
used to analyze the levels of p53 protein. An average of 10 fields
was observed for each specimen at a magnification of ×400. The
Motic Med 6.0 Digital Medical Image Analysis System (Motic
Instruments Inc., Richmond, Canada) was used for data analysis.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)
staining
Apoptosis was detected using TUNEL staining, in
accordance with the manufacturer’s instructions (TUNEL kit; Roche
Diagnostics). In brief, paraffin-embedded sections were
deparaffinized and rehydrated as previously described in the
immunohistochemistry analysis, prior to being pretreated with
proteinase K for 30 min at 37°C and rinsed with PBST three times
for 5 min each time. Samples were then incubated with 50 μl TUNEL
reaction mixture for 1 h at 37°C in a wet-box. Following a further
three washes, 4′,6-diamidino-2-phenylindole (DAPI) was applied for
nuclear staining. The sections were subsequently observed under a
fluorescence microscope (Olympus BX60; Olympus, Tokyo, Japan). at a
magnification of ×400. An average of 10 fields was observed for
each specimen. The degree of apoptosis was represented by the
percentage of positively stained cells. Slides that were treated in
the same manner, although without incubation with the TUNEL
reaction mixture, served as negative controls.
Statistical analysis
The data are presented as the mean ± standard
deviation and were analyzed using SPSS 17.0 statistical software
(SPSS, Inc., Chicago, IL, USA). Measurement data were compared
using a Dunnett’s t-test, while enumeration data were analyzed
using a χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of p53 in URSA
In order to validate the variability of p53
expression in females with URSA compared with that in females with
a normal pregnancy, qPCR and immunohistochemistry were performed to
evaluate the expression levels of p53 in the chorionic villi of the
URSA and control groups. The results showed that the mRNA and
protein expression levels of p53 were upregulated in the URSA group
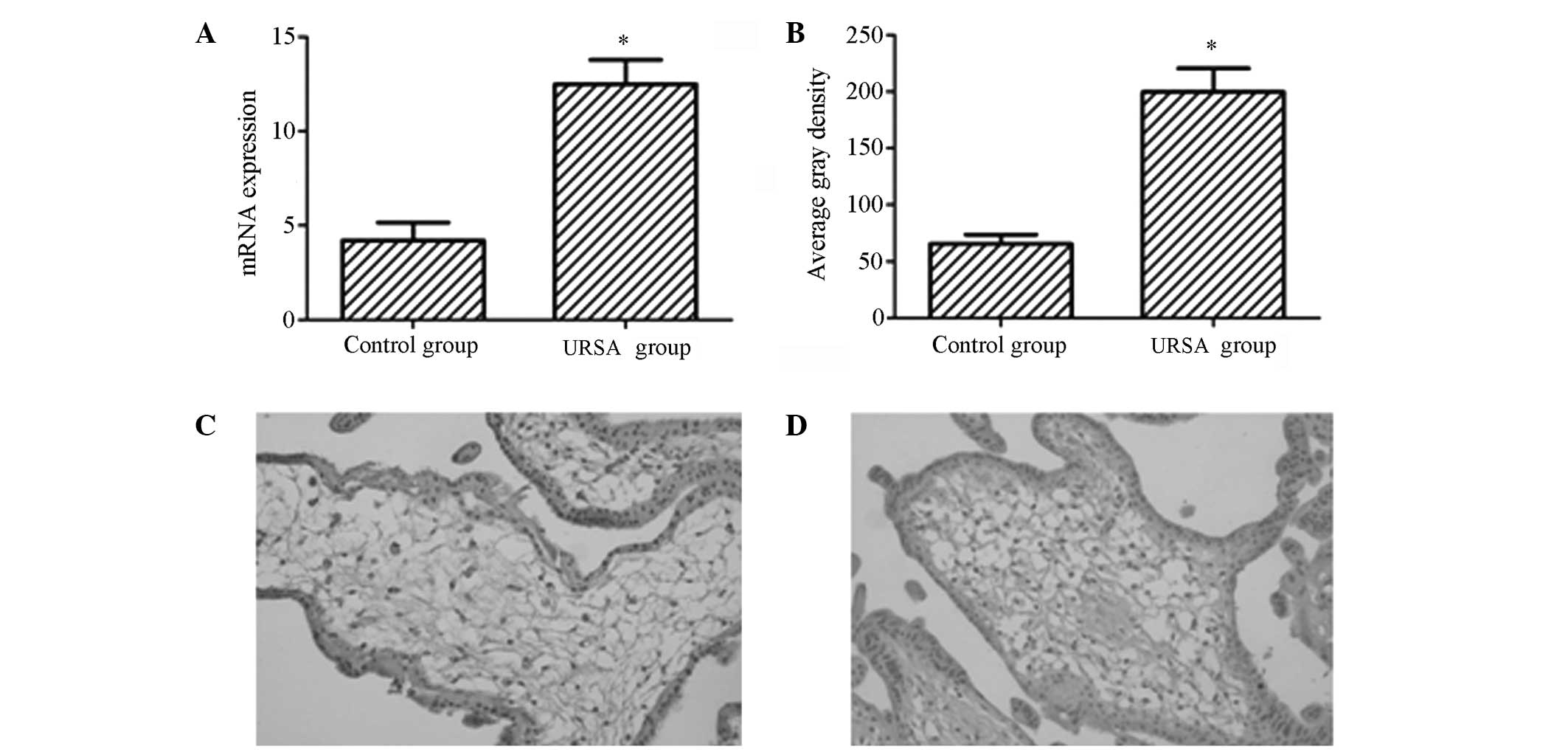
compared with those in the control group (Fig. 1A and B), with statistically
significant differences (P<0.05). As shown in Fig. 1C and D, the p53 protein was
observed to be predominantly distributed in the nucleus, appearing
as yellow or pale brown particles.
Apoptotic events in URSA
The level of apoptosis in the chorionic villi of
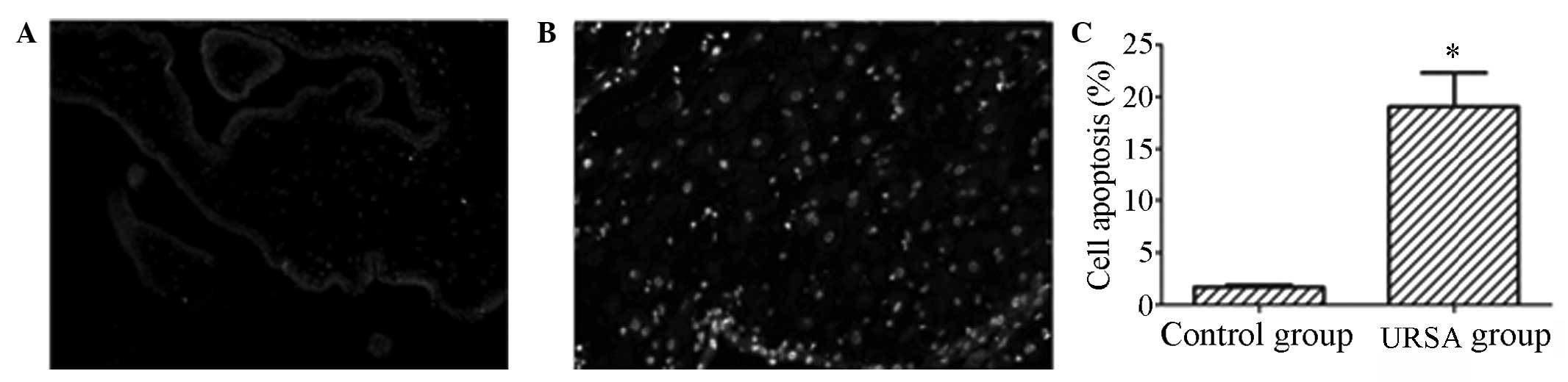
females with URSA was analyzed using a TUNEL assay. As shown in
Fig. 2A and B, a significant
increase in the number of apoptotic events was observed in the
chorionic villus tissues of the URSA group compared with the number
in the control group. The results of the statistical analysis are
shown in Fig. 2C: The level of
apoptosis was demonstrated to be 2.16% in the control group,
compared with 19.7% in the URSA group. The difference between the
groups was identified to be statistically significant
(P<0.05).
Discussion
RSA is a health problem that affects 1–5% of females
of a childbearing age. In ~50% of patients with RSA, the mechanisms
remain unexplained. It has increasingly been demonstrated that the
occurrence of URSA is associated with a high level of cell
apoptosis (6). Apoptosis, also
known as programmed cell death, is regulated by a series of genes
and is important for cell proliferation and differentiation. It may
be that a low level of apoptosis in placental villi and decidual
tissues is a normal physiological phenomenon (11) and that RSA occurs when levels of
apoptosis are high. A study by Shiraishi et al(12) of rat abortion models revealed that
the apoptosis level increased significantly in the chorionic villi
(placental tissue) of rats with URSA (12), indicating that the RSA may have
been due to the high level of apoptosis.
A number of studies have revealed that the abnormal
expression of genes involved in apoptosis, such as Fas/Fas ligand
(FasL) (13), transforming growth
factor (TGF)-β (14), tumor
necrosis factor (TNF)-α (15) and
Bcl-2/Bcl-2-associated X protein (Bax) (16), in placental villi and decidual
tissues is one of the causes of RSA. p53 is an important protein
involved in apoptosis and has been shown to participate in cell
cycle regulation (17,18). In the present study, the mRNA and
protein expression levels of p53 were investigated in the chorionic
villus (placental) tissues of females with URSA by qPCR and
immunohistochemical analysis. The mRNA and protein expression
levels of p53 were observed to be significantly higher in the URSA
group compared with those in the healthy control group. This
indicated that the RSA may have been due to the abnormal expression
of p53 in the chorionic villi. This result was consistent with
results from studies investigating other types of abortion
(19,20).
To validate the function of p53 in URSA, the levels
of apoptosis in the placental villi of patients with URSA were
detected using a TUNEL assay. TUNEL is an established method used
to detect DNA fragments; DNA fragmentation represents a
characteristic hallmark of apoptosis. It was observed that the
number of apoptotic events were significantly higher in the
chorionic villus tissues of the URSA group compared with the number
in the control group, which suggests that the high expression level
of p53 resulted in an increased number of apoptotic events, and
thus led to RSA.
In conclusion, a high level of p53 expression may
result in an elevated level of apoptosis, which may then lead to
RSA. However, the detailed regulatory mechanisms require further
study.
References
|
1
|
Saravelos SH, Cocksedge KA and Li TC: The
pattern of pregnancy loss in women with congenital uterine
anomalies and recurrent miscarriage. Reprod Biomed Online.
20:416–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Dahtory FA: Chromosomal abnormalities
as a cause of recurrent abortions in Egypt. Indian J Hum Genet.
17:82–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Todorova-Ananieva K: Autoimmune thyroid
disorders and reproductive failures. Akush Ginekol (Sofiia).
48(Suppl 2): 26–30. 2009.(In Bulgarian).
|
|
4
|
Shankarkumar U, Pradhan VD, Patwardhan MM,
Shankarkumar A and Ghosh K: Autoantibody profile and other
immunological parameters in recurrent spontaneous abortion
patients. Niger Med J. 52:163–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nair RR, Khanna A and Singh K: Association
of FAS −1377 G>A and FAS −670 A>G functional polymorphisms of
FAS gene of cell death pathway with recurrent early pregnancy loss
risk. J Reprod Immunol. 93:114–118. 2012.
|
|
6
|
Cinar O, Kara F and Can A: Potential role
of decidual apoptosis in the pathogenesis of miscarriages. Gynecol
Endocrinol. 28:382–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer JH and Helfand SL: New tricks of an
old molecule: lifespan regulation by p53. Aging Cell. 5:437–440.
2006. View Article : Google Scholar
|
|
8
|
Kaare M, Bützow R, Ulander VM, Kaaja R,
Aittomäki K and Painter JN: Study of p53 gene mutations and
placental expression in recurrent miscarriage cases. Reprod Biomed
Online. 18:430–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Shen D, Gu Y, Zhong P, Xie J and
Song Q: The diagnostic value of Ki-67, P53 and P63 in
distinguishing partial Hydatidiform mole from hydropic abortion.
Wien Klin Wochenschr. 124:184–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang Y, Kong B, Yang Q, Ma D and Qu X: The
p53-HDM2 gene-gene polymorphism interaction is associated with the
development of missed abortion. Hum Reprod. 26:1252–1258. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halperin R, Peller S, Rotschild M,
Bukovsky I and Schneider D: Placental apoptosis in normal and
abnormal pregnancies. Gynecol Obstet Invest. 50:84–87. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiraishi H, Hayakawa S and Satoh K:
Murine experimental abortion by IL-2 administration is caused by
activation of cytotoxic T lymphocytes and placental apoptosis. J
Clin Lab Immunol. 48:93–108. 1996.PubMed/NCBI
|
|
13
|
Ejima K, Koji T, Tsuruta D, Nanri H,
Kashimura M and Ikeda M: Induction of apoptosis in placentas of
pregnant mice exposed to lipopolysaccharides: possible involvement
of Fas/Fas ligand system. Biol Reprod. 62:178–185. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannubilo SR, Landi B, Pozzi V, et al:
The involvement of inflammatory cytokines in the pathogenesis of
recurrent miscarriage. Cytokine. 58:50–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Liu T and Wang Z: Association of
tumor necrosis factor-α gene promoter polymorphisms (−308G/A,
−238G/A) with recurrent spontaneous abortion: a meta-analysis. Hum
Immunol. 73:574–579. 2012.
|
|
16
|
Taylor DD and Gercel-Taylor C: Alterations
in T-cell signal transduction molecules associated with recurrent
spontaneous pregnancy loss. J Reprod Immunol. 63:137–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madan E, Gogna R, Kuppusamy P, Bhatt M,
Pati U and Mahdi AA: TIGAR induces p53-mediated cell-cycle arrest
by regulation of RB-E2F1 complex. Br J Cancer. 107:516–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng J, Zhang HH, Zhou CX, Li C, Zhang F
and Mei QB: The histone deacetylase inhibitor trichostatin A
induces cell cycle arrest and apoptosis in colorectal cancer cells
via p53-dependent and -independent pathways. Oncol Rep. 28:384–388.
2012.PubMed/NCBI
|
|
19
|
Chen YX, Shen DH, Gu YQ, et al:
Immunohistochemistry of p57 and p53 protein in differential
diagnosis of hydropic abortion, partial and complete hydatidiform
mole. Zhonghua Bing Li Xue Za Zhi. 40:694–697. 2011.(In
Chinese).
|
|
20
|
Savion S, Lepsky E, Orenstein H, et al:
Apoptosis in the uterus of mice with pregnancy loss. Am J Reprod
Immunol. 47:118–127. 2002. View Article : Google Scholar : PubMed/NCBI
|